Abstract
Introduction:
Nicotine’s interoceptive stimulus effects likely help explain smoking’s reinforcing efficacy, but human studies have been limited by difficulties controlling dosing via tobacco inhalation. Our objective was to describe a procedure to study nicotine discrimination via smoking.
Methods:
Dependent smokers abstinent overnight (>12 hours) were first “trained” to discriminate between two cigarettes differing in nicotine content, based on four puffs of exposure, and then tested on whether they successfully acquired that discrimination. After piloting with Quest brand commercial cigarettes, 29 subjects engaged in the main study with cigarettes available through NIDA (Spectrum; 16mg vs. 0.4mg nicotine content). Discrimination training first involved two trials, one with each cigarette, prior to six testing trials. Due to results with the first 20 subjects, the remaining nine received two training trials with each cigarette (four total). Subjective perceptions were also assessed during each testing trial, and puff choice between the two cigarettes available concurrently was assessed after testing, on the last two trials.
Results:
All five pilot subjects successfully discriminated Quest 1 versus Quest 3 (defined by at least five out of six trials correct, ie, >80%). Yet, only 10 of 20 subjects (50%) were able to discriminate the two Spectrum cigarettes based on two training trials. After changing to four training trials, eight of nine subjects were able to discriminate (89%). Subjective perceptions and puff choice differed between cigarettes more in those able versus unable to discriminate them.
Conclusions:
With sufficient training exposures, smokers can discriminate nicotine between cigarettes differing in nicotine contents.
Implications:
The interoceptive stimulus effects of nicotine are critical to understanding reinforcement from cigarette smoking behavior. Because of the very recent availability of Spectrum research cigarettes from NIDA, with specific known amounts of nicotine content, the study of nicotine discrimination in humans via cigarette smoking may now be feasible. Our results demonstrate that, with sufficient training, smokers can behaviorally discriminate nicotine from four puffs’ exposure between cigarettes differing in nicotine contents. Future research should evaluate human discrimination of nicotine from greater amounts of cigarette smoke exposure, as well as in response to other procedural variations.
Introduction
Nicotine’s interoceptive stimulus effects may be key to cigarette smoking’s reinforcing efficacy.1–3 Such stimulus effects are commonly assessed in nonhuman animal species via behavioral drug discrimination procedures.4,5 In humans, however, these effects are nearly always studied indirectly, via self-reports of subjective effects,6,7 which are similar but not synonymous with interoceptive stimulus effects.8 Crucially, self-reported subjective effects cannot be objectively verified (by definition) and so may reflect expectations of effects due to beliefs about the administered substance9 or other biases. In contrast, behavioral drug discrimination procedures do not suffer from these problems. Moreover, since subjective drug effects cannot be assessed in nonhuman species, directly comparing discriminative stimulus and subjective effects can only be done with humans.10–12 Behavioral responses in drug discrimination procedures can have other advantages, such as potentially indicating neural sites of a drug’s action13,14 and possibly occurring at doses below those generating significant subjective effects.15,16 The latter observation suggests that behavioral drug discrimination may be more sensitive to dose than self-reported subjective measures.
