Abstract
Scans of the Drosophila melanogaster genome have identified organophosphate resistance loci among those with the most pronounced signature of positive selection. In this study, the molecular basis of resistance to the organophosphate insecticide azinphos-methyl was investigated using the Drosophila Genetic Reference Panel, and genome-wide association. Recently released full transcriptome data were used to extend the utility of the Drosophila Genetic Reference Panel resource beyond traditional genome-wide association studies to allow systems genetics analyses of phenotypes. We found that both genomic and transcriptomic associations independently identified Cyp6g1, a gene involved in resistance to DDT and neonicotinoid insecticides, as the top candidate for azinphos-methyl resistance. This was verified by transgenically overexpressing Cyp6g1 using natural regulatory elements from a resistant allele, resulting in a 6.5-fold increase in resistance. We also identified four novel candidate genes associated with azinphos-methyl resistance, all of which are involved in either regulation of fat storage, or nervous system development. In Cyp6g1, we find a demonstrable resistance locus, a verification that transcriptome data can be used to identify variants associated with insecticide resistance, and an overlap between peaks of a genome-wide association study, and a genome-wide selective sweep analysis.
Keywords: azinphos-methyl, D. melanogaster, Drosophila Genetic Reference Panel, systems genetics, Cyp6g1
Genome-wide scans for positive selection have become possible over recent years, and reveal fascinating insights into recent evolution, with a global perspective afforded by whole genome analyses. These scans are becoming increasingly sophisticated as methods advance from a focus on hard sweeps to partial sweeps and soft sweeps. Whereas a locus with a hard sweep has a single haplotype surrounding a single adaptive variant, a locus with a soft sweep has multiple haplotypes containing one or more selected variants. Partial sweeps occur when adaptive variants have not reached fixation. Studies such as these lead to candidates of selection in a completely unbiased way; however, it is not always easy to deduce what selective force is driving the selection on identified genes, and the lack of phenotypic validation of candidates has been a major criticism of these approaches (Jensen et al. 2016). Resistance to insecticides is a compelling evolutionary model, due to the relatively recent introduction of these toxins, and the specific selective pressures they are capable of imparting. This model has, however, tended to focus on genes of major effect. The Drosophila Genetic Reference Panel (DGRP), a set of inbred Drosophila melanogaster lines with sequenced genomes and transcriptomes (Mackay et al. 2012; Huang et al. 2015), allows for the identification of both major and minor effect alleles contributing to resistance phenotypes, in the context of recent selection.
In 2015, Garud et al. identified regions of the D. melanogaster genome under strong, recent selection by interrogating the sequences of DGRP lines for signatures of selective sweeps (Garud et al. 2015). The top three regions identified in this screen, Cyp6g1, Ace, and CHKov1 had all been previously associated with resistance to insecticides (Daborn et al. 2001; Pralavorio and Fournier 1992; Aminetzach et al. 2005), and two of them to a particular insecticide: the organophosphate (OP) azinphos-methyl.
Resistance to OPs is arguably the best understood of any resistance to an insecticide class. Widespread use of OPs for more than half a century on a range of pests has resulted in many well-studied cases of resistance to members of this class of toxin (Siegfried and Scharf 2001). Acetylcholinesterase (Ace) is the molecular target of OPs. Bound in the postsynaptic membrane, it hydrolyses the ester bond in acetylcholine following neurotransmission, ending the signal. OPs bind irreversibly to Ace, causing a build-up of acetylcholine in the synapse, and continuous stimulation of the postsynaptic neuron. This results in paralyzing seizures, and the eventual death of the insect. Four substitutions in D. melanogaster Ace cause insensitivity of the enzyme to OPs. These mutations occur together in some alleles, in many cases acting cooperatively to increase resistance, with differing combinations maximizing resistance to different insecticides by either restricting access or affecting the position of key catalytic residues (Mutero et al. 1994; Menozzi et al. 2004). Additionally, duplications of Ace exhibit extreme population differentiation (Kolaczkowski et al. 2011), providing further evidence that selection is acting at this locus in D. melanogaster.
Another of Garud et al.’s top three candidate genes is CHKov1, originally identified in a screen of D. melanogaster transposable element polymorphisms under recent, positive selection (Aminetzach et al. 2005). The same study then linked the CHKov1-DOC allele (containing the insertion of doc1420 into the coding region of this uncharacterized gene) with resistance to the OP azinphos-methyl by comparing two strains differing by a single introgressed region. In 2011, Magwire et al. reported that resistance to the sigma virus Rhabdoviridae mapped to a region containing CHKov1, a result that was supported using a genome-wide association study (GWAS) in the DGRP population (Magwire et al. 2011).
A cytochrome P450 gene, Cyp6g1, is also one of Garud et al.’s top three candidates for positive selection (Garud et al. 2015). Naturally occurring alleles causing the overexpression of Cyp6g1 result in resistance to DDT and neonicotinoids (Daborn et al. 2001), which is attributable to Cyp6g1-limited metabolism of these toxins (Joussen et al. 2008; Hoi et al. 2014). Resistance to the OP diazinon in Australian populations was mapped to a region containing Cyp6g1 (Pyke et al. 2004). Daborn et al. (2007) subsequently reported, however, that transgenic Cyp6g1 overexpression was incapable of conferring resistance to diazinon.
