Abstract
The identification and mobilization of useful genetic variation from germplasm banks for use in breeding programs is critical for future genetic gain and protection against crop pests. Plummeting costs of next-generation sequencing and genotyping is revolutionizing the way in which researchers and breeders interface with plant germplasm collections. An example of this is the high density genotyping of the entire USDA Soybean Germplasm Collection. We assessed the usefulness of 50K single nucleotide polymorphism data collected on 18,480 domesticated soybean (Glycine max) accessions and vast historical phenotypic data for developing genomic prediction models for protein, oil, and yield. Resulting genomic prediction models explained an appreciable amount of the variation in accession performance in independent validation trials, with correlations between predicted and observed reaching up to 0.92 for oil and protein and 0.79 for yield. The optimization of training set design was explored using a series of cross-validation schemes. It was found that the target population and environment need to be well represented in the training set. Second, genomic prediction training sets appear to be robust to the presence of data from diverse geographical locations and genetic clusters. This finding, however, depends on the influence of shattering and lodging, and may be specific to soybean with its presence of maturity groups. The distribution of 7608 nonphenotyped accessions was examined through the application of genomic prediction models. The distribution of predictions of phenotyped accessions was representative of the distribution of predictions for nonphenotyped accessions, with no nonphenotyped accessions being predicted to fall far outside the range of predictions of phenotyped accessions.
Keywords: genomic prediction, soybean, genetic diversity
The foundation of plant breeding is genetic diversity, yet the success of modern scientific plant breeding is leading to an erosion of the very genetic diversity it relies upon as farmers discard landraces in favor of genetically improved and uniform cultivars derived from a limited ancestral base. This genetic erosion increases vulnerability to agricultural insect and disease epidemics, as well as diminishes gains from breeding and selection. Germplasm collections serve as an important source of variation for germplasm enhancement; that variation sustains long-term genetic gain in breeding programs. A stunning number of accessions—7.4 million—is being maintained ex situ by plant germplasm collections worldwide, also referred to as gene banks (FAO 2010). The largest number of accessions belongs to wheat, with ∼856,000 accessions held, followed by rice with nearly 774,000 accessions (FAO 2010). The USDA National Plant Germplasm System (NPGS) alone holds > 574,000 accessions for 14,965 species as of June 2015, ranging from 53,525 accessions for rice to 165 accession for quinoa (https://npgsweb.ars-grin.gov/gringlobal/query/accessionsbysite.aspx).
The identification and mobilization of useful genetic variation from germplasm banks for use in breeding programs is clearly a necessity, not only for sustaining current rates, but also for increasing future rates of crop genetic improvement (Sehgal et al. 2015). Nevertheless, there is evidence that these collections are woefully underutilized. In 2004, Carter and coworkers estimated that among ∼45,000 unique soybean accessions maintained in germplasm collections worldwide, only 1000 have been used in applied breeding programs (Carter et al. 2004). Beneficial alleles for traits like yield have been mined from exotic and wild germplasm (Tanksley et al. 1996; Fox et al. 2015), and breeders accept that landraces and exotic germplasm likely contain alleles that could enhance their germplasm, even for intensely selected traits, such as yield. However, efficiently mining such large germplasm collections with little knowledge on accession breeding values and the distribution of favorable alleles for complex traits like yield is a huge challenge, yet selecting exotic parents for yield improvement is just as critical as selecting elite parents.
Plummeting costs of next-generation sequencing (NGS) is revolutionizing the way in which researchers and breeders interface with plant germplasm collections. It is possible that all accessions held worldwide will be densely genotyped using NGS technologies. Some present examples of wide-scale genotypic characterization of the germplasm collections include the genotyping by sequencing of the CIMMYT maize collection (Hearne et al. 2015) and the sequencing of 3000 rice genomes (Li et al. 2014). This information will greatly benefit the selection of accessions for breeding and genetics research. Using genomic data, accessions could be selected that contain specific alleles of desired effect (McCouch et al. 2012), or all accessions representing all allelic variations at particular loci (such as maturity) could be selected. An allele-focused approach could be replaced or augmented by a genomic prediction approach to predict the breeding value of each accession held in the collection (Meuwissen et al. 2001; Habier et al. 2007; VanRaden 2008). Such predictions on breeding value, especially when compared to some well-known adapted checks, would greatly increase the value of germplasm collections by giving breeders a means to identify those accessions (of the thousands that are available) meriting their attention (Longin and Reif 2014).
The USDA Soybean Collection dates back to 1895, with record keeping formally starting in 1898. A large share of the accessions (∼5000) was collected as part of the expedition of P. H. Dorsett and W. J. Morse in Asia between 1924 and 1932 (Nelson 2011). The USDA Soybean Germplasm Collection (hereafter referred to as the Collection) is one of the most intensely used germplasm collections in the world, and the most intensely used in the NPGS (Nelson 2011). Remarkably, the entire collection has been genotyped with 50K single nucleotide polymorphisms (SNPs; Song et al. 2015), creating a tremendous resource for understanding the distribution of genomic variation in the Collection and how it relates to phenotypic variation.
We assessed the usefulness of the genomic and phenotypic data collected on 9171 records from the Collection for developing genomic prediction models to evaluate the genetic value of accessions held in the collection for the complex, yet economically important, traits of protein, oil, and yield. Moreover, we investigated factors affecting prediction accuracy such as training set composition, both in terms of subpopulation membership and trial locations. Our results are the first report on using comprehensive, extensive data gathered over time by the curators of a germplasm collection for making genomic predictions that will help breeders select accessions in a more rational manner.
Materials and Methods
Phenotypic and genotypic data
The USDA Soybean Germplasm Collection contains ∼18,500 accessions of G. max. The phenotypic data used in this study was obtained from the USDA Soybean Germplasm Collection evaluations conducted periodically to characterize newly acquired accessions for basic morphological, agronomic (including yield), and seed quality traits. Data from 25 trials were analyzed (Table 1). Dates of the data sets range from 1963 to 2003 and locations include Urbana, IL; St. Paul, MN; Lexington, KY; and Stoneville, MS. The majority (5731) of accessions was evaluated in just one trial, 2976 accessions were evaluated in two trials, 50 accessions were evaluated in three trials, 11 accessions were evaluated in four trials, and three accessions in five trials. Accessions originally classified as maturity group 0 were evaluated mostly in St. Paul, with a small number evaluated in Urbana. Classification of these accessions has now been refined to include 00 and 000 classification. Maturity groups (MG) I–III were evaluated predominantly in Urbana, with some MG I and II evaluated in St. Paul. MG IV were evaluated in Lexington, Urbana and, to a small extent, Stoneville. Accessions belonging to MGs V–IX were evaluated in Stoneville, with the exception of seven MG V accessions being evaluated in Urbana in 2001–2002 (Table 1). All trials were blocked by MG. The 1MN63 and 1IL64 trials included two replications planted within the same year. All other trials also included two replicates, but replicates were planted in two separate years. Field plots comprising the trials conducted between 1963 and 1966 were two rows per entry, 2.4 m long and 1 m apart, except for 1MN63 in which row spacing was 0.90 cm. Starting in 1980, trials consisted of four-row plots to minimize competition effects. Rows were 3 m long and 0.75 m apart at planting and end-trimmed to 2.4 m long. The only exceptions were the 1989–1990 trials in St. Paul and Urbana, where rows were planted to be 4.7 m long but later trimmed to 3.2 m. Data were collected only on the center two rows.