Although preclinical research on nicotine discrimination began nearly a half century ago,4,5 the biggest obstacle to studying nicotine discrimination in humans has been lack of methods to rapidly administer nicotine doses in controlled fashion. Discrimination testing with any drug requires careful manipulation of dose administration, to confirm differential responding is due to the difference in drug exposure.4 Cigarette smoking, the most common manner of human use, allows wide variability in nicotine dose exposure due to variations in a smoker’s puff topography, or the intensity and pattern of smoke inhalation.17,18 Even when cigarettes differing sharply in stated nicotine “yield” are used to manipulate intended dosing, their actual nicotine “content” may not differ, and so delivery of nicotine to smokers may not vary because of the ease with which smokers can alter smoking intensity per puff.19 Nicotine yield in commercial brands, as determined by the Federal Trade Commission (FTC method, via machines), is based on a specific amount of smoke for chemical analysis.20 Yet, most brands allow ventilation of smoke directly into ambient air through the holes engineered in the paper, lowering machine-based yield. Human smokers can “defeat” this ventilation, such as by covering over the holes to inhale a greater amount of the smoke,19,21,22 rendering these yield values of limited use in assessing a cigarette’s nicotine dose delivery. Furthermore, because cigarette smoke contains thousands of chemical constituents other than nicotine,23 some of which may be psychoactive,24 the discriminative stimulus effects of nicotine may be confounded with those of the other constituents, which can differ between cigarettes.25 In sum, control over the exteroceptive or nondrug-related interoceptive effects inherent in the vehicle used for drug delivery is as critical as control over drug dosing in conducting any drug discrimination research. As a result, early studies of nicotine discrimination in humans using commercial cigarettes varying in nicotine yield could not verify that discrimination behavior was based on the specific nicotine dose delivered by the administered cigarette, rather than on other cigarette factors.26,27
Other smoked nicotine tobacco products, such as cigars or hookah, etc., similarly lack the dosing control necessary for discrimination testing. Smokeless tobacco or other nonmedicinal nicotine products also lack control over dose, and some deliver nicotine very slowly28 or with nondrug stimulus effects specific to those products. These issues further lessen their appropriateness for examining behavioral discrimination of nicotine’s acute stimulus effects, especially in an effort to better understand cigarette smoking reinforcement. (Yet, slow administration using experimental oral nicotine capsules could be satisfactory).29 Most medicinal nicotine replacement products (ie, NRT), available since the 1980s, have also been problematic for research on nicotine discrimination for similar reasons. As outlined by Schneider et al.,30 they often provide nicotine delivery that is very slow (eg, patch) and/or lacking in control of differential dosing (eg, gum).
For this reason, we developed a nicotine nasal spray procedure (nonmedicinal) in the 1980s to conduct research on acute effects of specific nicotine doses rapidly absorbed by humans (as with smoking31). This spray preparation allowed virtually any amount of nicotine to be administered (under an IND monitored by the US Food and Drug Administration; FDA). We subsequently established a research program on discrimination of nicotine via nasal spray in humans.13,15,32 Yet, findings with a research nasal spray, or any non-inhaled method of administering nicotine, also may have uncertain relevance for understanding nicotine discrimination via cigarette smoking because of exteroceptive or interoceptive sensory effects possibly unique to that route of administration.33
Very recently, however, research cigarettes that differ across a range of specific nicotine contents have become available (through the National Institute on Drug Abuse, NIDA), potentially allowing study of nicotine discrimination in humans via smoking. These research cigarettes, labeled “Spectrum” (see Methods), differ from commercial low-yield brands (eg, “lights”) due to manipulations of the actual nicotine content of the tobacco used, rather than by engineering of the filter ventilation, etc.34 Thus, in contrast with commercial brands, smokers cannot easily obtain greater nicotine intake from Spectrum cigarettes with lower contents.
The objective of this article is to describe results from a procedure to assess discrimination of nicotine via cigarettes, as a starting point to guide future research. We initially adapted procedures from the only prior systematic research of nicotine discrimination in humans, that using nasal spray.15,33 These procedures were first pilot tested using commercial cigarettes differing in nicotine yield (Quest 1 and 3), while we awaited FDA review of our protocol for studying the Spectrum research cigarettes (as explained in the Methods). As noted below, the procedures appeared satisfactory during piloting and were implemented in the formal study to test discrimination of nicotine via Spectrum cigarettes. However, we found that additional training was necessary to increase the number of smokers able to demonstrate reliable nicotine discrimination. These observations, and the resulting procedure for testing nicotine discrimination via cigarette smoking, are described.