Here, we describe a systems genetics approach (Ayroles et al. 2009) that incorporates into a single model associations of phenotypic, genomic, and transcriptomic variation to investigate resistance to azinphos-methyl using the DGRP population. This study aimed to characterize resistance to this insecticide from a polygenic framework, with the added advantage of being able to assess the involvement of the peaks identified by selective sweep analysis in azinphos-methyl resistance, using the DGRP population in which they were detected.
Materials and Methods
Fly lines
The DGRP lines examined in this study were generated by Mackay et al. (2012), and were obtained from the Bloomington Drosophila stock center in Indiana. 6g1HR-GAL4, UAS-Cyp6g1 and Phi86 lines were generated by Chung et al. (2007). All fly stocks were maintained at 25° on rich medium containing, maltose (46 g/L), dextrose (75 g/L), yeast (35 g/L), soy flour (20 g/L), maize meal (73 g/L), agar (6 g/L), acid mix (14 ml/L), and tegosept (16 ml/L). The acid mix solution was made up of orthophosphoric acid (42 ml/L), and propionic acid (412 ml/L), while the tegosept solution was 50 g tegosept dissolved in 950 ml of 95% EtOH. Applicable quantities of azinphos-methyl were mixed into rich medium once it had cooled below 60°, to produce insecticide media.
Insect bioassays
First-instar larvae (< 24 hr old) were collected from laying plates and transferred onto insecticide media at a density of 20 larvae per vial. Controls were performed using media containing no insecticide. The number of fully formed pupae were scored after 7 d. Three biological replicates were performed for each dose.
Calculation of LD50
For each DGRP line, dose data were corrected for control mortality using Abbott’s correction, and linear models were fitted to dose-mortality data on a log-probit scale using ‘glm’ in the R statistical package (R Core Team 2015) and scripts from (Johnson et al. 2013). 50% lethal dose (LD50) values and 95% confidence intervals were calculated using Fieller’s method from fitted linear models (Finney 1971).
Genome-wide association studies
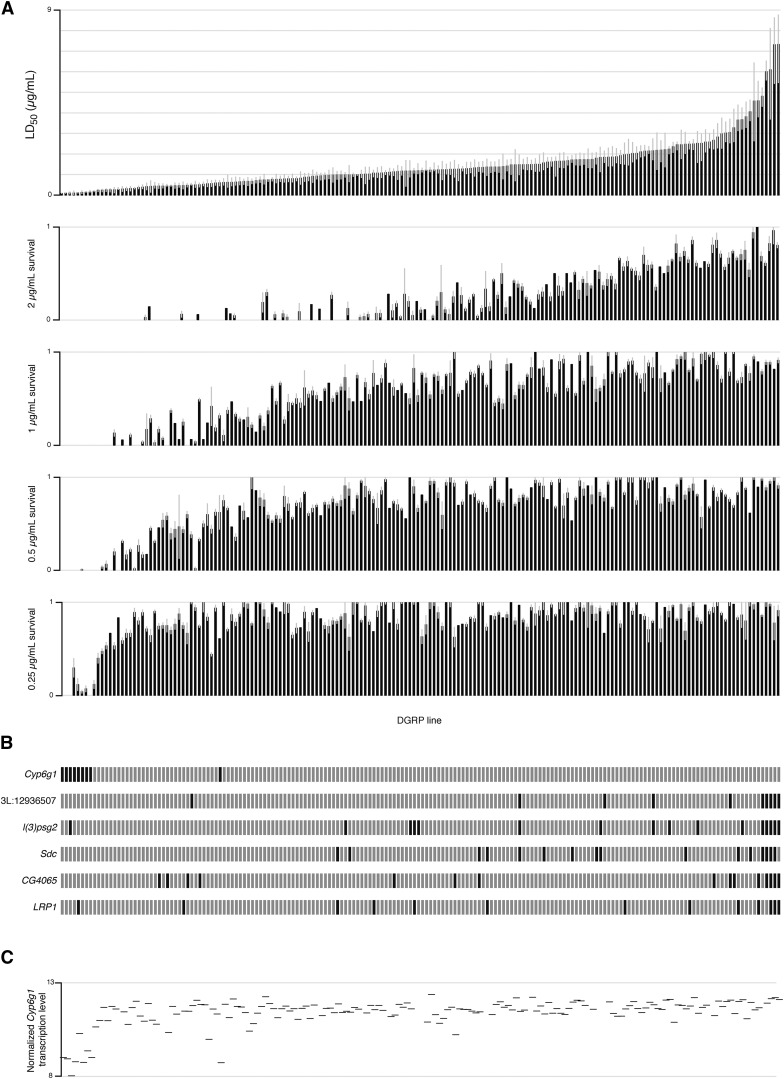
Phenotypes for 178 lines at each of the four common doses, and the LD50, were submitted to the Mackay Lab DGRP2 pipeline as five separate GWAS (Figure 1A; http://dgrp.gnets.ncsu.edu/; Huang et al. 2014).
Figure 1.
