Abstract
Introduction:
Cigarette smoking in cocaine users is nearly four times higher than the national prevalence and cocaine use increases cigarette smoking. The mechanisms underlying cigarette smoking in cocaine-using individuals need to be identified to promote cigarette and cocaine abstinence. Previous studies have examined the salience of cigarette and cocaine cues separately. The present aim was to determine whether cigarette attentional bias (AB) is higher in cigarettes smokers who smoke cocaine relative to individuals who only smoke cigarettes.
Methods:
Twenty cigarette smokers who smoke cocaine and 20 non-cocaine-using cigarette smokers completed a visual probe task with eye-tracking technology. During this task, the magnitude of cigarette and cocaine AB was assessed through orienting bias, fixation time, and response time.
Results:
Cocaine users displayed an orienting bias towards cigarette cues. Cocaine users also endorsed a more urgent desire to smoke to relieve negative affect associated with cigarette craving than non-cocaine users (g = 0.6). Neither group displayed a cigarette AB, as measured by fixation time. Cocaine users, but not non-cocaine users, displayed a cocaine AB as measured by orienting bias (g = 2.0) and fixation time (g = 1.2). There were no significant effects for response time data.
Conclusions:
Cocaine-smoking cigarettes smokers display an initial orienting bias toward cigarette cues, but not sustained cigarette AB. The incentive motivation underlying cigarette smoking also differs. Cocaine smokers report more urgent desire to smoke to relieve negative affect. Identifying differences in motivation to smoke cigarettes may provide new treatment targets for cigarette and cocaine use disorders.
Implications:
These results suggest that cocaine-smoking cigarette smokers display an initial orienting bias towards cigarette cues, but not sustained attention towards cigarette cues, relative to non-cocaine-using smokers. Smoked cocaine users also report a more urgent desire to smoke to relieve negative affect than non-cocaine users. Identifying differences in motivation to smoke cigarettes may provide new treatment targets for both cigarette and cocaine use disorders.
Introduction
Cocaine users smoke cigarettes at nearly four times greater prevalence than national estimates.1,2 Cocaine use is positively associated with cigarette smoking.3 Individuals meeting criteria for both tobacco and cocaine dependence use cocaine at an earlier age, use more grams per week, and use cocaine more frequently than non-cigarette smokers.4 Cigarette smoking also modestly increases craving for cocaine,5 predicts poorer treatment outcomes for cocaine-dependent individuals,6 and smoking cessation increases the likelihood of cocaine abstinence.7–9
The mechanisms underlying cigarette smoking in cocaine-using individuals need to be identified as a way to promote cigarette and cocaine abstinence.10 Cigarettes and cocaine are often used in close temporal proximity.11 Smoked cocaine and cigarettes share common discriminative stimuli (eg, lighters, smoke). Substance-related cues play a critical role in drug use and relapse.12–15 Repeated associative pairings between a substance and substance related cues results in attentional bias (AB), which is the allocation of a disproportionate amount of time attending to substance related stimuli.16,17 Cigarette and cocaine AB have been demonstrated using eye-tracking technology during the visual probe task.18–20
Despite a well-documented relationship between cigarette smoking and cocaine use, the salience of cigarette cues has not been assessed in cigarette-smokers who also smoke cocaine. To this end, the magnitude of cigarette and cocaine AB were compared using the visual probe task with eye-tracking technology in two groups: (1) cocaine-smoking cigarette smokers (2) non-cocaine-using cigarette smokers. Given the shared conditioning history and the activation common neuropathways,21,22 it was hypothesized that the magnitude of cigarette AB would be larger in cocaine-using cigarette smokers as compared to non-cocaine-using cigarette smokers.