Method
Participants
Subjects eligible for this research were dependent smokers who preferred non-menthol cigarettes (We limited the sample to non-menthol smokers to minimize variability in responding between subjects due to the non-nicotine constituents that could alter discrimination behavior, which warrant separate study in their own right.35). Presence of nicotine dependence was confirmed with DSM-V criteria36 assessed by a structured interview updated from Breslau et al.37 All also completed the Fagerstrom Test of Nicotine Dependence (FTND38). Subjects participating in the main study with Spectrum cigarettes (n = 29; 19M, 10 F) had means (SD) of 34.6 (12.3) years old, 16.8 (5.4) cigs/d, and 5.0 (1.9) FTND score. The five subjects in the piloting with Quest cigarettes (4M, 1 F) had corresponding means of 21.4 (2.6) years old, 15.6 (3.4) cigs/d, and 4.6 (1.5) FTND (These samples were completely separate, as subjects participated in only one of the studies.).
Cigarettes
The investigational research cigarettes for the main study were obtained from NIDA’s Drug Supply Program after submission of an application for an Investigational Tobacco Product to the Center for Tobacco Products (CTP) at the FDA (The 2009 Tobacco Control Act, creating CTP, mandates that CTP regulate new tobacco products, including Spectrum, while existing products need not undergo CTP regulation; see www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf.). They are labeled “Spectrum” and were manufactured by 22nd Century Group (Clarence, NY; www.xxiicentury.com/). Spectrum cigarettes were recently investigated in a 6-week trial to determine the impact of nicotine reduction in smokers not currently interested in quitting.39 The Spectrum cigarettes selected for this study were those differing the most on nicotine contents while matched on “tar” yield. As reported (http://grants.nih.gov/grants/guide/notice-files/NOT-DA-14-004.html), contents of the two were approximately 16mg versus 0.4mg of nicotine per gram of tobacco (“mg/g”), and yielded 10 and 9mg “tar,” respectively. The lower nicotine content cigarette, 0.4mg/g, is less than 5% of the content of typical commercial brands.40,41 When they are directly compared, the 16mg/g and 0.4mg/g cigarettes are sometimes labeled here as “moderate” and “very low” in nicotine, respectively, for simplicity (To compare these with commercial cigarettes, these Spectrum research cigarettes correspond to 0.8mg and 0.03mg nicotine yields by FTC method, while typical commercial brands yield about 0.9mg41).
Yet, because no prior study had formally tested nicotine discrimination via cigarette smoking (due to a lack of very low nicotine content cigarettes), we first piloted our procedures by assessing discrimination between two different commercially-purchased Quest brands. These were selected because their labeled nicotine and tar yields were comparable to those for the Spectrum research cigarettes (Quest 1 with 0.6mg and Quest 3 with 0.05mg nicotine, which were considered “moderate” and “very low,” respectively, in piloting; tar was 11 and 10mg, respectively, as reported in https://web.archive.org/web/20131104130634/http://www.ftc.gov/foia/frequentrequests/tarnicotineletter.pdf). Both were administered in blind fashion, as described below, to finalize session details such as setting the duration between trials, number of puffs per trial, etc.
Control Over Smoke Exposure
Smoke intake from all cigarettes was standardized at four puffs per trial, one every 30 seconds, via portable Clinical Research Support System (CReSS; Borgwaldt KC, Inc, Richmond, VA), and a new cigarette was used for each trial. This rate of exposure in initially deprived smokers allowed for repeated testing trials every 15 minutes while minimizing toxicity, since ad libitum smoking typically results in 10–12 puffs per cigarette.42,43 The exact timing and 2-second duration of each puff were guided by computer-presented instructions to standardize intake at about 60ml per puff (consistent with ad libitum puffing43). We also believed that testing ability to discriminate interoceptive effects of nicotine from smoking four puffs would capture the amount of exposure at the onset of one’s expectations about a cigarette, which may be a critical influence on subsequent self-administration of that cigarette (ie, reinforcement), as well as other responses.44–46 Prior tests on discriminating smoked marijuana similarly provided just two or four puffs per administration.47
Session Procedures
All subjects, in the pilot and main study, were instructed to abstain overnight (>12 hours) prior to the study session, confirmed by expired-air carbon monoxide (CO) ≤ 10ppm48 assessed by BreathCO CO monitor (Vitalograph, Lenexa, KS). Similar to prior drug discrimination research with humans,15,16 session procedures involved “training” trials for subjects to acquire the discrimination between the two drug conditions, and then “testing” trials to assess this acquisition of the discrimination. During each training trial, subjects were given one cigarette condition or the other and informed of the cigarette’s identification by letter code only (eg, “A” or “B”). Subjects were instructed to “evaluate these cigarettes based on your overall subjective feelings” since it was “important that you learn how to tell the difference between the two cigarettes.” One training trial for each cigarette initially comprised “training,” based on successful procedures in prior research with nicotine discrimination administered via nasal spray, showing a single training trial for each dose being discriminated was sufficient.15,33 Adequacy of a single training trial for each cigarette was confirmed in the piloting with Quest cigarettes (However, as noted in the Results, the number of training trials per cigarette was later doubled, increasing the total from 2 to 4, as a result of initial testing with the Spectrum cigarettes following the piloting with Quest cigarettes.).