Table 1. List of trials including accessions from the USDA Soybean Germplasm Collection.
| Trial | Location | Year(s) | Trial Entries | Trial MGs | No. per MG per Trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | VI | VII | VIII | IX | |||||
| SOYBEAN.EVALUATION.1MN63 | St. Paul, MN | 1963 | 170 | 1 | 170 | |||||||||
| SOYBEAN.EVALUATION.2MN81 | St. Paul, MN | 1980–1981 | 260 | 1 | 260 | |||||||||
| SOYBEAN.EVALUATION.3MN83.1 | St. Paul, MN | 1982–1983 | 136 | 1 | 136 | |||||||||
| SOYBEAN.EVALUATION.4MN87 | St. Paul, MN | 1986–1987 | 63 | 2 | 61 | 2 | ||||||||
| SOYBEAN.EVALUATION.5MN90 | St. Paul, MN | 1989–1990 | 31 | 1 | 31 | |||||||||
| SOYBEAN.EVALUATION.MN945 | St. Paul, MN | 1994–1995 | 257 | 3 | 136 | 109 | 12 | |||||||
| SOYBEAN.EVALUATION.MN0102 | St. Paul, MN | 2001–2002 | 422 | 3 | 176 | 241 | 5 | |||||||
| SOYBEAN.EVALUATION.1IL64 | Urbana, IL | 1964 | 125 | 3 | 1 | 76 | 48 | |||||||
| SOYBEAN.EVALUATION.1IL66 | Urbana, IL | 1965–1966 | 248 | 3 | 1 | 88 | 159 | |||||||
| SOYBEAN.EVALUATION.2IL81.1 | Urbana, IL | 1980–1981 | 570 | 4 | 9 | 175 | 174 | 212 | 0 | |||||
| SOYBEAN.EVALUATION.2IL81.2 | Urbana, IL | 1980–1981 | 519 | 2 | 24 | 495 | ||||||||
| SOYBEAN.EVALUATION.3IL83.1 | Urbana, IL | 1982–1983 | 433 | 2 | 1 | 432 | ||||||||
| SOYBEAN.EVALUATION.3IL83.2 | Urbana, IL | 1982–1983 | 153 | 3 | 2 | 86 | 65 | |||||||
| SOYBEAN.EVALUATION.3IL84 | Urbana, IL | 1983–1984 | 44 | 2 | 38 | 6 | 0 | |||||||
| SOYBEAN.EVALUATION.4IL87 | Urbana, IL | 1986–1987 | 367 | 5 | 1 | 65 | 91 | 59 | 151 | |||||
| SOYBEAN.EVALUATION.5IL90 | Urbana, IL | 1989–1990 | 379 | 5 | 2 | 12 | 51 | 89 | 225 | |||||
| SOYBEAN.EVALUATION.IL945 | Urbana, IL | 1994–1995 | 811 | 5 | 2 | 86 | 149 | 186 | 388 | |||||
| SOYBEAN.EVALUATION.IL0102 | Urbana, IL | 2001–2002 | 398 | 6 | 7 | 204 | 22 | 36 | 122 | 7 | ||||
| SOYBEAN.EVALUATION.MS923 | Stoneville, MS | 1992–1993 | 598 | 3 | 4 | 587 | 7 | |||||||
| SOYBEAN.EVALUATION.MS945 | Stoneville, MS | 1994–1995 | 653 | 5 | 1 | 5 | 318 | 328 | 1 | |||||
| SOYBEAN.EVALUATION.MS967 | Stoneville, MS | 1996–1997 | 974 | 6 | 45 | 233 | 243 | 208 | 236 | 9 | ||||
| SOYBEAN.EVALUATION.MS989 | Stoneville, MS | 1998–1999 | 307 | 6 | 9 | 102 | 80 | 69 | 46 | 1 | ||||
| SOYBEAN.EVALUATION.MS2000_02 | Stoneville, MS | 2000, 2002 | 564 | 3 | 485 | 76 | 3 | |||||||
| SOYBEAN.EVALUATION.MS2001_03 | Stoneville, MS | 2001, 2003 | 162 | 5 | 10 | 22 | 47 | 28 | 55 | |||||
| SOYBEAN.EVALUATION.2KY81 | Lexington, KY | 1980–1981 | 527 | 1 | 527 | |||||||||
| Total | 9171 | 993 | 1402 | 593 | 786 | 2196 | 854 | 1038 | 633 | 665 | 11 | |||
Protein and oil were also measured using seeds of accessions stored in cold room of the Urbana maintained Collection. This dataset is named SOYBEAN.CHEMICAL.NB.2009 and consists of 2721 samples. Soybean samples were sent from the Collection to St. Paul, MN where they were ground and scanned by NIR (Foss 6500) at the University of Minnesota. All accessions included in this set were also grown and phenotyped as part of other trials.
The traits analyzed for this study were seed yield, oil, protein, lodging, and early shattering. Seed yield was measured as the machine harvestable seed weight per plot adjusted to 13% seed moisture and expressed as Mg ha–1. From 1963 to 1966, protein concentration was determined using the Kjeldahl method, and oil concentration was determined using the Butt extraction method. From 1981 and beyond, oil and protein concentrations were determined using near-infrared reflectance on ground samples. Lodging is rated on a scale of 1–5, with 1 given to plots with 100% erect plants, and 5 given to plots with prostrate plants. Early shattering is scored at harvest on a 1–5 scale, where 1 = no shattering, 2 = 1–10% shattering, 3 = 10–25% shattering, 4 = 25–50% shattering, and 5 = greater than 50% shattering. More detailed trait descriptions and information on methods of measurement can be found at https://npgsweb.ars-grin.gov/gringlobal/descriptors.aspx, then choose “Soybean” from the dropdown menu.
In addition to the phenotypic data routinely collected by the USDA and collaborators on the collection, an independent data set on MGs I–V PIs was obtained to serve as an additional validation set. These data were collected by J. E. S. at the University of Nebraska in 2003 and 2004. Briefly, 101 accessions were selected from a larger set of ∼1500 accessions on the basis of acceptable lodging, seed shattering, disease resistance, and overall appearance. Most of these 101 accessions belong to MGs II and III. They were evaluated in field trials under two water regimes, dryland and full irrigation, at Lincoln, NE. Plots were arranged in a randomized complete block design with four replications per water regime. Replications receiving the same water treatment were blocked together in the field. Plots consisted of two rows 0.76 m apart and 2.90 m long. Plots were machine harvested, and seed yield was adjusted to 13% seed moisture. Protein and oil concentration were measured using near-infrared reflectance spectroscopy. For use here, the data were divided into four water-regime–year combinations. A linear model was fit to each dataset separately to calculate estimates of broad-sense heritability on an entry-mean basis. The linear model included rep (fixed) and accession (random).
The original genotype data set consisted of 52,041 SNPs scored using the Illumina Infinium SoySNP50K BeadChip as described by Song et al. (2013). The SNP data are publicly available at http://www.soybase.org/dlpages/index.php. SNPs with greater than 80% missing scores and minor-allele frequencies < 0.01 were removed from the data set, leaving 38,452 SNPs for analysis and genomic prediction model training.
Subpopulation assignment
The effect of predicting across and within subpopulations was investigated. Previous research found that country of origin and MG explain only a small proportion of the subpopulation structure (Bandillo et al. 2015). Accessions were clustered using ADMIXTURE (Alexander et al. 2009) to objectively assign accessions to more genetically differentiated subpopulations. ADMIXTURE provides model-based estimations of ancestry based on multi-locus genotype data. A number of subpopulations, K, is defined by the user. Each individual is assigned a membership probability to each subpopulation. For this study, the conversion from membership probabilities to discrete subpopulation memberships was accomplished by assigning each accession to the subpopulation that it had the highest probability of belonging to. Determining the value of K was accomplished using the differences from the estimated 10-fold cross-validation (CV) errors obtained from ADMIXTURE for successive K-values (ΔCV). Although the election of an optimal number of subpopulations is not a critical objective of this research, the K value at which ΔCV plateaued was chosen.
Models
The Bayesian models here presented include genetic and nongenetic (or structural) covariates. The nongenetic covariates were included to remove, as much possible, the phenotypic variance generated by environmental and population structural factors such as location and maturity group. Since all models have the same linear predictor form, at this point, only the general structure is shown and further specifications will be given to stress differences among models.
The linear predictor can be written as
| (1) |
where is the overall mean common to all phenotypes, is the effect of the jth trial (for j = 1,..,26); is the effect of the kth maturity group nested in the jth trial; is the additive genetic effect of the ith accession modeled using whole-genome markers; and is the residual. Residuals are assumed to be independent and identically distributed (IID) following a normal distribution with mean zero and variance . Since the effects of the maturity group are expected to change in accordance with the latitude of the trial locations, these effects were considered as nested within trials. Flat priors were given to the trial and maturity group effects to approximate fixed effects in maximum likelihood estimation.
