Abstract
Purpose
Risk stratification after surgery for colorectal cancer liver metastases (CRLM) is achieved using clinicopathologic variables, however is of limited accuracy. We sought to derive and externally validate a multigene expression assay prognostic of overall survival (OS) that is superior to clinicopathologic variables in patients with surgically resected CRLM.
Experimental Design
We measured mRNA expression in prospectively collected frozen tumor from 96 patients with surgically resected CRLM at Memorial Sloan Kettering Cancer Center (MSKCC, New York). We retrospectively generated a 20-gene molecular risk score (MRS) and compared its prognostic utility for overall survival (OS) and recurrence-free survival (RFS) with three common clinical risk scores (CRSs). We then tested the prognostic ability of the MRS in an external validation cohort (European) of 119 patients with surgically resected CRLM at the University Medical Center Utrecht (Netherlands) and Paul Brousse Hospital (France).
Results
For OS in the MSKCC cohort, MRS was the strongest independent prognosticator (HR 3.7–4.9, P<0.001) followed by adjuvant chemotherapy (HR 0.3, P≤0.001). For OS in the European cohort, MRS was the only independent prognosticator (HR 3.5, P=0.007). For RFS, MRS was also independently prognostic in the MSKCC cohort (HR 2.4–2.6, P≤0.001) and the European cohort (HR 1.6–2.5, P≤0.05).
Conclusion
Compared to CRSs, the MRS is more accurate, broadly applicable, and an independent prognostic biomarker of OS in resected CRLM. This MRS is the first externally validated prognostic multigene expression assay after metastasectomy for CRLM, and warrants prospective validation.
Keywords: Gene signature, metastasectomy, transcriptional profiling
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide.(1) Colorectal liver metastases (CRLM) develop in approximately 50% of patients with CRC(2) and are the major cause of death. Treatment for CRLM includes surgery, ablative techniques, regional, and systemic chemotherapy, with surgery offering the only possibility of cure. 5-year overall survival (OS) is approximately 50% with surgery combined with chemotherapy,(3) and 15–25% of patients are cured at 10 years.(4, 5) Outcomes after surgery are however heterogeneous, with up to 30% of patients dying from cancer within 2 years.(3) Hence accurate risk stratification is critical for patient selection to optimize therapeutic approaches.
Numerous groups, including our own, have incorporated standard clinical and pathologic parameters into clinical risk scores (CRS) to risk stratify patients.(6–10) However, CRSs have had limited success. Derived almost exclusively from single-institution cohorts reflecting local practice patterns and biases, they have not been successfully validated across institutions,(9, 11) in patients with longer follow-up,(12) or in the setting of neoadjuvant chemotherapy.(13, 14) Furthermore, none of these scores have achieved a level of prognostication sufficient to influence clinical decision-making.(15) This inability to accurately risk stratify patients results in a standard treatment approach applied to all patients, despite our knowledge that patient outcomes are very heterogeneous.
A genomic approach to prognostication has been adopted in many primary malignancies, proving both prognostic and predictive.(16–18) However a validated prognostic gene signature has not been developed to assess outcomes after metastasectomy for CRLM or any metastatic solid tumor. We previously reported two partially overlapping internally validated gene expression signatures prognostic of disease specific survival (DSS) and liver recurrence free survival (LRFS) after resection of CRLM, comprised of 19 and 115 genes respectively. We were however unable to identify a unifying gene signature prognostic of both recurrence free survival (RFS) and overall survival (OS), and had not validated our findings in an external cohort. (19) Herein, we aimed to identify a single multigene signature prognostic of both OS and RFS and externally validate this signature.
Methods
Study Design
We selected 96 patients who underwent liver resection between January 2000 and October 2007 for CRLM at Memorial Sloan Kettering Cancer Center (MSKCC) as previously described (derivation cohort).(19) Exclusion criteria included extrahepatic metastases, macroscopic residual disease (R2), missing CRS scores, inadequate follow-up, and insufficient amount or quality of RNA. The validation cohort (European cohort) was comprised of 119 patients who underwent liver resection for CRLM at the Paul Brousse Hospital (Villejuif, France) or the UMC Utrecht (Utrecht, Netherlands) between November 2000 and August 2010. In addition to the aforementioned exclusion criteria, patients with a history of non-colorectal malignancies, and those who received either prior local ablative therapy or chemoembolization in combination with surgery were also excluded in the validation cohort. Clinical information for both cohorts were collected prospectively and supplemented with retrospective review.(19, 20) The primary study endpoint was OS defined as time from liver resection to death or last follow-up. The secondary endpoint was RFS defined as the time from liver resection to cancer recurrence. This study was approved by institutional review boards at both institutions and informed consent to procure tissue for research purposes was obtained from all participants.