Methods
Participants
Fifty individuals were recruited from the community. Eight did not meet inclusion criteria and eye-tracking data were insufficient for two additional participants (ie, greater than 2 SDs below the mean for number of fixations recorded). Forty adults reporting smoking 10–20 cigarettes per day in the past 30 days completed the study. Individuals who had made a quit attempt (ie, any effort to reduce the number of cigarettes smoked) in past 30 days or intended to quit in the upcoming 30 days were excluded. Twenty participants reported using smoked cocaine as their preferred route within the past month and fulfilled diagnostic criteria for cocaine abuse or dependence as determined by a computerized Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (SCID)23. Twenty participants denied cocaine use in the past year and reported no more than five lifetime uses. Individuals were excluded based on criteria detailed previously.18 All participants provided written informed consent and completed screening questionnaires on current and past physical and mental health, measures of current psychological functioning, and detailed substance use history. The Institutional Review Board of the University of Kentucky approved all protocols and informed consent documents. Participants were compensated for their time.
Procedure
Data were gathered during routine screening for ongoing laboratory protocols during one outpatient session. Participants were instructed to abstain from drug use for 12 hours and caffeine use for 4 hours prior to testing to decrease the likelihood of being under the acute effects of a substance. Participants were also instructed to smoke their last cigarette at least 1 hour prior to their scheduled session. Upon arrival, all participants passed a field sobriety test and provided a breath sample negative for alcohol. Drug urine screens were conducted at the outset of the session as described previously.18 Participants provided an expired breath carbon monoxide (CO) sample on a Smokerlyser (Bedfont Scientific, Bedford, United Kingdom) and reported the time they smoked their last cigarette. Next, participants smoked one cigarette under staff supervision. Participants completed the visual probe task 1.5 hours following completion of the cigarette in order to reduce psychomotor stimulant effects24 on task performance and to standardize nicotine deprivation.25,26 Immediately following, participants completed questionnaires regarding cigarette craving and withdrawal (see below).
Visual Probe Task
Fixation data were collected using a Tobii X2-60 eye tracker (Tobii Technology, Sweden) as described previously.18 AB was measured using a visual probe task operated using E-prime software (Psychology Software Tools, Pittsburgh, PA) on a PC. For each trial, two 13cm × 18cm images (a substance-related and a matched neutral image) were presented side-by-side, 3cm apart, on a computer screen for 1000ms. Orienting bias (percent) and the amount of time (ms) fixating on the substance and neutral image was measured. Upon offset of the image pair, a visual probe (X) appeared either on the left or the right side of the screen, in the same location as one of the previously presented images. The amount of time (ms) to respond, by pressing one of two response keys indicating on which side of the screen the probe appeared, was measured. Response time data only included critical trials in which a correct response was made longer than 100ms after the probe appeared. Participants completed ten practice trials containing only neutral images to ensure that they understood the task requirements.
Critical task stimuli consisted of five cigarette images matched with five neutral images (ie, non-cigarette-related) and five cocaine images matched with five neutral images (ie, non-cocaine-related). Cigarette images depicted lit and unlit cigarettes. Cocaine images depicted crack or powder cocaine as well as related paraphernalia. Neutral images were matched on the number of objects in the image, size, and color scheme (eg, a cigarette matched with a stick or a crack pipe matched with a pen). Images were proximal cues (ie, no people/scenes)27 with muted color schemes and grayscale background. Cocaine and cigarette image pairs were analogous, but not directly matched. Cigarette images were never presented side-by-side with cocaine images. Images were presented four times, once for each of the four possible image/probe combinations for a total of 40 test trials, in random order (ie, left and right image locations and visual probe locations). Forty filler trials consisting of ten pairs of additional neutral images were intermixed with the test trials as described previously, for a total of 80 trials.18 The primary outcome variables were the difference in orienting direction and fixation time (ms) between (1) cigarette and neutral images and (2) cocaine and neutral images.
Questionnaires
The Minnesota Nicotine Withdrawal Scale—Revised consists of 15 items assessing withdrawal and negative affect over the last 24 hours using a five-point rating scale.28
The Questionnaire of Smoking Urges—Brief consists of 10 items assessing cigarette craving along a 100-unit scale.29 Factor 1 assesses desire to smoke to acquire rewarding effects. Factor 2 assesses the urgent desire to smoke to relieve negative affect.