These training trials were then followed by six testing trials (in which subjects were kept blind, or uninformed of cigarette identification) to assess this acquisition of discrimination. After the four puffs in each trial, subjects completed a short measure assessing eight different subjective perceptions of smoking the cigarette to determine which were associated with discrimination behavior. These eight items, commonly obtained in prior smoking studies,43 asked subjects to rate the cigarette on how “satisfying,” “strong,” “harsh,” “smooth,” and “similar to own brand” it was, and how much “nicotine,” “liking,” and “flavor” they experienced. Each was assessed via 0–100 visual analog scales (VAS), anchored by “not at all” to “very much.”43 They then circled “A” or “B” to identify the cigarette, according to the stimulus effects they perceived from each informed cigarette during acquisition of discrimination in the training trials. The two types of cigarettes were presented in random order during testing trials, one per 15 minutes. Successful discrimination was defined by the criterion of at least 80% correct identifications of the cigarettes (ie, on at least five out of six trials). To standardize subject motivation to learn this discrimination, subjects were told each correct cigarette identification would be reinforced by $1. The amount earned was added to the subject’s total participant payment of $20/hr for the 3-hour session. Those who “failed” to acquire discrimination (ie, less than five out of six correct trials) then repeated this testing on a different day, conducted in exactly the same manner as this first session (but with the cigarettes now labeled “C” and “D”), to confirm their inability to discriminate nicotine’s stimulus effects from these Spectrum cigarettes. Data analyses used the responses to cigarettes from this repeat session for such subjects.
Finally, after the sixth (last) testing trial, subjects in the main study (only) completed two final “choice” trials to gauge the relative reinforcing effects of the cigarettes, as in prior research on nicotine reinforcement via cigarettes.49 On each of these two trials, they were given both of the cigarettes and informed of the letter code for each. They were instructed to take a total of four puffs from some combination of the two cigarettes, based solely on their own choice (ie, all four puffs from one or from the other, or from a mix of the two). The timing and duration of each chosen puff was again controlled by computer instructions, as in the prior trials. The number of times the higher nicotine Spectrum cigarette (“moderate”; 16mg/g) was chosen out of the eight total puff opportunities from the two choice trials was the measure of nicotine reinforcement.