Procedures
We performed a gene expression microarray on resected CRLM in the derivation cohort to assess individual gene expression, as previously described.(19) Briefly, fresh frozen tumor specimens were obtained from archived tissue collected at the time of surgery from patients who underwent liver resection at MSKCC. Corresponding clinical information was obtained from a prospectively maintained hepatic resection database and supplemented by medical record review. After the above described exclusions, 187 frozen tissue samples were found to have at least 70% viable tumor cells based on histologic verification under hemotoxylin and eosin staining. These samples were macrodissected, RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), quality analyzed using an Agilent Bioanalyzer (Agilent Technology, Palo Alto, CA), and included for microarray analysis if the RNA integrity number (RIN) was ≥ 7 in both cohorts. Extracted total RNA was reverse-transcribed by a previously published method and the resulting complimentary DNA (cDNA) template was applied to gene expression analysis.(19) The target cDNAs were hybridized to the Illumina Human HT-12 Gene Chip containing a total of 47,231 annotated gene probe sets (Illumina, San Diego, CA). Arrays were scanned by using standard Illumina protocols and scanners. Microarray data are available in the ArrayExpress database under accession number E-MTAB-1951.
For the validation cohort, after the above described exclusions, frozen tumor samples were obtained and macrodissected as above, RNA extracted, cRNA synthesized and labeled, and mRNA generated and amplified as previously described (19). Gene expression was determined using the Human Array-Ready Oligo set (version 2.0, Qiagen, Limburg, Netherlands).(20) All data and protocols are available in Array Express under accession number E-TABM-1112. All microarray data were log transformed and quantile normalized.
Principal Component Analysis (PCA) is a mathematical technique commonly used to visualize datasets whose samples are characterized by a large number of variables. PCA reduces the dimensionality of the data while retaining most of the variation in the dataset by identifying directions along which the data variation is maximal, called principal components. This allows for each sample to be represented by a limited number of principal components, allowing for visual plotting of samples, and hence identifying similarities and differences.(21) Supervised PCA (SPCA) is similar to conventional PCA however uses only a subset of the predictors selected based on their high association with outcome, to overcome over fitting of highly correlated variables. Given our previous inability of identify a gene signature prognostic of OS using a ranking method based on signal-to-noise ratio, t-test statistic, Cox proportional hazard ratio, and leave-one-out cross validation,(20) as well as unsupervised PCA,(22) and given successful identification of prognostic gene signatures using the SPCA method(23–25), we chose to employ this mathematical approach to gene selection.
For gene selection, the derivation cohort was partitioned by a stratified random split into training and test sets. The training set contained 60% of the patients while the test set had 40% (Figure 1). We then performed a supervised principal component analysis to identify genes correlating with OS in the training set, with subsequent cross validation in the test set (Figure 1).(26) Variables in the training set were restricted (supervised) based on univariate Cox regression, followed by SPCA. The gene selection process was iteratively applied 1000 times with randomly generated training and test sets as described above. The reason for this repeated application was to identify the most reproducible associations and reduce the likelihood of selecting a potentially promising gene that appears significant in a single split due to random associations. Genes that significantly correlated with OS (P< 0.05) in both training and test sets with a frequency > 20% after 1000 iterations were selected for construction of the molecular risk score (MRS) (20 genes, Supplementary Figure 1). Following gene selection, standardized gene expression (SGE) was calculated as
Figure 1.