The additive genetic effect of the ith accession is modeled as a linear combination of random marker effects represented by , where p is the number of markers, xil is indicator variable for the lth marker scored on the ith accession, with bil being the marker effect. The election of the prior distribution of the random terms enables the model to perform different actions with respect to the treatment of these marker effects as described below. A comprehensive review of the five popular models used for genomic selection (GS) can be found in de los Campos et al. (2013), but a very brief description follows:.
Genomic best linear unbiased prediction (G-BLUP):
A convenient reparameterization to reduce the computational burden is given by considering with . From the properties of the multivariate normal distribution (MVN) where an symmetric matrix whose entries are given by and is the estimated allele frequency at the lth marker. This matrix is known as the genomic relationship matrix (GRM) whose entries describe genomic similarities among pairs of accessions. The posterior mean of g is the best linear unbiased predictor of g, where is obtained via restricted maximum likelihood (REML) methods.
Bayesian least absolute angle and selection operator (LASSO):
The structure of this model is very similar to that from the Bayes A model; however, the marker-specific prior variances are assumed IID exponential, where in this case is a regularization parameter that controls the shape of the exponential prior distribution. The marginal prior distribution of the marker effects becomes a double exponential distribution (DE).
Bayes B:
Bayes B is a variable selection model allowing some proportion (π) of marker effects to be null and the remaining (1 – π) to be non-null. This is captured with a mixture density: with as the proportion of markers with null effect. With this consideration, the marker-specific prior distributions of the non-null marker effects are a scaled inverted chi-squared distribution, . The prior distribution of the marker-specific variance parameter for the non-null proportion of marker effects is similar to the one used in Bayes A. To completely specify this model, a beta prior distribution is assumed for the proportion parameter such that with and . All of these assumptions result in the marker effects having a marginal prior distribution comprised of an IID mixture of a point mass at zero, and a scaled-t distribution.
Hyperparameters for all models were set using the rules described in de los Campos et al. (2013). All models were implemented using the BGLR package (Pérez and de los Campos 2014).
Cross-validation schemes
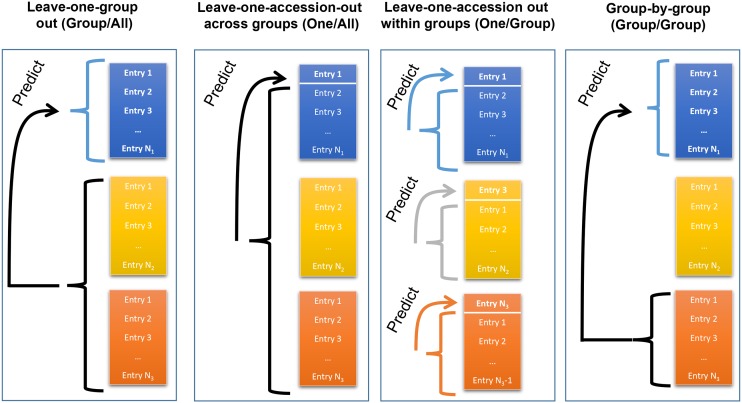
A series of CV schemes was designed to assess the usefulness of genomic predictions for selecting accessions as well as optimizing the construction of genomic prediction training sets. To accomplish the latter goal, several different grouping criteria for splitting the data were used in order to create variable training-testing relationships. The first grouping criteria involved splitting the data by trial (i.e., 26 trials for oil and protein; 25 trials for yield). A second grouping criteria used genetic criteria to split the entire population of accessions into nine subpopulations as described above. Finally, the training-testing sets were grouped by geographical location, which in this case was defined by state in which the evaluation trials were conducted (i.e., MN, IL, or MS). The KY data were dropped from this analysis since only one trial was conducted in KY.
Four CV schemes were applied to each grouping criteria. Each CV scheme mimicked the problem of predicting accessions without data. To accomplish this, all phenotypic records of any accession in the validation set was removed from the training set before model training. Each CV scheme is described individually.
Leave-one-accession-out within groups (One/Group): within each group (i.e., trial, subpopulation, state) each accession is predicted, one at a time, using as the training set the data from the remaining accessions in the same group. This procedure is repeated until all accessions in the group are predicted. To assess predictive ability, observations and predictions are compiled and correlated for each group separately.
Leave-one-accession-out across groups (One/All): this CV is the same as One/Group except training sets consists of data from all groups rather than only a single group.
Leave-one-group-out (Group/All): here, each group is predicted using a training set consisting of data from the other groups only. The training set does not include data from the group comprising the validation set.
Group-by-group (Group/Group): a whole group is predicted using the information from another, single group. This procedure is repeated for all possible combinations.
A schematic of these CV schemes is displayed in Figure 1.
Figure 1.
A diagram of the four cross-validation schemes used to validate genomic predictions. Each of the colors represents a different group. Groups are comprised of trials, states, or genetic clusters (see Materials and Methods). Arrows point from the training set to the validation set.
Predictive ability was assessed using Pearson’s product-moment correlation coefficient on the vectors of genomic predictions and observed phenotypes adjusted for trial and MG effects. Confidence intervals were computed using the bootstrap procedure with 10,000 bootstrap replicates.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
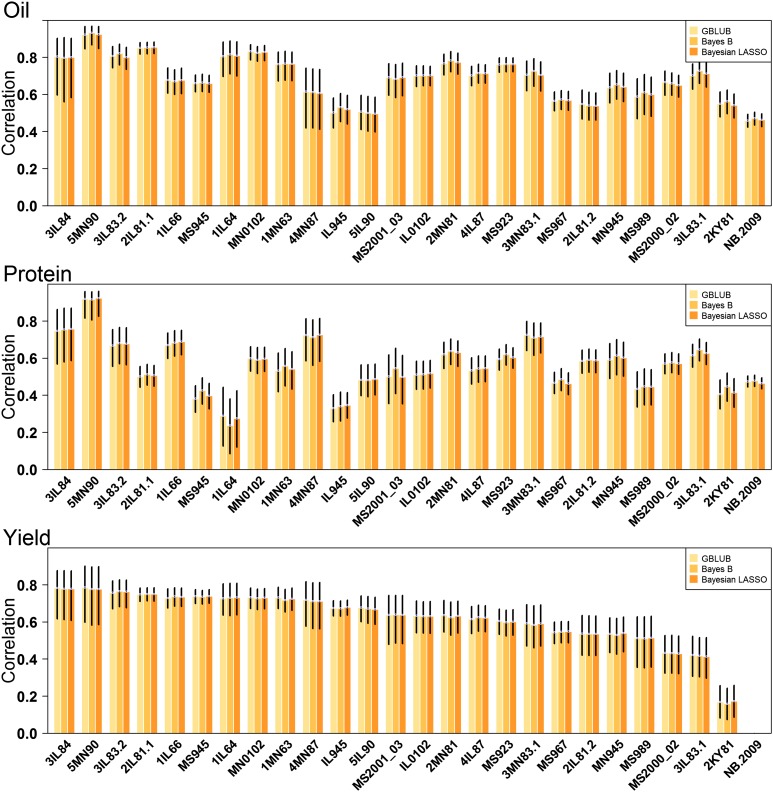
An initial assessment of predictive ability for oil, protein, and yield was made by performing Group/All CV and evaluating predictive abilities among the 25–26 trials with the five models described above. Average predictive abilities were moderate to very high for most trial and trait combinations (Figure 2, and Supplemental Material, Table S1). For oil, predictive ability ranged from 0.46 to 0.92, with a median across trials of 0.69. Predictive abilities for protein were lower, especially on the low end, ranging from 0.29 to 0.92 with a median of 0.56. Genomic prediction for accession yield, typically the most difficult trait to predict, was more successful than expected, yet highly variable, ranging from 0.17 to 0.79. The median predictive ability for yield was 0.64. Predictive abilities among the three models were compared for each trial and trait combination. Very little to no difference among models was observably evident (Figure 2). For this reason, G-BLUP was exclusively used for all subsequent analyses.
Figure 2.
Predictive abilities for oil, protein, and seed yield for each of the 25 trials. Predictions were made using the Group/All cross-validation scheme and three different models: genomic best linear unbiased prediction (G-BLUP), Bayes B, and Bayesian LASSO. The black bars display the 95% C.I.