Data Analyses
The piloting with five subjects involved simply determining how many were able to meet the criterion for successfully discriminating the Quest cigarettes (at least five out of six trials, or >80%, correct), so no formal analyses were conducted. In the main study (N = 29), the number of subjects able to discriminate the two Spectrum research cigarettes also was the primary result. We first conducted preliminary analyses of the smoking topography results between the two Spectrum cigarettes, to confirm equal exposure. No differences were found, with means (SD) of 260 (122) versus 261 (63) mls for moderate versus very low, respectively, from the four puffs per cigarette during the discrimination testing trials, F(1,28) < 1, ns, as expected. However, because the training procedures were revised, as described in Results, we conducted additional preliminary analyses between the two subgroups receiving the slightly different set of training procedures, comprising the first 20 and then the next nine subjects. The proportion of subjects in each subgroup who were able to discriminate the cigarettes was compared by chi-square analysis. For other responses to the cigarettes, analyses of variance indicated no differences between these subgroups in demographics, subjective perceptions, and choice responses, each F(1,27) < 1.2, all P > .30. So, the two subgroups were combined for comparisons of differences in subjective perceptions of the two cigarettes, and in choice behavior, between those able versus unable to correctly discriminate them (ie, at least five vs. less than five out of six trials correct). Thus, multivariate analyses of variance compared the differences between cigarettes in subjective responses as a function of whether or not subjects were able to discriminate, with follow-up univariate analyses of variances for the individual responses. For choice behavior, somewhat similarly, the number of choices for the moderate versus very low nicotine cigarettes were compared using paired-samples t tests (Because one choice trial was missing for one subject, N = 28 for the choice behavior analyses.).
Results
Piloting
All five subjects in the pilot testing with Quest brand commercial cigarettes were able to discriminate 0.6mg from 0.05mg nicotine (yield), as expected. Specifically, four subjects were correct on six of the six trials, while one was correct on four of six trials (67% correct) but retested on this discrimination in a second session with these procedures and discriminated them correctly on six of six trials.
In sum, subjects were successful in discriminating the two cigarettes with single training trials and these testing procedures, similar to prior studies of nicotine discrimination via nasal spray15,33 or very recently via oral capsules.29 Thus, we initially used these procedures to conduct the main study of nicotine discrimination with Spectrum research cigarettes.
Main Study
Discrimination Results
Initial Training Procedures.
Surprisingly, only 10 of the first 20 subjects (50%) were able to successfully discriminate the two Spectrum research cigarettes differing in nicotine content (16mg/g vs. 0.4mg/g). Just seven of 20 discriminated the cigarettes in the first session, while three others did not accurately discriminate them in the first session but succeeded in discriminating the two during a second, repeat session with the same procedures. Yet, the remaining 10 out of 20 failed to discriminate these cigarettes during both sessions.
Revised Training Procedures.
The greater difficulty of subjects in discriminating these two research cigarettes, which differ on nicotine “yield” (0.8 vs. 0.03mg) at least as widely as the Quest cigarettes used in piloting (0.6 vs. 0.05mg), was very unexpected. Reasons for this difficulty are uncertain, but discrimination could have been harder for the Spectrum research cigarettes if they were more closely matched on most non-nicotine constituents. Thus, we revised our procedures by adding a second training trial for each Spectrum cigarette, for a total of four training trials prior to the six testing trials. We hoped that this additional training exposure would be sufficient to enable most smokers to learn this discrimination, and so all other procedures outlined in Methods remained the same during subsequent sessions.
Discrimination Results With Revised Procedure.
As a result of this change for the subsequent participants, we found eight of nine subjects (89%) correctly discriminated the two Spectrum cigarettes, seven of nine in the first session. One subject failed in session 1 but successfully discriminated the cigarettes upon retesting in a second session, while the remaining subject failed to discriminate the cigarettes during both sessions. Thus, the ability of learning to discriminate the two Spectrum cigarettes at more than 80% accuracy (at least five out of six trials correct) was significantly greater when four training trials (two each cigarette) were provided, compared to when just two training trials (one each) were provided (ie, 89% vs. 50%; chi-square [1 df] = 3.99, P < .05).
Other Responses Associated With Ability to Discriminate
No further differences were seen in preliminary analyses of other responses as a function of the number of initial training trials subjects received. Thus, those data were combined between these two subgroups, in which 18 subjects were able to discriminate the two cigarettes, and 11 were not. We then examined those able versus unable to discriminate on demographics, finding no significant differences for age (means ± SEM of 33.4±2.9 vs. 36.5±3.7 years, respectively), cigarettes/d (16.3±1.3 vs. 17.6±1.7), and FTND (4.8±0.5 vs. 5.2±0.6), each F(1,27) < 1. We then compared them on subjective perceptions and choice behavior responses to the two cigarettes, which follows.