(A) Azinphos-methyl LD50 phenotype (error bars represent 95% C.I.) and four mean azinphos-methyl survival phenotypes at single doses (error bars represent SEM) for 178 DGRP lines, ordered by LD50 phenotype. (B) Lines carrying minor allele (black) of GWAS candidates. (C) Mean of male and female normalized Cyp6g1 transcription level as measured by Huang et al. (2015), data missing for some lines.
In silico genotyping
y; cn bw sp; assembled reference genome sequence version 5.33 was recovered from FlyBase (Millburn et al. 2016). DGRP line sequences from Illumina platforms were obtained from the Baylor College of Medicine website (https://www.hgsc.bcm.edu/content/dgrp-lines; Mackay et al. 2012). Reads were aligned to the y; cn bw sp; reference genome using Burrows-Wheeler Aligner (Li and Durbin 2009). Alignments of Illumina paired end reads to the y; cn bw sp; genome in regions containing CHKov1 and Cyp6g1 loci were analyzed with IGV 2.0 software (Robinson et al. 2011) to score structural variation and transposable element presence in each line. Alignments were used to identify and plot DGRP variation at each base in exons III and IV of Ace.
Preparation of transcriptome data
Transcriptome data for 1- to 3 d old adult flies from 185 DGRP lines were recovered from the DGRP website (http://dgrp.gnets.ncsu.edu/data.html; Huang et al. 2015). Mean transcription level was calculated for each gene from two biological replicates, for each of the 18,140 transcripts measured by Huang et al. (2015) for each sex and in each DGRP line.
Structural equation modeling
The ‘sem’ package (Fox et al. 2014) in R (R Core Team 2015) was used to generate a structural equation model incorporating factors associated below Bonferroni significance with azinphos-methyl resistance:
The six significantly associated single nucleotide polymorphisms (SNPs) from the LD50 GWAS as fixed variables.
The Cyp6g1-M allele identified by significantly associated SNPs from the 0.25 and 0.5 µg/ml survival phenotype GWAS as a fixed variable.
Expression of Cyp6g1 and Cyp6g2 (mean of male and female values) as random variables.
The azinphos-methyl LD50 phenotype as a random variable.
Cyp6g1 overexpression
Cyp6g1 overexpression using the GAL4/UAS system (Brand and Perrimon 1993) was originally described by Chung et al. (2007). 6g1HR-GAL4 females, in which GAL4 is regulated by Cyp6g1 upstream sequence originating from Hikone-R line flies, were crossed to UAS-Cyp6g1 males, which carry an additional copy of Cyp6g1 coding region under control of a UAS promoter. In the control cross Phi86 line males were used, which contain the UAS promoter but lack the additional Cyp6g1 coding region downstream.
Data availability
Strains are available upon request. Supplemental Material, File S1 contains detailed descriptions of all supplemental files. File S2 contains phenotypes for all five GWAS. Figure S1 contains plots of Cyp6g1 transcription level against LD50 phenotype. Figure S2 contains details of DGRP Ace variation in exons III and IV.
Results
GWAS of resistance to azinphos-methyl
A total of 178 DGRP lines was assayed for survival to pupation on rich medium containing azinphos-methyl at 0, 0.25, 0.5, 1, and 2 µg/ml, with additional doses (ranging from 0.0625 to 8 µg/ml) used to quantify the LD50 of lines with extreme phenotypes. LD50 values were calculated from probit models fit to survival data (corrected for control mortality using Abbott’s correction) from each line at each dose, and ranged from 0.083 µg/ml to 7.33 µg/ml. Phenotypes for 178 lines at each of the four common doses, and the LD50, were submitted to the Mackay Lab DGRP2 pipeline as five separate GWAS (Figure 1A; http://dgrp.gnets.ncsu.edu/; Huang et al. 2014).
Three of the five GWAS were able to identify phenotype-associated SNPs with P-values below the Bonferroni threshold for genome-wide significance (2.28 × 10−8; Figure 2). Considering results from all five GWAS, the strongest association (P = 6.6 × 10–24) is from the 0.25 µg/ml survival phenotype, and is located in an intron of Cyp6g1. All significant SNPs (below the Bonferroni threshold) in GWAS for both 0.25 µg/ml survival and 0.5 µg/ml survival phenotypes are in this same ∼70 kb region centered around Cyp6g1 (Table 1 and Figure 2). The three most significant Cyp6g1 SNPs are present in nine DGRP lines, eight of which are extremely susceptible to azinphos-methyl (Figure 1B). In silico genotyping methods reveal these nine lines to be the only DGRP lines that are homozygous for Cyp6g1-M—the ancestral allele of Cyp6g1—and the most susceptible to DDT (Schmidt et al. 2010).
Figure 2.
Manhattan plots for GWAS of LD50, 0.25 µg/ml survival, 0.5 µg/ml survival, 1 µg/ml survival, and 2 µg/ml survival azinphos-methyl phenotypes. The x-axis shows genomic location of variant, the y-axis shows –log10(P-value of association with phenotype). Bonferroni threshold for genome-wide significance (2.28 × 10−8) is shown.