Data Analysis
Independent samples t tests were conducted to compare demographics for continuous variables and chi-square analyses were conducted to compare categorical variables between groups. Orienting bias was calculated as the percent of trials in which the first fixation was directed towards the cigarette or cocaine image, respectively. Bias scores greater than 50% indicate a bias towards the cigarette or cocaine images. Fixation time (ms) was calculated by summing the total fixation time for each image type (cigarette, neutral-cigarette, cocaine, neutral-cocaine) and then dividing by the total number of trials (20). AB (orienting bias, fixation time, and response time) was assessed using a mixed-model analysis of variance (ANOVA; SPSS, IBM, Chicago, IL) with substance (cigarette and cocaine) and cue type (substance and neutral) as the within-groups factors and group (cocaine-using and non-cocaine-using) as the between-groups factor. The mean-square error term was used to conduct Fisher’s Protected LSD tests to determine potential differences between conditions. An effect size (Hedge’s g) was calculated for all significant effects.30 Statistical significance was set at P < .05.
Results
Table 1 presents descriptive and inferential statistics for comparisons between groups. Cocaine smokers endorsed higher Drug Abuse Screening Test (DAST)31 and Michigan Alcoholism Screening Test (MAST)32 scores than the non-cocaine users. The group of cocaine users also rated Factor 2 of the Questionnaire of Smoking Urges higher (urgent desire to smoke to relieve negative affect). The groups did not differ significantly on any other demographic characteristics or drug use history other than cocaine use.
Table 1.
Mean, SD, t Value, and Chi-Square Value for Comparisons Between Group Means
| Measure (range) | Group | Hedge’s g | ||||
|---|---|---|---|---|---|---|
| Cocaine-using | Non-cocaine-using | |||||
| Mean | SD | Mean | SD | Test of significance | ||
| Age | 41.2 | (7.0) | 38.3 | (12.4) | t (38) = 0.9 | |
| Females | 8 | 12 | χ2 (1) = 1.6 | |||
| Race | χ2 (2) = 5.0 | |||||
| African American | 15 | 8 | ||||
| Caucasian | 4 | 10 | ||||
| Other | 1 | 2 | ||||
| Years of education | 12.1 | (1.2) | 12.5 | (1.0) | t (38) = 1.3 | |
| DAST (0–28) | 12.9 | (5.6) | 3.0 | (2.1) | t (38) = 7.4* | 2.3 |
| MAST (0 – 53) | 10.2 | (10.3) | 3.6 | (4.2) | t (38) = 2.7* | 0.8 |
| Cigarette use | ||||||
| Cigarettes per day | 15.7 | (4.7) | 16.1 | (4.3) | t (38) = 0.3 | |
| Minutes since last cigarettea | 192.5 | (244.3) | 146.3 | (193.9) | t (38) = 0.7 | |
| Carbon monoxide level | 18.5 | (10.9) | 17.1 | (8.6) | t (38) = 0.5 | |
| FTND (0–10) | 5.3 | (2.1) | 4.5 | (1.6) | t (38) = 1.4 | |
| MNWS (total) (0–60) | 5.4 | (5.3) | 8.7 | (7.7) | t (38) = 1.6 | |
| QSU (0–100) | ||||||
| Factor 1 | 73.9 | (30.4) | 69.3 | (27.0) | t (38) = 0.5 | |
| Factor 2 | 40.9 | (28.8) | 24.2 | (22.3) | t (38) = 2.1* | 0.6 |
| Cocaine use | ||||||
| Days since last use | 2.6 | (4.0) | ||||
| Days used past month | 12.8 | (6.1) | ||||
| Days used past 3 months | 38.9 | (21.3) | ||||
| Years used | 16.7 | (7.3) | ||||
DAST = Drug Abuse Screening Test; FTND = Fagerström Test of Nicotine Dependence; MAST = Michigan Alcoholism Screening Test; MNWS = Minnesota Nicotine Withdrawal Scale; QSU = Questionnaire of Smoking Urges-Brief. Effect size (Hedge’s g) reported for significant group differences.
aMinutes since last cigarette prior to start of session.