It is important to remember that the validation phenotypes were adjusted for MG effects, and thus variation explained by the genomic prediction model is independent of any variation between maturity groups, and the predictive abilities calculated reflect the ability to predict within maturity groups. Moreover, reported predictive abilities are correlations between predictions and phenotypes corrected for MG, with no adjustment being made for the heritability of the validation phenotypes. Since the validation phenotypes are imperfect estimates of true additive genetic values, the reported predictive abilities are likely downwardly biased estimates of the true prediction accuracies being defined as the correlation between the predictions and true breeding values.
Since most of the accessions analyzed were unimproved landraces, an important consideration is the degree to which variation in lodging and shattering influence variation in machine harvestable seed yield. Shattering is a genetically simpler trait compared to yield (Funatsuki et al., 2014), and genomic prediction models trained using data from landraces might be simply predicting shattering rather than purely seed yield. An analysis of the phenotypic data did reveal that machine harvestable grain yield was negatively correlated with shattering and lodging, with mean correlation coefficients being –0.27 and –0.21, respectively (data not shown). In order to eliminate the influence of shattering and lodging on variation in seed yield, shattering and lodging scores were fit as fixed covariates both in the G-BLUP model and to calculate adjusted seed yield phenotypes for validation. Predictive ability was calculated as it was for Figure 2 using the 8517 records with available shattering and lodging scores. Predictive abilities for yield were reduced as expected when variation for lodging, shattering, or both was removed through the use of covariates (Table S2). The average reduction in seed yield predictive ability, expressed as a percentage of the original predictive ability, was near 10% when either lodging or shattering were accounted for (Table S2). When both traits were fit as covariates, the reduction in predictive ability was 23% on average across trials (Table S2). Predictive ability was not reduced at all, or very little, in some trials, whereas in others it was reduced by as much as 47%, indicating shattering and lodging affected seed yield to different degrees across trials. A similar analysis was performed on maturity date, but maturity date had a negligible effect on seed yield predictions after correction for MG effects (results not shown).
The value of training genomic prediction models for prediction of accession performance was further evaluated by using an independent set of MG II and III accessions evaluated in multiple environments, each with four replications, for the measured traits of oil, protein, and seed yield. Entry-mean heritabilities were high due to the highly replicated design, ranging from 0.83 on average for yield to 0.91 on average for protein and oil. Genomic predictive abilities, on average, were 0.58–0.59 for protein and oil, and 0.49 for yield (Table 2). These values are somewhat lower than the predictive abilities estimated for the GRIN trials, perhaps because the Nebraska trials included less genetic variability because accessions were preselected on the basis of agronomic performance. They were, however, similar to the predictive abilities for seed yield in the GRIN trials when seed yield values were adjusted for shattering and lodging. This result indicates that the genomic prediction models can still discriminate among relatively poor and good performing accessions within sets previously selected for agronomic performance. A comparison was made between the genomic predictive ability and correlations between the data available from the GRIN trials, and the phenotypes collected in the highly replicated Lincoln, NE, trials. Genomic predictive ability was consistently better than the GRIN phenotypes for protein and oil, although the confidence intervals did overlap (Table 2). For yield, the two methods were similar for three of the four Lincoln, NE, trials, and genomic prediction was numerically better than the phenotypic data in the fourth comparison (DRY–2003; Table 2). This result suggests that the genomic predictions are at least as good as the phenotypic data in GRIN, and thus may be a useful tool for choosing among the nonphenotyped accessions held in the Collection or newly collected accessions.
Table 2. Genomic predictive abilities using the Lincoln, NE, data as validation data, and correlations between the phenotypic data available in GRIN and the Lincoln, NE, data.
| Genomic Predictive Ability | Correlation: GRIN Data vs. NE Trials | |||||
|---|---|---|---|---|---|---|
| Oil | Protein | Yield | Oil | Protein | Yield | |
| DRY-2003 | 0.47 [0.30; 0.63] | 0.52 [0.37; 0.65] | 0.42 [0.16; 0.64] | 0.46 [0.31; 0.60] | 0.43 [0.29; 0.56] | 0.27 [0.06; 0.41] |
| DRY-2004 | 0.62 [0.49; 0.73] | 0.63 [0.51; 0.73] | 0.50 [0.35; 0.65] | 0.46 [0.30; 0.59] | 0.45 [0.30; 0.58] | 0.49 [0.33; 0.64] |
| IRR-2003 | 0.63 [0.50; 0.75] | 0.58 [0.47; 0.67] | 0.52 [0.38; 0.66] | 0.51 [0.38; 0.63] | 0.41 [0.23; 0.55] | 0.53 [0.38; 0.67] |
| IRR-2004 | 0.64 [0.51; 0.74] | 0.59 [0.46; 0.71] | 0.53 [0.38; 0.70] | 0.53 [0.42; 0.64] | 0.46 [0.32; 0.58] | 0.52 [0.36; 0.65] |
Each year two trials were conducted with two water regimes, dryland (DRY) and irrigated (IRR). Results for oil, protein, and yield are displayed. Values displayed in brackets are the 95% C.I. of the correlation coefficient estimates.
A key question when using predictions for accession selection relates to the enrichment of the selected set. While correlations are a good indicator of how successful genomic predictions could be used for this purpose, we desired to look directly at this by calculating the frequency of “selected” accessions observed to be better than the mean or in the bottom 10% based on actual field trial data. The top 10% of accessions were chosen on the basis of their genomic predictions using G-BLUP and Group/All as described above. Shattering and lodging were not adjusted for in this analysis, as we assumed breeders would want select accessions with high machine harvestable seed yield. We found that, on average, a high percentage of accessions among the top 10% based on predictions were observed to be better than the trial mean. This value was 89% for oil, 80% for protein, and 88% for yield (Table 3). In the case of yield, 100% of the selected accessions were observed to be better than the population mean in five trials. Another key outcome would be the avoidance of poorly performing accessions. The top 10% based on predictions very rarely included accessions observed to be in the bottom 10%. The average observed frequency across trials was only 0–2% depending on the trait (Table 3). A frequency of 0% was observed for more than half the trials. This result indicates that predictions can very effectively eliminate the worst- performing accessions.
Table 3. Percentages of accessions among the top 10% of accessions based on predictions observed to be in the bottom 10% or greater than the mean based on phenotypic data from each listed trial.
| Bottom 10% | Greater Than Mean | |||||
|---|---|---|---|---|---|---|
| Oil | Protein | Yield | Oil | Protein | Yield | |
| USDA evaluations | ||||||
| 1MN63 | 0 | 0 | 0 | 100 | 76.5 | 94.4 |
| 2MN81 | 0 | 0 | 3.8 | 83.3 | 92.3 | 73.1 |
| 3MN83.1 | 0 | 0 | 0 | 93.3 | 73.7 | 86.7 |
| 4MN87 | 0 | 0 | 0 | 85.7 | 100 | 100 |
| 5MN90 | 0 | 0 | 0 | 75 | 100 | 100 |
| MN945 | 0 | 0 | 3.8 | 85.2 | 81.5 | 88.5 |
| MN0102 | 0 | 0 | 0 | 90.9 | 93 | 88.6 |
| 1IL64 | 0 | 7.7 | 0 | 92.3 | 69.2 | 84.6 |
| 1IL66 | 0 | 0 | 0 | 96 | 92 | 100 |
| 2IL81.1 | 0 | 0 | 0 | 100 | 82.5 | 96.5 |
| 2IL81.2 | 1.7 | 0 | 3.8 | 76.7 | 88.7 | 79.2 |
| 3IL83.1 | 0 | 0 | 2.2 | 85.4 | 88.6 | 68.9 |
| 3IL83.2 | 0 | 0 | 0 | 100 | 82.4 | 100 |
| 3IL84 | 0 | 0 | 0 | 100 | 80 | 100 |
| 4IL87 | 0 | 0 | 0 | 86.8 | 75 | 92.1 |
| 5IL90 | 0 | 0 | 0 | 94.7 | 78.9 | 87.2 |
| IL945 | 2.4 | 9.9 | 0 | 85.4 | 72.8 | 96.3 |
| IL0102 | 0 | 2.5 | 5.3 | 92.5 | 77.5 | 94.7 |
| MS923 | 1.5 | 0 | 0 | 80.6 | 98.3 | 77.4 |
| MS945 | 1.4 | 1.5 | 0 | 88.6 | 64.2 | 93.8 |
| MS967 | 2 | 2.1 | 1 | 83.2 | 79.4 | 88.8 |
| MS989 | 0 | 6.3 | 6.3 | 93.5 | 59.4 | 87.5 |
| MS2000_02 | 0 | 0 | 3.4 | 87.7 | 75.8 | 76.3 |
| MS2001_03 | 0 | 0 | 5.9 | 94.1 | 52.9 | 82.4 |
| 2KY81 | 0 | 4.7 | 5.6 | 78.3 | 64.1 | 61.1 |
| Mean | 0.4 | 1.4 | 1.6 | 89.2 | 79.9 | 87.9 |
| Lincoln, NE, trials | ||||||
| 2003-DRY | 0 | 0 | 9.1 | 72.8 | 90.9 | 63.7 |
| 2003-IRR | 0 | 0 | 9.1 | 91 | 81.8 | 72.8 |
| 2004-DRY | 0 | 9.1 | 9.1 | 81.8 | 72.8 | 81.8 |
| 2004-IRR | 0 | 0 | 9.1 | 81.9 | 63.7 | 72.8 |
| Mean | 0 | 2.3 | 9.1 | 81.9 | 77.3 | 72.8 |
Data for both the USDA Soybean Germplasm Collection evaluations and J.E. Specht trials conducted in Lincoln, NE, are presented.