Table 1. Variants with P-values below the Bonferroni threshold for genome-wide significance (2.28 × 10−8) from GWAS of five azinphos-methyl phenotypes.
| Phenotype | Candidate | Site Class | No. Variants | Location | Minimum P-Value |
|---|---|---|---|---|---|
| 0.25 µg/ml survival | Cyp6g1 | Various | 45 | 2R:12131954-2R:12202171 | 6.579 × 10−24 |
| 0.5 µg/ml survival | Cyp6g1 | Various | 8 | 2R:12185332-2R:12202171 | 1.02 × 10−9 |
| LD50 | Unannotated | Intergenic | 2 | 3L:12936507-3L:12936514 | 2.62 × 10−14 |
| LD50 | l(3)psg2 | Nonsynonymous | 1 | 3L:5586237 | 9.93 × 10−10 |
| LD50 | Sdc | Intronic | 1 | 2R:21457715 | 3.98 × 10−9 |
| LD50 | CG4065 | Synonymous | 1 | 2R:24135649 | 7.80 × 10−9 |
| LD50 | LRP1 | Intronic | 1 | 2R:8191283 | 8.15 × 10−9 |
Multiple variants indicating a single region are grouped together.
SNPs in and around Cyp6g1 were not detected by the LD50 GWAS, which identified instead six other Bonferroni-significant SNPs (Table 1).
Phenotype to transcriptome associations
A linear model was fit between azinphos-methyl LD50 values from 159 DGRP lines, and mean transcription level of each gene measured by Huang et al. (2015). Of the 18,140 transcripts in this dataset, a single transcript for each sex was found to be associated with the phenotype with a P-value below the Bonferroni threshold for transcriptome-wide significance (2.76 × 10−6). In the case of both male and female associations, this transcript mapped to Cyp6g1 (P = 1.93 × 10−6, P = 2.75 × 10−7 respectively; Figure 1C and Figure S1). Transcriptome associations with the four single-dose phenotypes yielded similar results (data not shown). This supports the finding from our GWAS that alleles of Cyp6g1, which have been demonstrated to increase transcription level and hence resistance to DDT, imidacloprid and nitenpyram (Daborn et al. 2001, 2002, 2007; Schmidt et al. 2010), are associated with resistance to azinphos-methyl in DGRP lines.
Structural equation model
Structural equation modeling was used to test the involvement of Bonferroni-significant factors from GWAS and transcriptome-phenotype associations in the azinphos-methyl LD50 phenotype (Cyp6g2 expression level was included due to its correlation with Cyp6g1 expression), and the model explained the data significantly well (χ2 = 6.83, d.f. = 10, P = 0.74; Figure 3). The model did not show a significant influence by two SNPs (3L:12936507 and 3L:12936514), but supported the influence of the other four Bonferroni-significant SNPs on the phenotype, and showed their involvement was independent of Cyp6g1, as no systematically significant path was found connecting these SNPs to the phenotype indirectly, through Cyp6g1 expression. Systematically significant paths were found connecting the Cyp6g1-M allele to expression of both Cyp6g1 and Cyp6g2, but only Cyp6g1 expression was found to have a significant influence on the phenotype.
Figure 3.
Structural equation model showing the influence of Bonferroni-significant factors from GWAS, and transcriptome-phenotype associations in the azinphos-methyl LD50 phenotype. Standardized coefficients are shown on paths; only statistically significant (P < 0.05) paths are shown. Standardized coefficients account for substitution of homozygous major allele by homozygous minor allele. The involvement of 3L:12936507 and 3L:12936514 SNPs was rejected by the model.
Verification of Cyp6g1 as an azinphos-methyl resistance mechanism
Flies transgenically overexpressing Cyp6g1 using the GAL4-UAS system, driven by upstream elements from a DDT-resistant Cyp6g1 allele (Chung et al. 2007), were phenotyped on azinphos-methyl laced media. The LD50 of these flies was significantly higher, and 6.5-fold greater, than controls that did not overexpress the enzyme (Figure 4).
Figure 4.
Azinphos-methyl LD50 of Cyp6g1-overexpression flies (UAS-Cyp6g1 × 5′HR) compared with the relevant control (Phi86 × 5′HR). Error bars represent 95% C.I.
Cyp6g1-AA and Cyp6g1-BA alleles
DDT-resistant Cyp6g1-AA and Cyp6g1-BA alleles are both present in the DGRP. Cyp6g1-BA has been shown to confer tissue-specific expression differences, and a slight increase in male DDT resistance, over Cyp6g1-AA (Schmidt et al. 2010). We find no significant difference between the mean azinphos-methyl LD50 values for each of these alleles (Figure 5A).
Figure 5.
Mean azinphos-methyl LD50 phenotypes for structural variants at (A) Cyp6g1, and (B) CHKov1. Note there is significant difference in mean LD50 between Cyp6g1-AA and Cyp6g1-BA alleles, or between CHKov1 and CHKov1-DOC alleles (Student’s t-test; P > 0.05).
CHKov1 alleles
It was previously reported that insertion of the doc1420 transposable element into the coding region of CHKov1 increases resistance to azinphos-methyl (Aminetzach et al. 2005). DGRP lines were genotyped for this structural variation, and the mean azinphos-methyl LD50 for each class was compared. There was no significant difference identified between the groups (Figure 5B).