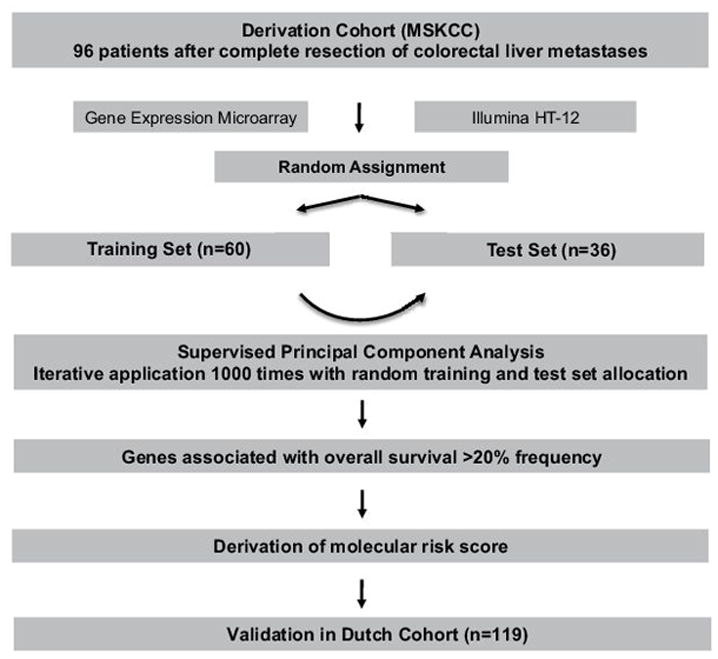
Investigational design.
MRS was calculated as
The derivation cohort was then partitioned into a low and high-risk group based on median MRS, to eliminate the effect of extreme values in the cohort. Clinicopathologic variables not incorporated into CRSs and that have been demonstrated to be independently prognostic of outcome following resection of CRLM, the 3 most widely used CRSs (Fong(7), Nordlinger(27), and Iwatsuki(6)), and the MRS were included as covariates in univariate analysis of OS and RFS. For the Fong CRS, the factors that comprise it include lymph node status of the primary tumor (negative or positive), disease free interval (<12 months or >= 12 months), serum CEA level prior to liver resection (>200 ng/ml or <= 200 ng/ml), number of hepatic tumors (1 or >1) and tumor size (<=5cm or >5cm). Patients receive 1 point for each adverse factor, and the CRS represents the sum. Patients were then divided into two groups: high risk (CRS >= 3) and low risk (CRS<3) based on established definitions.(13, 28, 29) Covariates with P<0.05 on univariate analysis were included in multivariate analysis, with a maximum of 7 degrees of freedom as per Harrell’s guidelines.(30) Survival probabilities were estimated using the Kaplan-Meier method.
Statistical methods
We compared categorical variables using the χ2 test and continuous variables using a two-sided t-test. We performed univariate analysis using the log-rank test and multivariate analysis using the Cox regression model. Gene expression data were analyzed using R statistical software (version 3.0). We performed all other statistical analysis using StataSE (version 13.1, TX, USA) and Prism (version 6, CA, USA). P<0.05 was considered statistically significant.
Results
Patient characteristics
Ninety-six patients formed the derivation cohort (Table 1). Sixty-nine patients (72%) received neoadjuvant chemotherapy, 79 (83%) adjuvant chemotherapy, and 35 (36%) adjuvant hepatic artery infusion (HAI) chemotherapy. Fifty-two patients (54%) had > 3 segments resected, and 58 (60%) had > 1 tumor. Median follow-up for survivors was 89 months. Sixty-six patients (69%) developed recurrence, and 70 patients (73%) died during the study period. Median OS, and RFS in the derivation cohort were 52 and 13 months respectively (Supplementary Figure 2A, 2B).
Table 1.
Clinicopathologic variables in derivation and validation cohorts.
| Derivation (MSKCC) (%) | Validation (European) (%) | p-value | |
|---|---|---|---|
| Total number of patients | 96 | 119 | |
| Age (median, range) | 60 (29–88) | 62 (33–85) | NS |
| Sex | |||
| Male | 33 (34%) | 77 (65%) | < 0.0001 |
| Female | 63 (66%) | 42 (35%) | |
| Largest Tumor Size > 5cm | 22 (23%) | 38 (32%) | NS |
| Primary Nodal Status | NS | ||
| N+ | 56 (58%) | 59 (50%) | |
| N− | 40 (42%) | 51 (43%) | |
| Missing | 0 | 9 (8%) | |
| > 1 tumor | 58 (60%) | 63 (53%) | NS |
| DFI < 12 months | 51 (53%) | 72 (61%) | NS |
| CEA > 200 | 8 (8.3%) | 13 (11%) | NS |
| Neoadjuvant chemotherapy | 69 (72%) | 64 (54%) | < 0.01 |
| Adjuvant chemotherapy | 79 (83%) | 68 (57%) | < 0.001 |
| Adjuvant HAI chemotherapy | 34 (35%) | 0 (0%) | < 0.001 |
| Type of resection | < 0.01 | ||
| Minor (≤ 3 segments) | 44 (46%) | 76 (64%) | |
| Major (> 3 segments) | 52 (54%) | 43 (36%) | |
| Median follow up (months) | 89 | 25 | < 0.01 |
One hundred and nineteen patients formed the validation cohort (Table 1). Sixty-four patients (54%) received neoadjuvant chemotherapy, 68 (57%) adjuvant chemotherapy, and none received HAI chemotherapy. Forty-three patients (36%) had > 3 segments resected, and 63 (53%) had > 1 tumor. Median follow-up for survivors was 25 months. Compared to the derivation cohort, the validation cohort had a significantly smaller fraction of female patients (35% vs. 66%, P<0.0001) and resections of > 3 segments (36% vs. 54%, P<0.01). The validation cohort had a lower fraction of patients who received neoadjuvant (54% vs. 72%, P<0.01), adjuvant (57% vs. 83%, P<0.001), and HAI chemotherapy (0% vs. 35%, P<0.001). Ninety-eight patients (82%) developed recurrence, and 29 patients (24%) died during the study period. Median OS, and RFS in the validation cohort were 55 and 10 months respectively (Supplementary Figure 2A, 2B).