Trials
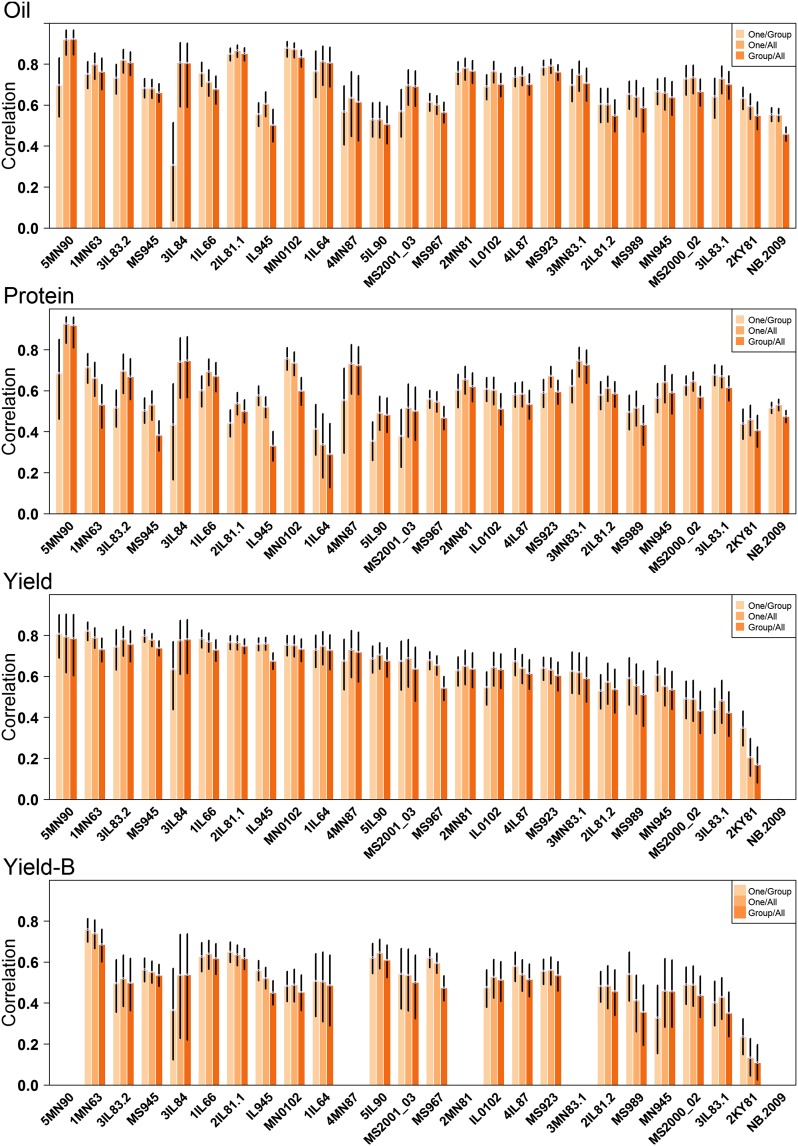
The effect of combining vs. separating trial data was evaluated by performing within-trial CV (One/Group), between-trial CV (Group/All), and combining data across all trials (One/All) (Figure 1).
Within-trial predictive abilities were moderate to high for all traits, being greater than 0.60 in most cases for oil and yield (Figure 3 and Table S1). Predictive abilities were slightly lower for protein. Only a very subtle improvement was observed when data across all other trials was added to the training set (One/All), with differences ranging from 0.04 (protein) to –0.01 (yield) (Table S1). While, on average, there was very little difference, the range across trials was considerable, and it appeared that there were benefits to including all data in the extreme cases. In the case of the 3IL84 trial, for example, it was observed that predictive ability could be increased from 0.31 to 0.81 for oil and 0.44 to 0.75 for protein when data were combined across all trials compared to a within-trial training set only. On the negative side, we observed that predictive ability was decreased by 0.05 for oil and 0.06 for protein, in the case of IL66 and IL945 trials, respectively. It appeared that, for protein and oil, benefits to combining across trials were much more dramatic compared to any reductions in predictive ability (Figure 3 and Table S1). The differences between One/Group and One/All were more uniformly distributed in the case of yield, with a reduction of 0.06 for MN945 and a gain of 0.14 for 2KY81.
Figure 3.
Predictive abilities for oil, protein, and seed yield for each of the 25 trials. Predictions were made using the One/Group, One/All, and Group/All cross-validation schemes. The black bars display the 95% C.I.
Using data from the same trial(s) in both training and validation sets creates the unrealistic advantage of including the trial-specific G × E effects contained in the validation data. Because the exact same environmental conditions specific to individual trials would not be observed again, a better assessment of the usefulness of these GRIN training sets for predicting future trial performance would be attained using the Group/All CV. The Group/All CV correlations were very close, on average, to the One/Group and One/All CV correlations (Figure 3 and Table S1), indicating that the sheer volume of data can overcome any lack of shared G × E effects.
A trial-by-trial CV (Group/Group) results in highly variable predictive abilities. In many cases, the predictive abilities between trials was zero, but, in some cases, the predictive ability reached as high as 0.90 (e.g., oil, 2MN81 predict 5MN90) (Table S3). The average predictive ability for the Group/Group CV was 0.49 for oil, 0.30 for protein, and 0.45 for yield, which is far less than the predictive abilities observed using Group/All CV. This illustrates the expected advantage of combining data across many trials to form a training set.
By ordering the trials by state, it is apparent that the northern locations of MN and IL predicted one another relatively well as compared to be predictive ability between MS and the northern locations. This pattern was more prominent for yield (Table S3).