Ace resistance substitutions in the DGRP
Menozzi et al. (2004) identify four common substitutions near the active groove of Ace that reduce sensitivity to various organophosphate and carbamate insecticides. Analysis of DGRP sequence data reveals that three of these four substitutions (I161V, G265A and F330Y) are polymorphic in the DGRP at moderate frequencies, while one (G368A) is entirely absent (Figure S2).
Discussion
Cyp6g1
Here we have shown that the strongest genome-wide association detected out of five azinphos-methyl resistance phenotypes (four single doses and the LD50) identifies Cyp6g1—a gene previously associated with resistance to insecticides. Cyp6g1 was first described as a DDT resistance gene by Daborn et al. (2001), who found that DDT-resistant lines of D. melanogaster contain an Accord transposable element insertion upstream of the gene (Daborn et al. 2002), which correlates with increased Cyp6g1 expression. Chung et al. (2007) showed this increased expression to be in specific tissues, important for insecticide detoxification. Cyp6g1 cross-resistance was additionally described to the neonicotinoids imidacloprid (Daborn et al. 2001) and nitenpyram (Daborn et al. 2007), and, in 2008, the capacity of the enzyme to metabolize both DDT and imidacloprid was demonstrated in cell culture by Joussen et al. (2008).
Four alleles of Cyp6g1 were described by Schmidt et al. (2010); the previously identified Cyp6g1-Accord allele was found to also involve a tandem duplication of the gene (Cyp6g1-AA), and two additional resistant alleles were described, characterized by two successive transposable element insertion events (Cyp6g1-BA and Cyp6g1-BP). The most derived of these, Cyp6g1-BP, is also the most DDT-resistant; however, it is absent from the DGRP. Both Cyp6g1-AA and Cyp6g1-BA confer resistance to DDT relative to the ancestral Cyp6g1-M allele, but the work of Schmidt et al. (2010) suggests this to be the smallest step, phenotypically, of the allelic series. Significant differences between Cyp6g1-AA and Cyp6g1-BA alleles were shown in DDT LD50 for males but not females, and in expression in the midgut but not the fat body. We found no difference between the mean azinphos-methyl LD50 values of Cyp6g1-AA and Cyp6g1-BA alleles in the DGRP (Figure 5A), which, given the subtleties in the phenotypes identified by Schmidt et al. (2010), is not surprising.
Cyp6g1 was also associated with resistance to azinphos-methyl by comparing the LD50 phenotype to transcriptome data from 185 DGRP lines gathered by Huang et al. (2015). While this is consistent with our findings that alleles increasing Cyp6g1 expression are associated with resistance, it also provides further evidence that candidate genes may be identified by associations between phenotype and transcriptome. This supports the work of Ayroles et al. (2009), who found, using the original 40 DGRP transcriptomes, that verifiable associations can be detected between phenotype and transcription level. This additional dimension to the analysis of the molecular basis of phenotypic variation in the DGRP should prove more powerful when the phenotype used matches the transcriptome data of Huang et al. (2015), specifically by sex and lifestage.
Comparing transcription level directly with a phenotype is powerful, as it relies on the measurement of a functionally relevant attribute. Thus, evolutionary unrelated variants can be pooled together based on transcription level, thereby alleviating the issue of allelic heterogeneity that can confound GWAS. This may be especially significant when the variants that are pooled are too rare to be picked up by GWAS.
Validation that increased Cyp6g1 expression confers increases in azinphos-methyl resistance comes from our finding that transgenic overexpression of Cyp6g1, using the GAL4-UAS system and regulatory elements from the Cyp6g1-AA allele, is sufficient to confer a 6.5-fold increase in LD50. While we may speculate that this is due to improved metabolism of the insecticide by increased Cyp6g1 enzyme concentration in metabolic tissues, the ability of Cyp6g1 to metabolize azinphos-methyl remains to be demonstrated, as in the case of DDT and imidacloprid (Joussen et al. 2008; Hoi et al. 2014).
OP resistance has previously been linked to the chromosomal region containing Cyp6g1. Ogita (1958) described dominant cross-resistance between DDT and parathion. Kikkawa (1961) then mapped parathion resistance in the Hikone-R strain to a region on chromosome 2 also associated with DDT resistance, and also described cross-resistance to malathion. Pyke et al. (2004) mapped diazinon resistance to this same region, and found evidence of what Schmidt et al. (2010) would later describe as Cyp6g1-AA and Cyp6g1-BP alleles among resistant individuals. The findings of Pyke et al. (2004) were seemingly contradicted, however, by Daborn et al. (2007), who found transgenic overexpression of Cyp6g2, but not Cyp6g1, sufficient to confer diazinon resistance. The DGRP transcriptome data (Huang et al. 2015) demonstrates that expression of Cyp6g1 is correlated with that of its tandem paralog Cyp6g2 (R2 = 0.52 and 0.44 for male and female adults, respectively). So one tentative hypothesis is that diazinon resistance was mapped to Cyp6g1 in a natural population due to the collateral upregulation of Cyp6g2 in natural resistance alleles, which explains why transgenic overexpression of Cyp6g1 alone failed to confer resistance. Our findings of azinphos-methyl resistance in this study differ from those with diazinon, as we were able to verify that Cyp6g1 alone is capable of conferring high levels of resistance when transgenically overexpressed. While we do not know the capacity of overexpressed Cyp6g2 to confer resistance to azinphos-methyl, structural equation model analysis suggests that Cyp6g2 expression does not independently influence LD50 in DGRP lines (Figure 3).