Gene selection for MRS
Using iterative supervised principle component analysis, we identified 20 genes that were associated with OS at a frequency of > 20% (Supplementary Figure 1). Of these 20 genes, 6 genes overlapped with the previously reported 19-gene signature for DSS, and 5 genes overlapped with the previously reported 115-gene signature for LRFS (19). Only 3 genes were common to all three signatures. Gene expression hierarchical clustering revealed 13 first order-clustering groups (Supplementary Figure 3).
MRS is the strongest independent prognosticator of OS in the derivation and validation cohorts
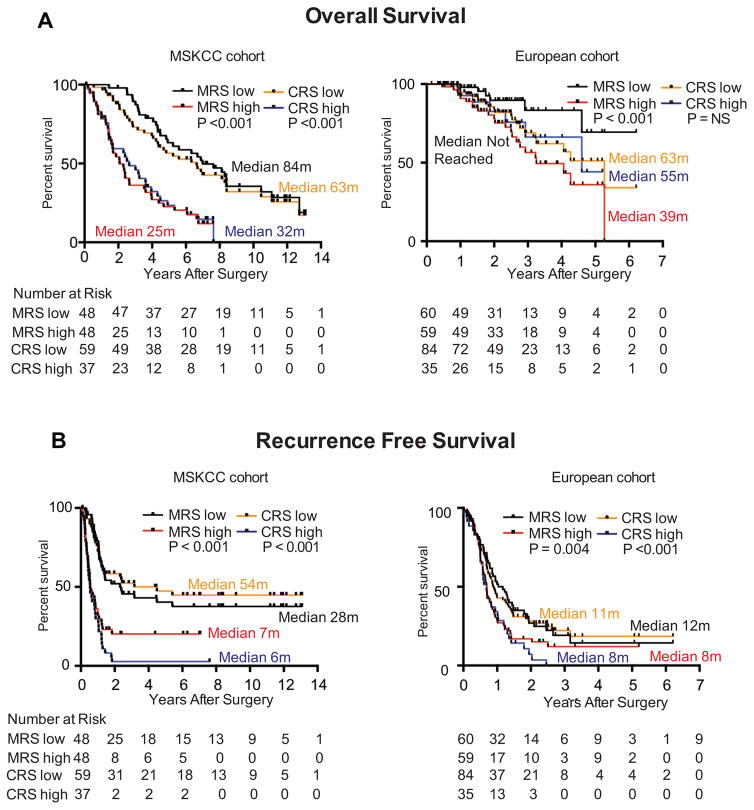
Consistent with the MSKCC cohort forming the basis of the MRS, on univariate analysis, a high MRS (HR 3.8, P<0.001) was prognostic of decreased OS (Figure 2A, Table 2). Additionally, a high CRS (Fong HR 2.7, P<0.001; Nordlinger HR 2.1, P=0.002) was prognostic of decreased OS, and adjuvant chemotherapy was prognostic of increased OS (HR 0.5, P=0.01) (Table 2). On multivariate analysis, a high MRS was prognostic of decreased OS (HR 3.7–4.9, P≤0.001) and adjuvant chemotherapy was prognostic of increased OS (HR 0.3, P≤0.001) (Table 2).
Figure 2.
A. Overall survival in derivation (MSKCC) and validation (European) cohorts stratified by MRS (median) and CRS (<3, low; ≥ 3, high).