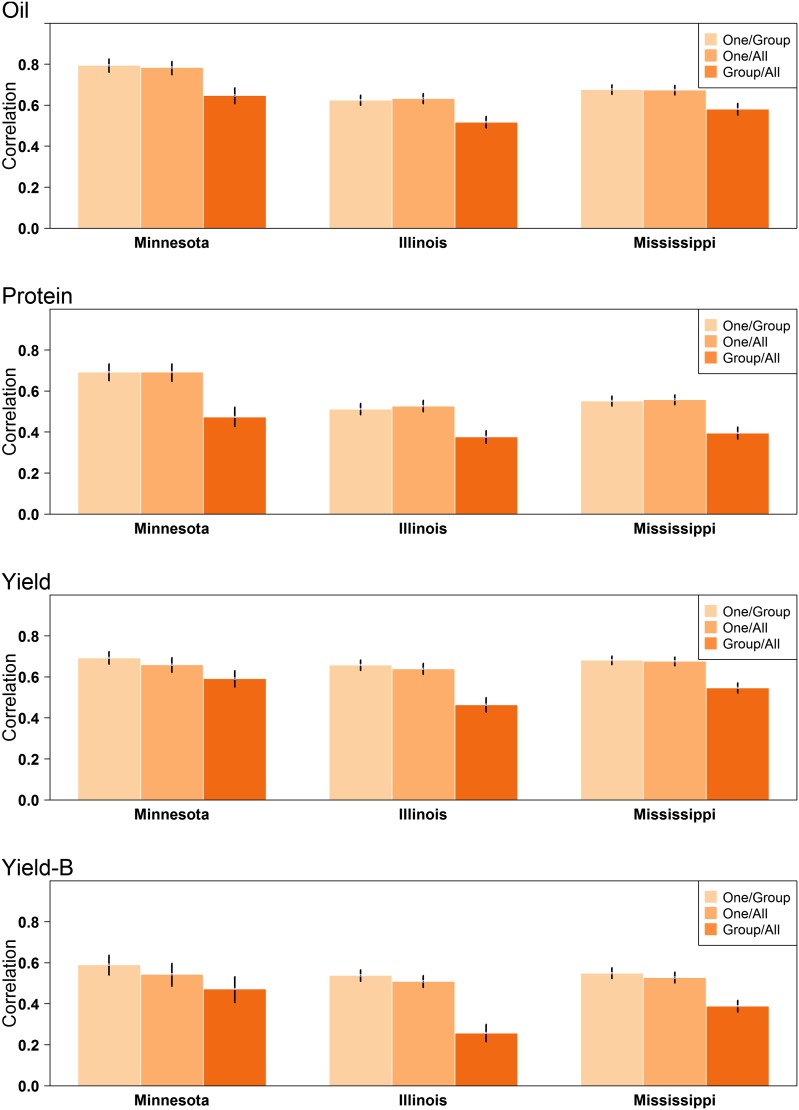
States
Given the pattern observed when predicting between trials conducted in different states, we desired to look at this more closely by setting up a CV based on trial geographical location. The distribution of data points across states is as follows: 4047 records from 11 IL trials; 1339 records from seven MN trials; 3258 records from six MS trials (Table 1). The MN trials consisted of only MG 0–II accessions with the majority (72%) belonging to MG 0. The IL trials were predominantly comprised of accessions from MGs I–IV, with < 1% being from MGs 0 and V. The MS trials consisted only of accessions from MGs IV–IX.
As expected, a training set including data from the state being predicted (One/Group or One/All) performed much better than training sets not including data from the state being predicted (Group/All) (Figure 4 and Table 4). A key question we wanted to address with this analysis was whether training sets should be created by dividing data among states, or if a universal training set including all data—regardless of state—would perform just as well or better. Little to no differences were observed between these two CV schemes for any trait and state combination (Figure 4 and Table 4). This finding suggests that predictive abilities are not improved by maximizing training set size by combining across states, nor are they reduced by including data from environments as different as MS when predicting relative performance of early MG accessions in MN. A similar pattern was observed when variation for lodging and shattering was removed through inclusion of covariates.
Figure 4.
Predictive abilities for oil, protein, and seed yield for each of the three states. Predictions were made using the One/Group, One/All, and Group/All cross-validation schemes. The black bars display the 95% C.I.
Table 4. Predictive abilities for oil, protein, and yield estimated using state as the grouping factor.
| MN | IL | MS | |
|---|---|---|---|
| Oil | |||
| MN | 0.80 | 0.71 | 0.53 |
| IL | 0.56 | 0.68 | 0.52 |
| MS | 0.43 | 0.51 | 0.62 |
| Protein | |||
| MN | 0.70 | 0.43 | 0.42 |
| IL | 0.31 | 0.55 | 0.39 |
| MS | 0.21 | 0.37 | 0.52 |
| Yield | |||
| MN | 0.69 | 0.59 | 0.44 |
| IL | 0.51 | 0.68 | 0.43 |
| MS | 0.40 | 0.45 | 0.66 |
One/Group estimates are on the diagonal and Group/Group estimates are on the off-diagonal. Columns display the states that were used as the calibration set to perform predictions of those states that appear in rows.
Clusters
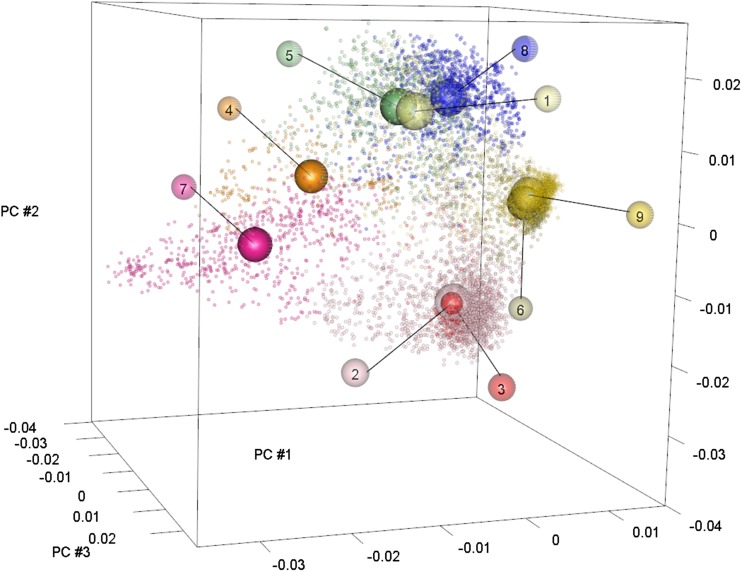
The ADMIXTURE analysis suggested the presence of nine genetic clusters within the population of accessions used for this study (Figure S1). A visual inspection of the principal component analysis plot displayed in Figure 5 suggests that the diversity within clusters varies, and structure among the clusters exists, with some clusters being more closely related than other clusters. The proximity of clusters to one another can be partially explained by MG. Clusters four, five and eight are comprised mainly of early maturity groups (0–II), whereas early and medium MGs appear in Cluster 1 (Table S4). Clusters 2, 3, 6, 7 and 9 belong to medium and late MGs. Most clusters include good representation of at least three MGs except for cluster 3, which is dominated by MG VIII.
Figure 5.
A three-dimensional plot of accession values for principal components one, two, and three. The centroid of each cluster is indicated by an empty sphere. The spheres containing numbers label each centroid by its corresponding cluster designation.
In general, predictive abilities were lower for the Group/All scheme based on cluster compared to the One/Group and One/All schemes (Figure 6 and Table 5). Without correction for shattering and lodging, the One/All scheme tended to produce the highest predictive abilities, although the difference between One/Group and One/All were very small. Correcting seed yield for shattering and lodging produced a different outcome where the One/Group scheme was markedly better for four of the nine clusters (Figure 6 and Table 5). A pattern between predictive ability and relationship between clusters was not readily apparent. The only consistent result was the poor predictive ability of cluster 3, which was expected based on its limited size and variation. These results combined indicate that within-cluster information is the most valuable information. We tested whether compiling a training set by only including related clusters improved predictive abilities. To do this, clusters 1, 4, 5, and 8 were grouped, and clusters 2, 3, 6, 7, and 9 were grouped. Grouping clusters by genetic similarity did not improve predictions compared to the universal One/All scheme (data not shown).
Figure 6.
Predictive abilities for oil, protein, and seed yield for each of the nine genetic clusters (CL). Predictions were made using the One/Group, One/All, and Group/All cross-validation schemes. The black bars display the 95% C.I.