*Significant difference between groups, P < .05.
For orienting bias, the ANOVA revealed a significant interaction of cue type and group, F (1,38) = 5.0, P < .05. Cocaine users displayed an orienting bias towards cigarette (59%, SD = 11, g = 1.5) and cocaine cues (61%, SD = 11, g = 2.0). Non-cocaine users did not display a statistically significant orienting bias towards either cue (54%, SD = 14, cigarettes; 54%, SD = 8, cocaine).
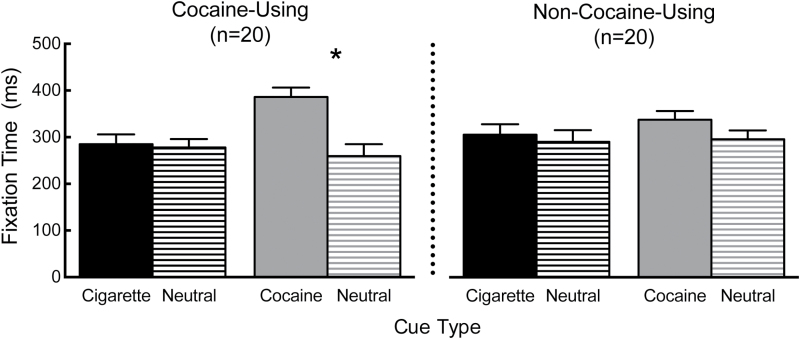
Figure 1 presents cigarette and cocaine AB scores for the cocaine-using and non-cocaine-using groups. For fixation time, the ANOVA revealed a significant interaction of substance, cue type, and group, F (1,38) = 4.3, P < .05. Cocaine users fixated on cocaine images 126.5ms longer than neutral images (g = 1.2), indicating a significant cocaine AB. In contrast, non-cocaine users did not fixate on cocaine images longer than neutral images. Fixation time to cigarette and neutral images did not significantly differ in either the cocaine-using or the non-cocaine-using group (Ps > .05), indicating the absence of a cigarette AB. No significant interactions or main effects were detected for response time data (Ps > .05).
Figure 1.
Mean (standard error mean) fixation time to cigarette, neutral-cigarette, cocaine, and neutral-cocaine cues for cocaine-using (left panel) and non-cocaine-using (right panel) cigarette smokers. An asterisk indicates a significant difference between fixation time to cocaine and neutral cues for the cocaine-using cigarette smokers.
Discussion
This study compared cigarette and cocaine AB in cocaine-smoking and non-cocaine-using cigarette smokers. Cocaine users displayed an orienting bias, but not fixation time bias, towards cigarette cues. Non-cocaine users did not display an orienting bias or fixation time cigarette AB. This indicates that initial orienting, but not sustained attention to cigarette cues, is greater in cocaine-using than non-cocaine-using cigarette smokers, under these experimental parameters (ie, smoking a cigarette 1.5 hours prior to task completion). Consistent with prior studies, cocaine users also displayed a large magnitude cocaine AB as measured by orientation bias and fixation time, which was not present in non-cocaine users.18 Response time did not differ between substance or group.
Although the groups did not differ on indices of cigarette use or negative affect, cocaine-using cigarette smokers endorsed a more urgent desire to smoke to relieve negative affect than non-cocaine users, as measured by the Questionnaire for Smoking Urges. This suggests that the incentive motivation underlying cigarette smoking differs in cocaine-smoking and non-cocaine-using individuals. For the cocaine users, in addition to not having smoked a cigarette for 1.5 hours, individuals reported last using cocaine approximately 2.6 days prior. As such, cocaine smokers might experience negative affect associated with cigarette craving differently than non-cocaine users, as a result of their cocaine use.