B. Recurrence free survival in derivation (MSKCC) and validation (European) cohorts stratified by MRS (median) and CRS (<3, low; ≥ 3, high).
Circles and squares are censored events. P values were determined by log rank method. NR = Not Reached.
Table 2.
Univariate and multivariate analysis of clinicopathologic features, clinical risk scores, and molecular risk score with overall survival in derivation cohort. Multivariate analyses represent individual comparisons of molecular risk score with each clinical risk score.
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P-value | HR | CI | P-value | HR | CI | P-value | HR | CI | P-value | |
| Age | ||||||||||||
| ≤75 | Ref | - | - | |||||||||
| >75 | 1.1 | 0.5–2.1 | 0.8 | |||||||||
| Sex | 1 | 0.7–1.9 | 0.6 | - | - | - | ||||||
| Neoadjuvant chemotherapy | 1.1 | 0.7–1.9 | 0.6 | - | - | - | ||||||
| HAI chemotherapy | 0.6 | 0.4–1 | 0.06 | - | - | - | ||||||
| Adjuvant chemotherapy | 0.5 | 0.3–0.9 | 0.01 | 0.3 | 0.2–0.5 | <0.001 | 0.3 | 0.2–0.6 | 0.001 | 0.3 | 0.2–0.6 | 0.001 |
| Fong Score | ||||||||||||
| Low | Ref | - | - | Ref | - | - | - | - | ||||
| High | 2.7 | 1.6–4.4 | <0.001 | 2.3 | 1.3–3.9 | 0.003 | ||||||
| Nordlinger Score | ||||||||||||
| 1 | Ref | - | - | Ref | - | - | ||||||
| 2 | 2.1 | 1.3–3.5 | 0.002 | - | 2.2 | 1.4–3.6 | 0.001 | - | ||||
| 3 | 2.2 | 0.5–9.5 | 0.3 | 2.1 | 0.5–9 | 0.3 | ||||||
| Iwatsuki Score | ||||||||||||
| 1 | Ref | - | - | Ref | - | - | ||||||
| 2 | 1.9 | 0.7–5.6 | 0.2 | - | - | 1.4 | 0.5–3.9 | 0.6 | ||||
| 3 | 2.9 | 1–8.3 | 0.05 | 2.2 | 0.8–6.5 | 0.1 | ||||||
| 4 | 11.4 | 1.9–66 | 0.007 | 2.9 | 0.5–18 | 0.2 | ||||||
| Molecular Risk Score | ||||||||||||
| Low | Ref | - | - | Ref | - | - | Ref | - | - | - | - | - |
| High | 3.8 | 2.2–6.4 | <0.001 | 3.7 | 2.1–6.6 | <0.001 | 4.9 | 2.8–8.6 | <0.001 | 4.4 | 2.5–7.8 | <0.001 |
In the validation cohort, on univariate analysis, the MRS (HR 3.7, P=0.004) and the Nordlinger score (HR 3.4, P=0.04) were prognostic of decreased OS (Figure 2A, Table 3). On multivariate analysis, only the MRS and none of the CRSs remained prognostic of decreased OS (HR 3.5, P=0.007) (Table 3).
Table 3.
Univariate and multivariate analysis of clinicopathologic features, clinical risk scores, and molecular risk score with overall survival in validation cohort.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | CI | P-value | HR | CI | P-value | |
| Age | ||||||
| ≤75 | Ref | - | - | |||
| >75 | 1.1 | 0.1–8.6 | 0.9 | |||
| Sex | 1.1 | 0.5–2.5 | 0.7 | - | - | - |
| Neoadjuvant chemotherapy | 0.8 | 0.4–1.7 | 0.5 | - | - | - |
| Adjuvant chemotherapy | 0.5 | 0.2–1 | 0.07 | - | - | - |
| Fong Score | ||||||
| Low | Ref | - | - | - | - | - |
| High | 1 | 0.4–2.4 | 0.9 | |||
| Nordlinger Score | ||||||
| 1 | Ref | - | - | Ref | - | - |
| 2 | 1.7 | 0.5–5.1 | 0.4 | 1.6 | 0.5–4.8 | 0.4 |
| 3 | 3.4 | 1.1–10.9 | 0.04 | 2.9 | 0.9–9.3 | 0.06 |
| Iwatsuki Score | ||||||
| 1 | Ref | - | - | |||
| 2 | 0.7 | 0.2–2.5 | 0.6 | |||
| 3 | 0.9 | 0.2–2.9 | 0.8 | - | - | - |
| 4 | 1 | 0.3–3.5 | 0.9 | |||
| Molecular Risk Score | ||||||
| Low | Ref | - | - | Ref | - | - |
| High | 3.7 | 1.5–9.1 | 0.004 | 3.5 | 1.4–8.6 | 0.007 |
MRS is independently prognostic of RFS in the derivation and validation cohorts
Consistent with the MSKCC cohort forming the basis for derivation of the MRS, on univariate analysis, a high MRS (HR 2.5, P<0.001) was prognostic of decreased RFS (Figure 2B, Supplementary Table 1). Additionally, neoadjuvant chemotherapy (HR 1.8, P=0.04), and a high CRS (Fong, Nordlinger, and Iwatsuki) were prognostic of decreased RFS (Supplementary Table 1). On multivariate analysis, the MRS remained independently prognostic of decreased RFS (HR 2.4–2.6, P≤0.001, Supplementary Table 1) when compared to each CRS.