LD50 GWAS candidates
Although a verifiable azinphos-methyl resistance mechanism, Cyp6g1 was identified by only two of the four single-dose GWAS, and not the LD50 GWAS. This demonstrates that the genetic architecture of related phenotypes, like a range of doses of the same insecticide, may vary significantly. In contrast to Cyp6g1, the six SNPs identified by the LD50 GWAS with P-values below the Bonferroni threshold (Table 1) are all low frequency variants enriched among resistant individuals (Figure 1B). Although integrated haplotype scores give no indication that these variants are under recent selection (data not shown), their identification may be informative of the biology of azinphos-methyl toxicity. Structural equation modeling supports the influence of four of these six SNPs on the LD50 phenotype, as factors independent of Cyp6g1 expression (Figure 3).
A nonsynonymous SNP in the second exon of lethal (3) persistant in salivary gland 2 (l(3)psg2) is predicted to cause a serine to threonine substitution at amino acid 726 of the protein. l(3)psg2 is expressed in response to ecdysone, and involved in regulation of programmed cell death in the salivary glands during metamorphosis (Wang et al. 2008; Ihry and Bashirullah 2014). Although its role in the salivary gland has been specifically studied, l(3)psg2 is expressed in a range of tissues, most highly, in larvae, in the central nervous system (Celniker et al. 2009; Chintapalli et al. 2007).
Syndecan (Sdc) is a heparin sulfate proteoglycan that is involved in axon guidance in central nervous system development by facilitating Slit-Robo binding (Chanana et al. 2009), and also in neuromuscular junction morphogenesis (Johnson et al. 2006). These functions of Sdc are reflected in its high expression in the larval central nervous system (Celniker et al. 2009; Chintapalli et al. 2007). However, Sdc is expressed in larvae at higher levels in the fat body (Chintapalli et al. 2007), where natural Sdc alleles have been found to affect variation in fat storage (De Luca et al. 2010). Given that azinphos-methyl binds its target in the neuromuscular junction, and exerts its effect through the nervous system, the development of these systems could certainly be involved in differences in sensitivity to the insecticide. Also intriguing in relation to insecticide resistance is Sdc’s involvement in fat storage, as the fat body is a key metabolic tissue, and, in fact, one of the tissues in which Cyp6g1 is upregulated in resistant alleles (Chung et al. 2007). Fat storage is also relevant given the ultimate cause of death due to azinphos-methyl toxicity is likely to be a depletion of energy supplies.
Little is known about the function of CG4065 in D. melanogaster. It contains a region homologous with the Mak10 subunit of the NatC complex, shown in Zebrafish to be developmentally controlled, and required for cell proliferation and vessel formation in early development (Wenzlau et al. 2006). It is expressed in a range of larval tissues, but most highly in the central nervous system (Chintapalli et al. 2007).
LDL receptor protein 1 (LRP1) is expressed in most cell types, but is highest in hepatocyte-like cells and neurons (Herz and Bock 2002). Its role in hepatocytes has been characterized in its mouse homolog, where it functions as a receptor for lipoproteins that carry lipids from the gut to the liver (Rohlmann et al. 1998). In the D. melanogaster brain, it has been demonstrated to facilitate transport across the blood–brain barrier of lipoprotein LTP, in order to regulate insulin-like peptide production in response to dietary lipid intake (Brankatschk et al. 2014). The role of LRP1 as a blood–brain barrier transporter is of particular interest in reference to azinphos-methyl, given the insecticide must enter the central nervous system to exert its effect. LRP1 was also identified in a previous DGRP GWAS of a food intake phenotype (Garlapow et al. 2015), with RNAi verification demonstrating LRP1 knockdown significantly increases food uptake in males.
CHKov1
Insertion of a doc1420 transposable element into the coding region of CHKov1 has previously been associated with resistance to azinphos-methyl in a single, introgressed D. melanogaster line (Aminetzach et al. 2005). More recently, Magwire et al. (2011) found, through linkage mapping and a subsequent DGRP GWAS, that the CHKov1-DOC allele was associated with resistance to the Sigma virus. Given that Magwire et al. (2011) were able to detect CHKov1-DOC in their GWAS from a haplotype of SNPs in linkage disequilibrium with the insertion, we may have expected to find the same haplotype significantly associated in any of our azinphos-methyl GWAS. To verify that CHKov1-DOC is not associated with this phenotype, we genotyped DGRP lines for the insertion and found no significant difference between LD50 values of lines carrying ancestral or CHKov1-DOC alleles (Figure 5B). In this study we found no evidence to support the involvement of CHKov1 in resistance to azinphos-methyl, although we cannot rule out its effect on resistance in the adult life stage—the stage at which Aminetzach et al. (2005) performed toxicology studies.