Table 5. Genomic predictive abilities using the One/Group, Group/All, and One/All cross-validation schemes for oil, protein, and seed yield. Data was grouped by genetic cluster.
| Oil | Protein | Yield | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One/Group | Group/All | One/All | One/Group | Group/All | One/All | One/Group | Group/All | One/All | ||||||||||
| Cluster | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a | Est. | 95% C.I.a |
| 1 | 0.34 | [0.25; 0.42] | 0.48 | [0.40; 0.56] | 0.52 | [0.44; 0.58] | 0.40 | [0.32; 0.48] | 0.51 | [0.44; 0.57] | 0.55 | [0.49; 0.61] | 0.35 | [0.25; 0.46] | 0.43 | [0.30; 0.54] | 0.47 | [0.36; 0.57] |
| 2 | 0.54 | [0.51; 0.56] | 0.44 | [0.41; 0.47] | 0.54 | [0.51; 0.57] | 0.50 | [0.47; 0.53] | 0.36 | [0.32; 0.40] | 0.50 | [0.47; 0.53] | 0.57 | [0.53; 0.60] | 0.50 | [0.45; 0.55] | 0.58 | [0.54; 0.62] |
| 3 | 0.13 | [0.00; 0.30] | 0.32 | [0.11; 0.51] | 0.35 | [0.12; 0.54] | 0.32 | [0.17; 0.46] | 0.28 | [0.10; 0.45] | 0.27 | [0.09; 0.44] | 0.18 | [0.01; 0.35] | 0.27 | [0.11; 0.43] | 0.25 | [0.08; 0.41] |
| 4 | 0.41 | [0.30; 0.51] | 0.39 | [0.28; 0.50] | 0.45 | [0.35; 0.55] | 0.34 | [0.24; 0.43] | 0.32 | [0.21; 0.41] | 0.45 | [0.35; 0.53] | 0.44 | [0.33; 0.54] | 0.35 | [0.25; 0.45] | 0.41 | [0.31; 0.51] |
| 5 | 0.42 | [0.37; 0.47] | 0.42 | [0.37; 0.46] | 0.48 | [0.43; 0.52] | 0.46 | [0.40; 0.52] | 0.40 | [0.35; 0.44] | 0.51 | [0.46; 0.55] | 0.32 | [0.26; 0.39] | 0.38 | [0.31; 0.45] | 0.44 | [0.37; 0.50] |
| 6 | 0.52 | [0.49; 0.55] | 0.38 | [0.34; 0.42] | 0.53 | [0.49; 0.55] | 0.48 | [0.45; 0.51] | 0.30 | [0.27; 0.34] | 0.50 | [0.46; 0.52] | 0.44 | [0.40; 0.48] | 0.35 | [0.31; 0.39] | 0.45 | [0.42; 0.49] |
| 7 | 0.45 | [0.38; 0.51] | 0.4 | [0.34; 0.45] | 0.46 | [0.39; 0.52] | 0.40 | [0.34; 0.46] | 0.22 | [0.14; 0.29] | 0.37 | [0.30; 0.44] | 0.43 | [0.37; 0.49] | 0.40 | [0.34; 0.45] | 0.44 | [0.38; 0.49] |
| 8 | 0.55 | [0.50; 0.59] | 0.52 | [0.48; 0.57] | 0.58 | [0.53; 0.62] | 0.48 | [0.44; 0.52] | 0.41 | [0.38; 0.45] | 0.52 | [0.48; 0.55] | 0.45 | [0.39; 0.51] | 0.44 | [0.38; 0.49] | 0.49 | [0.43; 0.54] |
| 9 | 0.52 | [0.48; 0.56] | 0.38 | [0.32; 0.43] | 0.53 | [0.49; 0.56] | 0.44 | [0.40; 0.48] | 0.24 | [0.19; 0.30] | 0.46 | [0.42; 0.50] | 0.34 | [0.29; 0.39] | 0.22 | [0.15; 0.28] | 0.34 | [0.28; 0.39] |
| Mean | 0.43 | 0.42 | 0.50 | 0.42 | 0.34 | 0.46 | 0.39 | 0.37 | 0.43 | |||||||||
One/All, leave-one-accession-out within groups; Group/All, leave-one-group-out; One/All, leave-one-accession-out across groups; Est, estimated; C.I., confidence interval.
Obtained by Bootstrapping 10,000 the adjusted phenotypes and predicted values.
Prediction of nonphenotyped accessions
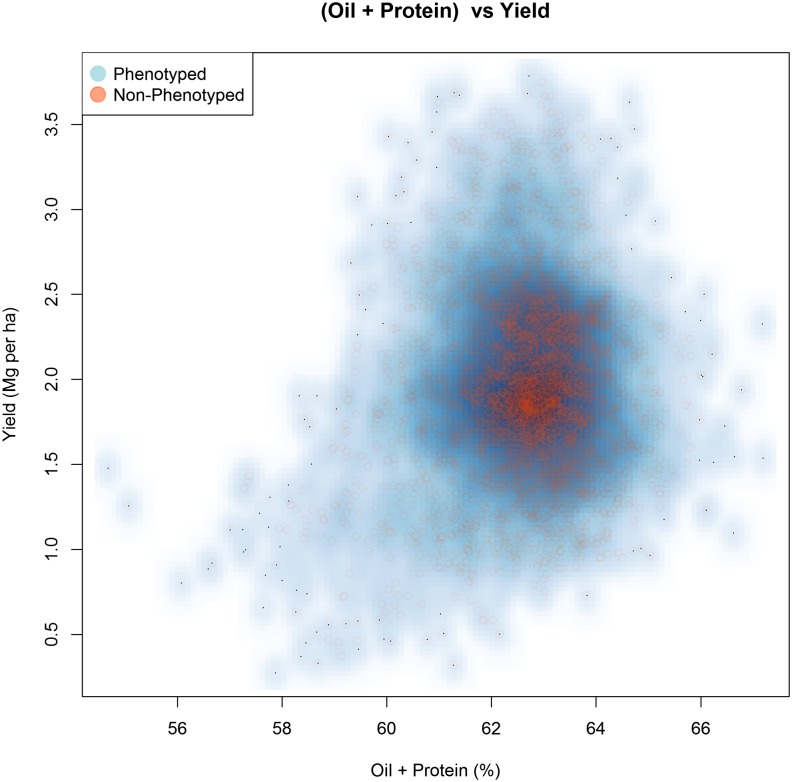
A total of 8771 accessions housed in the Collection has been phenotyped at least once in the 26 trials (Table S4), but no phenotypic data were available for 7608 accessions from GRIN when this study was designed. Genomic predictions were calculated for the nonphenotyped accessions using the full training set (i.e., all clusters, all environments) in order to assess differences in distributions between phenotyped and nonphenotyped accessions. Phenotyped accessions were predicted with the One/All cross-validation scheme. More specifically, we wanted to know if any nonphenotyped accessions would be predicted to be superior to the phenotyped accessions. Substantial differences were not observed with the predictions of the nonphenotyped accessions falling within the range of the phenotyped accessions (Figure 7). Nevertheless, using information in the form of genomic predictions will help breeders choose among those accessions that have no accompanying information, opting for those that would be expected to be above average for yield, protein, and oil, and thus avoiding those accessions predicted to be inferior for these traits. Table S5 and Table S6 contain genomic predictions for the phenotyped and nonphenotyped accessions.
Figure 7.
Scatter plot of genomic predictions for grain yield vs. the sum of oil and protein. The intercept of each trait included in the prediction to place values back on the original trait measurement scale. Phenotyped accessions are represented by the blue density cloud, and nonphenotyped accessions are represented by the red circles.
Discussion
Crop germplasm collections hold valuable genetic diversity to help protect society against the genetic erosion of agriculturally important species for which only a limited number of genotypes are actually cultivated at any given time. It is imperative that these collections exist as dynamic, utilized sources of variation rather than as “gene morgues”, as they are sometimes referred to (Hoisington et al. 1999). One obstacle to utilization is reliable phenotypic characterization of collections, as phenotyping collections consisting of tens of thousands of accessions can be difficult and expensive. High density genotyping of entire germplasm collections, however, has become more feasible than thorough phenotyping, even with the advent of phenomics platforms. This study demonstrated that historical data on accessions held in collections, when combined with high density SNP data, can be used to develop predictive models for important and complex traits of soybean. Genomic prediction models explained an appreciable amount of the variation in accession performance in independent trials, with correlations between predictions and observations reaching up to 0.92 for oil and protein, and 0.79 for machine-harvestable seed yield. Predictive abilities for seed yield were reduced when variation for lodging and shattering was accounted for. Nevertheless, estimates of prediction accuracy calculated using data from a highly replicated, independent trial of only accessions with previously determined acceptable performance (i.e., minimal shattering and lodging) also gave an optimistic outcome for using predictions to assist in the selection of superior accessions. Based on a comparison of predictions and observed field performance in each trial, a soybean breeder could select the top 10% of accessions based on genomic prediction of yield, and expect 88% of the selected accessions to be better than average for yield. This example demonstrated that genomic predictions can be used to enrich field trials of accessions with accessions that perform better than a randomly selected set. Looking at the extremes, we found that the top 10% for each trait rarely contained accessions that performed in the bottom 10% according to actual trial data, indicating that using predictions very effectively eliminates the accessions that hold little promise, ultimately saving field resources to evaluate more of those that do hold promise.