In contrast to previous studies, fixation time did not detect a cigarette AB. Smoking demographics (eg, Fagerström Test for Nicotine Dependence33) were comparable with prior studies that detected a bias.19,25,34 As such, the discrepancy may be attributed to experimental design. For example, nicotine deprivation increases fixation time to cigarette cues.25 Smoking a cigarette 1.5 hours prior task completion might have attenuated AB or mitigated group differences. Studies detecting a cigarette AB with fixation time have used longer image presentations (ie, 2000–6000ms), more trials, and varied analytical strategies (ie, duration of initial fixation).19,25,35 Studies detecting a cigarette AB through response time have required a more complex choice response (ie, locate and identify the probe)19,36–39 than those that have not detected a bias.40,41 Furthermore, cigarette AB might be uniquely sensitive task context (ie, priming or carry-over effects). In a modified Drug Stroop task, response time to smoking words was slower following alcohol priming words relative to neutral words.42 In future studies, cigarette AB should be assessed independently of cocaine AB. A larger sample size would facilitate further analysis of individual differences and enable broader conclusions to be drawn.
These results suggest that with comparable rates of smoking, cocaine-smoking cigarette smokers display greater initial orienting bias towards cigarette cues than non-cocaine-using cigarette smokers. Furthermore, cocaine smokers report a more urgent desire to smoke to relieve negative affect than non-cocaine users, when nicotine deprived for 1.5 hours. Identifying differences in cue salience and motivation to smoke cigarettes may provide new treatment targets for both cigarette and cocaine use disorders, at least in this group of cocaine-smoking subjects. Whether these results generalize to individuals preferring powder cocaine is a direction for future research. Future studies should also identify the parameters (eg, nicotine or cocaine administration) under which cigarette AB, as measured by fixation time, might differ in cocaine-using and non-cocaine-using individuals.
Funding
This research was supported by the National Institute on Drug Abuse (R01 DA025032 and R01 DA025591 to CRR) (T32 DA035200 to CRR, KRM, JLA III); National Center for Advancing Translational Sciences (TL1 TR000115 to KRM); and internal funding (University of Kentucky to WWS). These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript.
Declaration of Interests
None declared.
Acknowledgments
KRM, WWS, and CRR developed the study concept. Data were collected by KRM. KRM and JLA III performed the data analysis and interpretation under the supervision of WWS and CRR. KRM drafted the manuscript, and JLA III, WWS, and CRR provided critical reviews. All authors approved the final version of the manuscript for submission.
References
- 1. Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5(2):117–130. doi:10.1016/0899-3289(93)90056-H. [DOI] [PubMed] [Google Scholar]
- 2. United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health, 2013. ICPSR35509-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2014. www.icpsr.umich.edu/cgi-bin/sdaterms Accessed July 21, 2015. [Google Scholar]
- 3. Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav. 2010;35(4):318–324. doi:10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40(3):195–201. doi:10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 5. Brewer AJ, III, Mahoney JJ, III, Nerumalla CS, Newton TF, De La Garza R. II The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacol Biochem Behav. 2013;106:132–136. doi:10.1016/j.pbb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrell PT, Montoya ID, Preston KL, Juliano LM, Gorelick DA. Cigarette smoking and short-term addiction treatment outcome. Drug Alcohol Depend. 2011;115(3):161–166. doi:10.1016/j.drugalcdep.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav. 1996;21(3):409–412. doi:10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 8. Shoptaw S, Rotheram-Fuller E, Yang X, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317–1328. doi:10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 9. Winhusen TM, Kropp F, Theobald J, Lewis DF. Achieving smoking abstinence is associated with decreased cocaine use in cocaine-dependent patients receiving smoking-cessation treatment. Drug Alcohol Depend. 2014;134:391–395. doi:10.1016/j.drugalcdep.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10(2):189–195. doi:10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- 11. Wiseman EJ, McMillan DE. Combined use of cocaine with alcohol or cigarettes. Am J Drug Alcohol Abuse. 1996;22(4):577–587. doi:10.3109/00952999609001682. [DOI] [PubMed] [Google Scholar]