Subjective Perceptions.
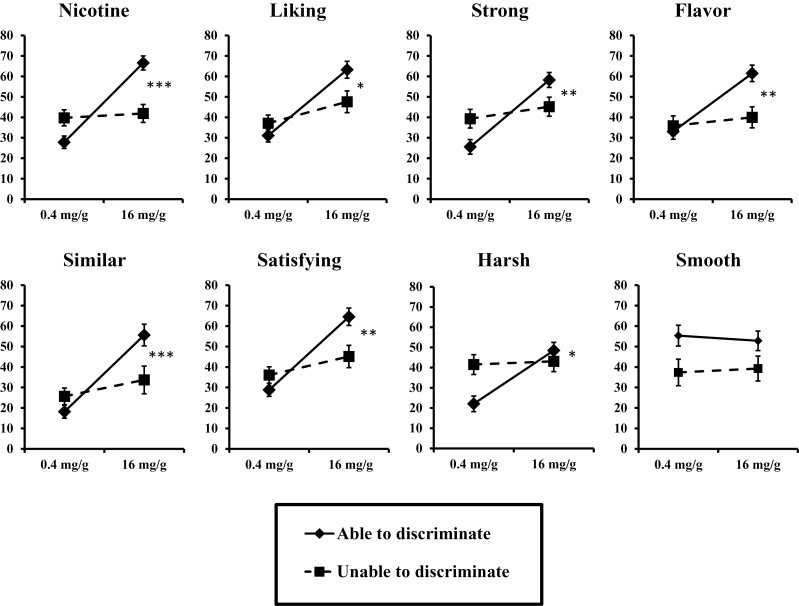
As hypothesized, subjective responses differed between the two cigarettes more among those able versus those unable to behaviorally discriminate them (ie, interaction of nicotine content by discrimination ability, F(8,20) = 3.37, P = .01 in multivariate analyses of variance). As shown in Figure 1, significant interactions for all the individual subjective perceptions, except “smooth,” were found in the follow-up analyses of variances.
Figure 1.
Mean subjective perception items in response to smoking the “moderate” (16mg/g) and “very low” (0.4mg/g) nicotine Spectrum cigarettes, between those able (n = 18) versus unable (n = 11) to discriminate the cigarettes (*P < .05, **P < .01, and ***P < .001 for interaction of able/unable to discriminate by moderate/very low nicotine cigarette in univariate analyses of variances [ANOVAs]).
Nicotine Choice Behavior.
Also as expected, mean (SEM) puffs chosen was greater for the moderate versus very low nicotine cigarette among the entire sample (N = 28), 5.1±0.3 versus 2.9±0.3 puffs, with t(27) = 4.47, P < .001 for the difference between cigarettes. Somewhat similar to results above for subjective perceptions, further paired-samples t tests showed that this difference in puff choice between cigarettes was significant for those able to discriminate them, 5.3±0.3 versus 2.7±0.3 puffs, t(16) = 4.07, P < .001, but only marginal for those unable to discriminate, 4.9±0.4 versus 3.1±0.4 puffs, t(10) = 2.09, P = .06. In an exploratory examination among those 11 unable to meet the criterion of five or more correct trials to show ability to discriminate, as the number of correct discrimination test trials (out of six) increased, so did the number of puffs chosen (out of eight) for the moderate nicotine cigarette during the subsequent choice trials: two correct trials (3.3±0.3 puffs), three correct (4.7±0.3), and four correct (6.3±0.6).
Discussion
This study describes the initial development of a procedure to acutely test whether smokers can discriminate between cigarettes differing in nicotine contents. Research on discrimination of nicotine in humans may increase understanding of the reinforcing efficacy of tobacco smoking.4,5,29,32 Our procedures, primarily adapted from prior studies of discriminating nicotine administered by nasal spray in smokers and nonsmokers,33 were found largely applicable to the study of nicotine from smoking these Spectrum research cigarettes, other than one clear exception. That exception in procedures was the need for two training trials of exposure for each of the administered cigarettes, rather than just one, for a clear majority of smokers to meet the criterion for showing ability to discriminate between these research cigarettes differing in their nicotine contents. When this increase in training trials was implemented, the proportion of subjects able to discriminate the Spectrum cigarettes significantly increased, from 50% to 89%.