In the validation cohort, on univariate analysis, neoadjuvant chemotherapy (HR 1.7, P=0.009), high MRS (HR 1.5, P=0.04), and a high CRS (Fong, Nordlinger, and Iwatsuki) were prognostic of decreased RFS (Figure 2B, Supplementary Table 2), whereas adjuvant chemotherapy was prognostic of increased RFS (HR 0.5, P=0.003) (Supplementary Table 2). On multivariate analysis, the MRS remained independently prognostic of RFS (HR 1.6–2.5, P≤0.05) (Supplementary Table 2).
Discussion
Outcomes after surgery for CRLM remain highly heterogeneous, ranging from death within 2 years to long-term cure,(4, 5) highlighting the need to accurately risk stratify patients to aid in pre- and post-operative decision making. Currently, only CRS systems utilizing standard pathologic and clinical variables are available to risk stratify patients with resected CRLM, as proposed by Fong(7), Nordlinger(27), Iwatsuki(6), and others(8–10). However, these systems have failed to validate across different patient cohorts, and lack prognostic accuracy in the era of modern chemotherapy. This underscores the need for novel prognostic biomarkers that are superior to CRSs. We successfully derived and externally validated the first gene expression assay in resected CRLM that achieves this.
CRSs are not prognostic in all patient cohorts. Zakaria demonstrated that the Fong, Nordlinger, and Iwatsuki scores did not validate in 662 patients with resected CRLM at the Mayo clinic, with concordance estimates less than 0.6 for DSS and RFS and hence stratification roughly equivalent to chance.(9) Roberts similarly showed in 286 patients with resected CRLM, that 6 of 7 CRSs identified cancer specific deaths at a frequency of less than 65% frequency.(12) Nomograms estimating outcomes after resection of CRLM are also poorly prognostic, achieving a maximum concordance index of only 0.61, and hence not identifying patients who will experience disease recurrence or death nearly 40% of the time (15, 31) Our findings concur with these studies – neither the Fong, Nordlinger, nor Iwatsuki CRSs were independently prognostic of OS in the validation cohort (Table 3). Notably, atleast 1 of the 3 CRSs failed to achieve significance on univariate analysis in both cohorts for both endpoints. In contrast, the MRS remained independently prognostic of both OS and RFS in the validation cohort. Furthermore, the MRS prognosticated outcomes with equivalent magnitude in both cohorts (derivation cohort OS HR 3.7–4.9, validation cohort OS HR 3.5, Tables 2,3) despite significant differences in the frequency of female patients, neoadjuvant, adjuvant, and regional chemotherapy, and major resections (Table 1). Hence the MRS is superior to CRSs in prognostic ability, and appears consistent in its magnitude of stratification in differing patient cohorts.
Due to data demonstrating a clinical benefit to first(32, 33) and second-line(34, 35) chemotherapy in metastatic colorectal cancer, patients are increasingly heavily pre-treated at the time of evaluation for surgery. This necessitates a biomarker that remains prognostic independent of chemotherapy. Currently, CRSs do not achieve this. Ayez and colleagues found that 3 CRSs, including the Fong score, did not stratify patients when applied prior to neoadjuvant chemotherapy.(13) Other groups have arrived at similar conclusions when examining the prognostic ability of CRSs in the setting of chemotherapy.(14) In contrast, the MRS remained prognostic and independent of chemotherapy for both OS and RFS in the validation cohort where up to 54% received neoadjuvant chemotherapy and 57% received adjuvant chemotherapy. Hence, unlike CRSs, the MRS appears independently prognostic of both RFS and OS despite neoadjuvant, adjuvant, or regional chemotherapy, highlighting its utility in the modern era.