Ace
Another expected resistance mechanism, absent from our GWAS results, is variation in the target site of OP insecticides, Ace. Four substitutions in Ace have been demonstrated, in vitro, to affect binding of azinphos-methyl and other insecticides to the enzyme (Menozzi et al. 2004). In their genotyping of the four insensitivity substitutions in Ace alleles worldwide, Karasov et al. (2010) identified three substitutions at moderate frequencies, but found the fourth, G368A, absent. We found a similar pattern in DGRP genotypes, with G368A likewise absent (Figure S2). According to the binding kinetics analysis of Menozzi et al. (2004), G368A is required for high levels of Ace insensitivity to azinphos-methyl, and, although combinations of substitutions present in the DGRP are capable of reducing Ace sensitivity by as much as 4.3-fold, we do not see significant differences in mean LD50 values of lines grouped by substitution haplotype (data not shown). The insensitivity to azinphos-methyl by Ace in the DGRP is relatively small, given the spectrum of insensitivities achieved by ‘resistant’ Ace substitution haplotypes containing G368A, which are as high as 77-fold for azinphos-methyl (Menozzi et al. 2004).
Conclusions
In this study, we utilized a systems genetics approach to uncover the molecular basis of resistance to azinphos-methyl—a strong candidate for a selective agent in the DGRP population according to a recent selective sweep scan. We find no evidence to support the involvement of CHKov1-DOC in resistance to azinphos-methyl, and we find that, although insecticide-resistant Ace alleles are present in the DGRP, alleles conferring high levels of insensitivity to azinphos-methyl are absent. However, we detect strong associations between our azinphos-methyl phenotype and both genomic and transcriptomic DGRP data, indicating that alleles of Cyp6g1, which confer resistance to DDT and neonicotinoids, also confer resistance to azinphos-methyl. This finding is validated by transgenic overexpression of the gene in key metabolic tissues. While we cannot directly implicate azinphos-methyl as a selective agent in the DGRP population, we find that Cyp6g1’s range of substrates among insecticides is larger than previously thought, which may explain the strong signature of selection at this locus. This study demonstrates the utility of genomic, transcriptomic, and positive selection scans in developing a more complete picture of a phenotype.
Supplementary Material
Acknowledgments
The authors thank Philip Batterham, Owain Edwards, and Llewellyn Green for their discussions and assistance. This work was supported by the Australian Research Council discovery grant (DP0985013), the University of Melbourne “near-miss grants” scheme, and the Commonwealth Scientific and Industrial Research Organization support of Paul Battlay.
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.031054/-/DC1
Communicating editor: S. I. Wright
Literature Cited
- Aminetzach Y. T., Macpherson J. M., Petrov D. A., 2005. Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science 309: 764–767. [DOI] [PubMed] [Google Scholar]
- Ayroles J. F., Carbone M. A., Stone E. A., Jordan K. W., Lyman R. F., et al. , 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat. Genet. 41: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Brankatschk M., Dunst S., Nemetschke L., Eaton S., 2014. Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling. eLife 3: e02862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanana B., Steigemann P., Jäckle H., Vorbrüggen G., 2009. Reception of slit requires only the chondroitin–sulphate-modified extracellular domain of syndecan at the target cell surface. Proc. Natl. Acad. Sci. USA 106: 11984–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using flyatlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Chung H., Bogwitz M. R., McCart C., Andrianopoulos A., Ffrench-Constant R. H., et al. , 2007. Cis-regulatory elements in the accord retrotransposon result in tissue-specific expression of the Drosophila melanogaster insecticide resistance gene cyp6g1. Genetics 175: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daborn P., Boundy S., Yen J., Pittendrigh B., 2001. DDT resistance in Drosophila correlates with cyp6g1 over-expression and confers cross-resistance to the neonicotinoid imidacloprid. Mol. Genet. Genomics 266: 556–563. [DOI] [PubMed] [Google Scholar]
- Daborn P., Yen J., Bogwitz M., Le Goff G., Feil E., et al. , 2002. A single p450 allele associated with insecticide resistance in Drosophila. Science 297: 2253–2256. [DOI] [PubMed] [Google Scholar]
- Daborn P. J., Lumb C., Boey A., Wong W., Ffrench-Constant R. H., et al. , 2007. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome p450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 37: 512–519. [DOI] [PubMed] [Google Scholar]
- De Luca M., Klimentidis Y. C., Casazza K., Chambers M. M., Cho R., et al. , 2010. A conserved role for syndecan family members in the regulation of whole-body energy metabolism. PLoS One 5: e11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney D. J., 1971. Probit Analysis, Ed. 3 Charles Griffin and Company Ltd., London. [Google Scholar]
- Fox, J., Z. Nie, and J. Byrnes, 2014 sem: Structural Equation Models R package version 3.1–5. Available at: http://CRAN.R-project.org/package=sem. Accessed: March 17, 2016.