Compiling historical data on accession evaluations conducted across four states going back to 1963 provided us with a very large training dataset consisting of over 9000 accessions. Soybeans adapted to different latitudes belong to different MGs. The trials used as a source of data ranged from trials conducted on early MGs in Minnesota to late MGs conducted in MS. We explored the optimal use of such a large and diverse training set for calibrating genomic prediction models. Our results can be summarized by two basic findings. First of all, the population and target environment being predicted should be well represented in the training set. The poorest predictive abilities were observed when we attempted to predict between states, or between genetic clusters. Second, genomic prediction training sets appear to be very forgiving to the presence of data from diverse geographical locations and genetic clusters. It was surprising to observe that adding data from very different geographical locations had no effect on predictive ability. For example, the prediction of performance in MN environments was not affected by the presence of training data collected in MS on MG VII–IX accessions. This may partially be an artifact stemming from the tendency of accessions from similar MGs to genetically cluster, and the partitioning of MGs across the states used for evaluation. In BLUP, information from closer relatives is weighted more heavily, while less weight is given to information from distant relatives (de los Campos et al. 2013), meaning data from MS was probably weighted less heavily in the prediction of early MG accession performance in MN.
Building a training set by adding accessions from different and diverse genetic clusters did not improve nor harm predictive ability when the goal was to predict accession performance within a single cluster. One exception included the prediction of yield corrected for shattering and lodging across diverse clusters. Our general results are not consistent with results from barley that suggested that the addition of unrelated individuals to a training set can potentially reduce predictive ability (Lorenz and Smith 2015), but they are consistent with results in maize where training sets were formed by combining data across heterotic groups (Technow et al. 2013). The underlying reasons for the neutral effect of adding genetically distant individuals to the soybean accession training sets could relate to the flow of information from historical LD and pedigree relationships to prediction accuracy (Habier et al. 2013). In the barley case (Lorenz and Smith 2015), where there is substantive family structure and a high degree of relatedness among lines from the same breeding program, it is likely that pedigree relationships, captured by G, are the predominant source of accuracy. The addition of less related individuals can reduce the accuracy provided by this source of information (Habier et al. 2013). In the case of the soybean germplasm collection, where many individuals do not share close pedigree relationships, and where common ancestors likely go back many generations, the predominant source of accuracy is likely historical LD. The large training populations and high marker densities may have allowed the capturing of this information (Habier et al. 2013; Hickey et al. 2014), offsetting any possible detrimental effect on the genetic relationships source of information.
In conclusion, this study demonstrates that historical data collected as part of plant germplasm collection characterizations can be used to develop predictive models to help breeders select accessions for introgressing useful genetic variation. We found that, in the case of the soybean germplasm collection, these models are robust to the inclusion of diverse sources of data, but training sets should include data from populations and environments representative of the target populations and environments. This data has already been collected and made freely available, and therefore nothing is preventing the use of these models for enhancing utilization of this genetic resource. Genomic predictions might also be used to develop trait-specific “core collections” that could be used for deeper phenotyping for detailed studies on physiological mechanisms and high-resolution QTL mapping. It is anticipated that the genomics revolution will create similar data resources for germplasm collections of other agriculturally important species, and that genomic prediction will serve as a key tool for making practical use of the genomic data.
Supplementary Material
Acknowledgments
This study was funded by a grant from the North Central Soybean Research Program titled “Acceleration of Soybean Yield and Composition Improvement through Genomic Selection”. We are grateful for their support.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.031443/-/DC1
Communicating editor: S. A. Jackson
Literature Cited
- Alexander D. H., Novembre J., Lange K., 2009. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandillo N., Jarquin D., Song Q., Nelson R., Cregan P., et al. , 2015. A population structure and genome-wide association analysis on the USDA Soybean Germplasm Collection. Plant Genome . 10.3835/plantgenome2015.04.0024 [DOI] [PubMed] [Google Scholar]
- Carter T. E., Nelson R. L., Sneller C. H., Cui Z., 2004. Genetic diversity in soybean, pp. 303–416 in Soybeans: Improvement, Production, and Uses, edited by Boerma H. R., Specht J. E. Crop Science Society of America, Madison, WI. [Google Scholar]
- de los Campos G., Hickey J. M., Pong-Wong R., Daetwyler H. D., Calus M. P. L., 2013. Whole-genome regression and prediction methods applied to plant and animal breeding. Genetics 193: 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2010 The second report on the state of the World’s plant genetic resources for food and agriculture. Food and Agriculture Organization (FAO), Rome, Italy.
- Fox C. M., Cary T. R., Nelson R. L., Diers B. W., 2015. Confirmation of a seed yield QTL in soybean. Crop Sci. 55: 992–998. [Google Scholar]
- Funatsuki H., Suzuki M., Hirose A., Inaba H., Yamada T., et al. , 2014. Molecular basis of a shattering resistance boosting global dissemination of soybean. Proc. Natl. Acad. Sci. USA 111: 17797–17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habier D., Fernando R. L., Dekkers J. C. M., 2007. The impact of genetic relationship information on genome-assisted breeding values. Genetics 177: 2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habier D., Fernando R. L., Garrick D. J., 2013. Genomic BLUP decoded: a look into the black box of genomic prediction. Genetics 194: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne, S., J. Franco, J. Chen, C. P. Sansaloni, C. D. Petroli et al., 2015 Genome wide assessment of maize genebank diversity; synthesis of next generation technologies and GIS based approaches. Proceedings of Plant and Animal Genome XXIII, San Diego, CA. [Google Scholar]
- Hickey J. M., Dreisigacker S., Crossa J., Hearne S., Babu R., et al. , 2014. Evaluation of genomic selection training population designs and genotyping strategies in plant breeding programs using simulation. Crop Sci. 54: 1476–1488. [Google Scholar]
- Hoisington D., Khairallah M., Reeves T., Ribaut J.-M., Skovmand B., et al. , 1999. Plant genetic resources: what can they contribute toward increased crop productivity? Proc. Natl. Acad. Sci. USA 96: 5937–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Wang J., Zeigler R. S., 2014. The 3,000 rice genomes project: new opportunities and challenges for future rice research. Gigascience 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longin C. F. H., Reif J. C., 2014. Redesigning the exploitation of wheat genetic resources. Trends Plant Sci. 19: 631–636. [DOI] [PubMed] [Google Scholar]
- Lorenz A. J., Smith K. P., 2015. Adding genetically distant individuals to training populations reduces genomic prediction accuracy in barley. Crop Sci. 55: 2657–2667. [Google Scholar]
- McCouch S. R., McNally K. L., Wang W., Hamilton R. S., 2012. Genomics of gene banks: a case study in rice. Am. J. Bot. 99: 407–423. [DOI] [PubMed] [Google Scholar]
- Meuwissen T. H. E., Hayes B. J., Goddard M. E., 2001. Prediction of total genetic value using genome-wide dense marker maps. Genetics 157: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., 2011. Managing self-pollinated germplasm collections to maximize utilization. Plant Genet. Resour. 9: 123–133. [Google Scholar]
- Pérez P., de los Campos G., 2014. Genome-wide regression and prediction with the BGLR statistical package. Genetics 198: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal D., Vikram P., Sansaloni C. P., Ortiz C., Pierre C. S., et al. , 2015. Exploring and mobilizing the gene bank biodiversity for wheat improvement. PLoS One 10: e0132112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Hyten D. L., Jia G., Quigley C. V., Fickus E. W., et al. , 2013. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS One 8: e54985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Hyten D. L., Jia G., Quigley C. V., Fickus E. W., et al. , 2015. Fingerprinting soybean germplasm and its utility in genomic research. G3 (Bethesda) 5: 1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D., Grandillo S., Fulton T. M., Zamir D., Eshed Y., et al. , 1996. Advanced backcross QTL analysis in a cross between an elite processing line of tomato and its wild relative L. pimpinellifolium. Theor. Appl. Genet. 92: 213–224. [DOI] [PubMed] [Google Scholar]
- Technow F., Bürger A., Melchinger A. E., 2013. Genomic prediction of northern corn leaf blight resistance in maize with combined or separated training sets for heterotic groups. G3 (Bethesda) 3: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRaden P. M., 2008. Efficient methods to compute genomic predictions. J. Dairy Sci. 91: 4414–4423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.