We also evaluated other responses to determine their possible association with ability versus inability to discriminate the nicotine content in these research cigarettes. No differences in smoking history or dependence were seen, but we found differential patterns of responding to the cigarettes on measures of subjective perceptions and choice behavior as a function of subjects’ discrimination ability/inability. These observations are consistent with the notions that the interoceptive stimulus effects guiding this behavioral discrimination of cigarettes varying in nicotine are associated with their concomitant subjective perceptions and subsequent choice behavior. Choice of the moderate versus very low nicotine cigarette during the choice trials at the end of the session was greater among the entire sample, consistent with greater reinforcing effects of cigarettes containing “moderate” amounts of nicotine content versus very low amounts.39,49 Notably, this choice behavior was significant only for those able to discriminate while marginal for those unable to discriminate. Yet, choice increased as the number of correct discrimination trials increased in those failing to meet the criterion of more than 80% correct, or at least five of the six trials. Thus, the subjects correct on four out of six trials may have perceived differences between the cigarettes, explaining their high rate of choosing the moderate nicotine cigarette, but not sufficiently to meet the discrimination criterion.
We note that, despite single training trials for each comparison cigarette tested, discrimination between the two Quest commercial cigarettes differing in nicotine “yield” during piloting was apparently easier than discrimination between the two Spectrum research cigarettes during the main study. Although both brands have been reported to be similar in tar content for the cigarettes tested here (see Methods), each brand may not necessarily be matched on all constituents. The Spectrum research cigarettes were explicitly matched as closely as possible on non-nicotine contents, but some differences may still be apparent. For example, the difference between cigarettes in subjective perceptions based on ability to discriminate them included items not expected to differ due to interoceptive stimulus effects of nicotine per se, specifically “harsh” and “flavor,” but not “smooth” (Figure 1). The basis for responses to these individual subjective items is not known, and associations of discriminability with responses on these two items raises the possibility that the differential nicotine contents of these cigarettes, or constituents besides tar, may alter their exteroceptive, as well as interoceptive stimulus effects.50,51 Thus, carefully manipulating only the drug per se while controlling the nondrug factors, important when assessing discriminative stimulus effects of nicotine, may be challenging with any available tobacco cigarettes. In any case, our results should inform subsequent research methods for evaluating behavioral discrimination between research cigarettes differing in nicotine contents.
Nevertheless, further evaluation of procedures for testing discrimination of nicotine via smoking is clearly warranted, to maximize the likelihood of smokers being able to acquire an ability to discriminate cigarettes differing in nicotine contents. Our starting point was the limited prior research on human discrimination of nonsmoked nicotine, other drug administration, or smoked marijuana.8,15,16,32,47 Among our study limitations, smoke exposure was restricted to four puffs/cigarette on each trial, due to the number of trials needed for discrimination training and testing, and for the puff choice testing. Based on prior research showing significant subjective and other effects from just a few puffs,45–47 our procedure of providing four puffs/trial was intended to allow sufficient smoke exposure by which to discriminate nicotine’s interoceptive effects while minimizing chances of toxic responses that could lead to avoidance of nicotine. This aim apparently was successful, since choice was higher for the moderate versus very low nicotine cigarettes in the session’s last two trials. Similarly, trials were separated by just 15 minutes, to enable completion of all testing within a reasonable session duration (3 hours), comparable to the 20-minute inter-trial interval in our prior discrimination studies with nasal spray nicotine.33 Yet, alternative procedures, such as increasing the dose to a full cigarette, for example, 8–12 puffs/trial as in typical ad libitum cigarette smoking,42,43 would likely increase the proportion of subjects able to discriminate these cigarettes under the original procedures, involving just one training trial per cigarette. Also, a longer time between trials could provide more separation of the interoceptive effects between cigarettes, perhaps improving discrimination behavior. However, these alternatives raise serious practical limitations of their own, as increasing each exposure per cigarette may require reducing the total number of trials within one session to avoid toxicity, and extending the duration of trials may require conducting discrimination training and testing across multiple sessions.