Recent data have demonstrated no OS benefit to perioperative chemotherapy in addition to surgery in patients with CRLM.(3, 36) However, certain subgroups of patients may experience benefit. In the validation cohort, in patients who did not receive neoadjuvant chemotherapy, adjuvant chemotherapy was associated with improved OS in the low MRS group (HR 8.5, 95% CI 1.4–118, P=0.02) but not the high MRS group (P=0.38). Although this result is based on a small sample size and must be explored in a larger, prospective dataset, it suggests that the MRS may have clinical applications akin to multigene assays in breast cancer by identifying subsets of patients that derive greater benefit from chemotherapy, and shifting risk stratification from a TNM to a multigene system.(18) It may also enhance our ability to molecularly stratify and select patients for clinical trial inclusion to assess the benefit of novel systemic therapies.
The success of Oncotype Dx, a multigene assay to risk stratify patients with resected primary breast cancer has shown the validity of a molecular approach to prognostication,(16) and has been applied to other primary tumors.(17) However, a prognostic molecular signature has yet to be developed for resected CRLM, or for metastasectomy of any solid tumor. Using a non-iterative supervised principle components method, we previously reported a 19-gene score prognostic of DSS and a 115-gene score prognostic of LRFS. However, in that study, we were unable to identify a prognostic gene score for RFS, or a single gene score prognostic of all endpoints (19). Using a multiple sampling approach with leave-one-out cross-validation, Snoeren and colleagues attempted to derive a prognostic gene signature based on expression data from the European validation cohort however were unsuccessful. (37) In the present study, using an iterative supervised principle component analysis not used in our previous report, we derived a new 20-gene molecular score that was prognostic of not only OS, but also RFS, in both cohorts, highlighting the strength of the iterative supervised principle components method for gene selection. It is noteworthy that unlike other multigene assays that only assess recurrence(16, 38, 39), we identified a MRS that significantly stratifies both RFS and OS with just 20 genes. Hence, as has been successfully performed with other gene expression signatures (Oncotype Dx, 21 genes), the MRS has the potential to be developed into clinically applicable and practical PCR assays with relative ease. Our findings are hence both novel as the first externally validated multigene assay to prognosticate outcomes after metastasectomy for any solid tumor, and practical with potential for clinical application as a biomarker in resected CRLM.
This study is retrospective, limited to resected patients, and fails to capture high risk, resectable candidates who progress to unresectability on initial systemic treatment. Conversely, it does not capture patients who have a complete pathologic response to systemic treatment, as seen in up to 37% of patients.(40) The study was also performed on banked frozen tissue and omitted patients who had poor quality RNA, or no banked tissue, providing additional sources of selection bias. It is unknown if the MRS remains prognostic when expression is measured on tissue embedded in paraffin, and this is currently under investigation. Finally, this study is limited by small sample size and mandates validation in larger prospective datasets.
In summary, we report a novel, externally validated prognostic gene expression signature for OS in resected CLRM that is superior to existing CRSs. Our data highlights the prognostic potential of transcription profiling in patients undergoing resection of CRLM and warrants prospective evaluation of its ability to identify patient subgroups that may derive benefits from specific therapeutic strategies. A molecular approach to risk assess patients treated with metastasectomy in other solid tumors appears promising and merits further investigation.
Supplementary Material
Statement of translational relevance.
Colorectal liver metastases (CRLM) develop in 50% of patients with CRC and are the major cause of death. The primary treatment includes surgery and chemotherapy, however outcomes remains heterogeneous. Currently, clinicopathologic risk scores are used to estimate prognosis yet these scores have poor accuracy across institutions and in the setting of chemotherapy.
A genomic approach to risk assessment has been shown to be both prognostic and predictive in primary malignancies however has yet been applied after metastasectomy for CRLM or any solid tumor. In this study, we report the first externally validated prognostic gene expression signature for OS in resected CLRM that is superior to clinical risk scores.
Our findings are novel as the first externally validated multigene expression assay to prognosticate outcomes after metastasectomy for CRLM and validates a molecular approach to risk assess patients treated with metastasectomy in solid tumors.
Acknowledgments
Grant support: This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant).