- 12. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi:10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–650. doi:10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 14. Sinha R, Li CS. Imaging-stress and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi:10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 15. Volkow N, Wang GJ, Telang F, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39(3):1266–1273. doi:10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20. doi:10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 17. Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. J Psychopharmacol. 2003;17(3):310–316. doi:10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- 18. Marks KR, Roberts W, Stoops WW, Pike E, Fillmore MT, Rush CR. Fixation time is a sensitive measure of cocaine cue attentional bias. Addiction. 2014;109(9):1501–1508. doi:10.1111/add.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98(6):825–836. doi:10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- 20. Marks KR, Pike E, Stoops WW, Rush CR. Test-retest reliability of eye tracking during the visual probe task in cocaine-using adults. Drug Alcohol Depend. 2014;145:235–237. doi:10.1016/j.drugalcdep.2014.09.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35(1):12–17. doi:10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–1326. doi:10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 24. Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93(3):317–320. doi:10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- 25. Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacology (Berl). 2004;173(1–2):116–123. doi:10.1007/s00213-003-1689-2. [DOI] [PubMed] [Google Scholar]
- 26. Baschnagel JS. Using mobile eye-tracking to assess attention to smoking cues in a naturalized environment. Addict Behav. 2013;38(12):2837–2840. doi:10.1016/j.addbeh.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 27. Miller MA, Fillmore MT. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction. 2010;105(5):883–890. doi:10.1111/j.1360-0443.2009.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi:10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 29. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi:10.1080/14622200124218. [DOI] [PubMed] [Google Scholar]
- 30. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 31. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi:10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 32. Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127(12):1653–1658. doi:http://dx.doi.org.ezproxy.uky.edu/10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 33. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34. Vollstädt-Klein S, Loeber S, Winter S, et al. Attention shift towards smoking cues relates to severity of dependence, smoking behavior and breath carbon monoxide. Eur Addict Res. 2011;17(4):217–224. doi:10.1159/000327775. [DOI] [PubMed] [Google Scholar]
- 35. Kang OS, Chang DS, Jang GH, et al. Individual differences in smoking-related cue reactivity in smokers: an eye-tracking and fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):285–293. doi:10.1016/j.pnpbp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 36. Bradley BP, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol. 2004;15(1):29–36. doi:10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 37. Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychol Addict Behav. 2003;17(1):66–72. doi:10.1037/0893-164X.17.1.66. [DOI] [PubMed] [Google Scholar]
- 38. Field M, Duka T, Tyler E, Schoenmakers T. Attentional bias modification in tobacco smokers. Nicotine Tob Res. 2009;11(7):812–822. doi:10.1093/ntr/ntp067. [DOI] [PubMed] [Google Scholar]
- 39. Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol. 2002;16(4):385–392. doi:10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- 40. Begh R, Munafò MR, Shiffman S, et al. Lack of attentional retraining effects in cigarette smokers attempting cessation: a proof of concept double-blind randomized controlled trial. Drug Alcohol Depend. 2015;149:158–165. doi:10.1016/j.drugalcdep.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McHugh RK, Murray HW, Hearon BA, Calkins AW, Otto MW. Attentional bias and craving in smokers: the impact of a single attentional training session. Nicotine Tob Res. 2010;12(12):1261–1264. doi:10.1093/ntr/ntq171. [DOI] [PubMed] [Google Scholar]
- 42. Oliver JA, Drobes DJ. Cognitive manifestations of drinking-smoking associations: preliminary findings with a cross-primed Stroop task. Drug Alcohol Depend. 2015;147:81–88. doi:10.1016/j.drugalcdep.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]