- Garlapow M. E., Huang W., Yarboro M. T., Peterson K. R., Mackay T. F., 2015. Quantitative genetics of food intake in Drosophila melanogaster. PLoS One 10: e0138129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud N. R., Messer P. W., Buzbas E. O., Petrov D. A., 2015. Recent selective sweeps in North American Drosophila melanogaster show signatures of soft sweeps. PLoS Genet. 11: e1005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Bock H. H., 2002. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 71: 405–434. [DOI] [PubMed] [Google Scholar]
- Hoi K. K., Daborn P. J., Battlay P., Robin C., Batterham P., et al. , 2014. Dissecting the insect metabolic machinery using twin ion mass spectrometry: a single p450 enzyme metabolizing the insecticide imidacloprid in vivo. Anal. Chem. 86: 3525–3532. [DOI] [PubMed] [Google Scholar]
- Huang W., Massouras A., Inoue Y., Peiffer J., Rámia M., et al. , 2014. Natural variation in genome architecture among 205 Drosophila melanogaster genetic reference panel lines. Genome Res. 24: 1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Carbone M. A., Magwire M. M., Peiffer J. A., Lyman R. F., et al. , 2015. Genetic basis of transcriptome diversity in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 112: E6010–E6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihry R. J., Bashirullah A., 2014. Genetic control of specificity to steroid-triggered responses in Drosophila. Genetics 196: 767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. D., Foll M., Bernatchez L., 2016. The past, present and future of genomic scans for selection. Mol. Ecol. 25: 1–4. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Tenney A. P., Ghose A., Duckworth A. M., Higashi M. E., et al. , 2006. The HSPGs syndecan and dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49: 517–531. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Dahlgren L., Siegfried B. D., Ellis M. D., 2013. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS One 8: e54092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen N., Heckel D. G., Haas M., Schuphan I., Schmidt B., 2008. Metabolism of imidacloprid and DDT by P450 CYP6G1 expressed in cell cultures of Nicotiana tabacum suggests detoxification of these insecticides in Cyp6g1-overexpressing strains of Drosophila melanogaster, leading to resistance. Pest Manag. Sci. 64: 65–73. [DOI] [PubMed] [Google Scholar]
- Karasov T., Messer P. W., Petrov D. A., 2010. Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genet. 6: e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa H., 1961. Genetical studies on the resistance to parathion in Drosophila melanogaster. Annu Rep Sci Wks Osaka Univ 9: 1–20. [Google Scholar]
- Kolaczkowski B., Kern A. D., Holloway A. K., Begun D. J., 2011. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics 187: 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F., Richards S., Stone E. A., Barbadilla A., Ayroles J. F., et al. , 2012. The Drosophila melanogaster genetic reference panel. Nature 482: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwire M. M., Bayer F., Webster C. L., Cao C., Jiggins F. M., 2011. Successive increases in the resistance of Drosophila to viral infection through a transposon insertion followed by a duplication. PLoS Genet. 7: e1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi P., Shi M., Lougarre A., Tang Z., Fournier D., 2004. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millburn G. H., Crosby M. A., Gramates L. S., Tweedie S., Consortium F., et al. , 2016. Flybase portals to human disease research using Drosophila models. Dis. Model. Mech. 9: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutero A., Pralavorio M., Bride J.-M., Fournier D., 1994. Resistance-associated point mutations in insecticide-insensitive acetylcholinesterase. Proc. Natl. Acad. Sci. USA 91: 5922–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita Z., 1958. The genetical relation between resistance to insecticides in general and that to phenylthiourea (PTU) and phenylurea (PU) in Drosophila melanogaster. Botyu-Kagaku 23: 188–205. [Google Scholar]
- Pralavorio M., Fournier D., 1992. Drosophila acetylcholinesterase: characterization of different mutants resistant to insecticides. Biochem. Genet. 30: 77–83. [DOI] [PubMed] [Google Scholar]
- Pyke F. M., Bogwitz M. R., Perry T., Monk A., Batterham P., et al. , 2004. The genetic basis of resistance to diazinon in natural populations of Drosophila melanogaster. Genetica 121: 13–24. [DOI] [PubMed] [Google Scholar]
- R Core Team , 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Robinson J. T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E. S., et al. , 2011. Integrative genomics viewer. Nat. Biotechnol. 29: 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlmann A., Gotthardt M., Hammer R. E., Herz J., 1998. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101: 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. M., Good R. T., Appleton B., Sherrard J., Raymant G. C., et al. , 2010. Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet. 6: e1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried B. D., Scharf M. E., 2001. Mechanisms of organophosphate resistance in insects, pp. 269–291 in Biochemical Sites of Insecticide Action and Resistance, edited by Ishaaya I. Springer, Berlin. [Google Scholar]
- Wang L., Evans J., Andrews H. K., Beckstead R. B., Thummel C. S., et al. , 2008. A genetic screen identifies new regulators of steroid-triggered programmed cell death in Drosophila. Genetics 180: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzlau J. M., Garl P. J., Simpson P., Stenmark K. R., West J., et al. , 2006. Embryonic growth-associated protein is one subunit of a novel n-terminal acetyltransferase complex essential for embryonic vascular development. Circ. Res. 98: 846–855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Supplemental Material, File S1 contains detailed descriptions of all supplemental files. File S2 contains phenotypes for all five GWAS. Figure S1 contains plots of Cyp6g1 transcription level against LD50 phenotype. Figure S2 contains details of DGRP Ace variation in exons III and IV.