Footnotes
Conflict of interest: None
Presented at: Society of Surgical Oncology Annual Cancer Symposium, March 2015.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan D, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA. 2014;311:1863–9. doi: 10.1001/jama.2014.3740. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. The lancet oncology. 2013;14:1208–15. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 4.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Annals of Surgery. 2002;235:759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. Journal of the American College of Surgeons. 1999;189:291–9. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of Surgery. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–14. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 9.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–91. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–35. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 11.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–64. 64–6. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, et al. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. The British journal of surgery. 2014;101:856–66. doi: 10.1002/bjs.9471. [DOI] [PubMed] [Google Scholar]
- 13.Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J, et al. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Annals of surgical oncology. 2011;18:2757–63. doi: 10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis. Surg Today. doi: 10.1007/s00595-014-1108-9. Epub 2015 Jan 7. [DOI] [PubMed] [Google Scholar]
- 15.Kattan MW, Gonen M, Jarnagin WR, DeMatteo R, D’Angelica M, Weiser M, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–7. doi: 10.1097/SLA.0b013e31815ed67b. [DOI] [PubMed] [Google Scholar]
- 16.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 17.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito H, Mo Q, Qin L-X, Viale A, Maithel SK, Maker AV, et al. PLOS ONE: Gene Expression Profiles Accurately Predict Outcome Following Liver Resection in Patients with Metastatic Colorectal Cancer. PloS one. 2013;8:e81680. doi: 10.1371/journal.pone.0081680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snoeren N, van Hooff SR, Adam R, van Hillegersberg R, Voest EE, Guettier C, et al. Exploring gene expression signatures for predicting disease free survival after resection of colorectal cancer liver metastases. PloS one. 2012;7:e49442. doi: 10.1371/journal.pone.0049442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringner M. What is principal component analysis? Nature biotechnology. 2008;26:303–4. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Mo Q, Qin LX, Viale A, Maithel SK, Maker AV, et al. Gene expression profiles accurately predict outcome following liver resection in patients with metastatic colorectal cancer. PloS one. 2013;8:e81680. doi: 10.1371/journal.pone.0081680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LD, Coffman LG, Chou JW, Black MA, Bergh J, D’Agostino R, Jr, et al. An iron regulatory gene signature predicts outcome in breast cancer. Cancer research. 2011;71:6728–37. doi: 10.1158/0008-5472.CAN-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Xiao G, Coombes KR, Behrens C, Solis LM, Raso G, et al. Robust gene expression signature from formalin-fixed paraffin-embedded samples predicts prognosis of non-small-cell lung cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:5705–14. doi: 10.1158/1078-0432.CCR-11-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Xiao G, Behrens C, Schiller J, Allen J, Chow CW, et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non-small cell lung cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:1577–86. doi: 10.1158/1078-0432.CCR-12-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bair E, Tibshirani R. Semi-supervised methods to predict patient survival from gene expression data. PLoS biology. 2004;2:E108. doi: 10.1371/journal.pbio.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 28.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 29.Roberts KJ, White A, Cockbain A, Hodson J, Hidalgo E, Toogood GJ, et al. Performance of prognostic scores in predicting long-term outcome following resection of colorectal liver metastases. The British journal of surgery. 2014;101:856–66. doi: 10.1002/bjs.9471. [DOI] [PubMed] [Google Scholar]
- 30.FEH . Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer Verlag; 2001. [Google Scholar]
- 31.Reddy SK, Kattan MW, Yu C, Ceppa EP, de la Fuente SG, Fong Y, et al. Evaluation of peri-operative chemotherapy using a prognostic nomogram for survival after resection of colorectal liver metastases. HPB (Oxford) 2009;11:592–9. doi: 10.1111/j.1477-2574.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 33.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 35.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 36.Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–11. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 37.Snoeren N, van Hooff SR, Adam R, van Hillegersberg R, Voest EE, Guettier C, et al. Exploring Gene Expression Signatures for Predicting Disease Free Survival after Resection of Colorectal Cancer Liver Metastases. PloS one. 2012;7:e49442. doi: 10.1371/journal.pone.0049442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29:4611–9. doi: 10.1200/JCO.2010.32.8732. [DOI] [PubMed] [Google Scholar]
- 39.Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12:245–55. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auer RC, White RR, Kemeny NE, Schwartz LH, Shia J, Blumgart LH, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116:1502–9. doi: 10.1002/cncr.24912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.