Finally, improvements in the procedures for testing nicotine discrimination may have wider applicability in similar research, perhaps including that to guide regulation policy. They may be effective with many of the other methods of administering nicotine in fairly rapid fashion, if dosing can be carefully controlled. Given the proliferation of diverse tobacco products (eg, small cigars, hookah, snus, other smokeless52) and nontobacco nicotine products (especially e-cigarettes) in the marketplace, nicotine discrimination behavior may vary across and within these products. Such procedures may also be appropriate for use in testing other responses to acute nicotine per se administered repeatedly, particularly via research cigarettes differing in nicotine content. Moreover, if results for nicotine discrimination are shown to closely predict those for nicotine reinforcement (eg, via choice behavior), it is conceivable that cigarettes with nicotine contents too low to be discriminated may also be too low to support reinforcement. Such an outcome could impact policy decisions to minimize risks of reinforcement and onset of dependence from regulated tobacco products approved for sale in the marketplace.39,53,54
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse and Food and Drug Administration Center for Tobacco Products (CTP) (U54 DA031659). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Declaration of Interests
None declared.
References
- 1. Harvey DM, Yasar S, Heishman SJ, et al. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacol. 2004;175:134–142. doi:10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- 2. Rose JE, Behm FM, Westman EC, et al. PET studies of the influences of nicotine on neural systems in cigarette smokers. Amer J Psychiatry. 2003;160:323–333. doi:http://dx.doi.org/10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- 3. Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacol. 1995;117:2–10. doi:10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- 4. Glennon RA, Young R. Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York, NY: John Wiley & Sons; 2011. [Google Scholar]
- 5. Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. In: Henningfield JE, London E, Pogun S, eds. Nicotine Psychopharmacology. New York, NY: Springer-Verlag; 2009:295–333. [DOI] [PubMed] [Google Scholar]
- 6. Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi:10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 7. Perkins KA, Jetton C, Keenan J. Common factors across acute subjective effects of nicotine. Nicotine Tob Res. 2003;5:869–875. doi:10.1080/14622200310001614629. [DOI] [PubMed] [Google Scholar]
- 8. Preston KL, Bigelow GE. Subjective and discriminative effects of drugs. Behav Pharmacol. 1991;2:293–313. [PubMed] [Google Scholar]
- 9. Perkins KA, Sayette M, Conklin CA, Caggiula AR. Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res. 2003;5:695–709. doi:10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- 10. Holtzman SG. Discriminative stimulus effects of drugs: relationship to potential for abuse. In: Modern Methods in Pharmacology Vol 6. Testing and Evaluation of Drugs of Abuse. New York, NY: Wiley-Liss, Inc; 1990:193–210. [Google Scholar]
- 11. O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi:10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stolerman IP. Inter-species consistency in the behavioural pharmacology of nicotine dependence. Behav Pharmacol. 1999;10:559–580. [DOI] [PubMed] [Google Scholar]
- 13. Perkins KA, Sanders M, Fonte C, et al. Effects of central and peripheral nicotinic blockade on human nicotine discrimination. Psychopharmacol. 1999;142:158–164. doi:10.1007/s002130050875. [DOI] [PubMed] [Google Scholar]
- 14. Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, vanHuijsduijnen RH, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi:10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 15. Perkins KA, DiMarco A, Grobe JE, Scierka A, Stiller RL. Nicotine discrimination in male and female smokers. Psychopharmacol. 1994;116:407–413. doi:10.1007/BF02247470. [DOI] [PubMed] [Google Scholar]
- 16. Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. [DOI] [PubMed] [Google Scholar]
- 17. Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epid Biomarkers Prev. 2005;14:1370–1375. doi:10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- 18. Pomerleau OF, Pomerleau CS, Rose JE. Controlled dosing of nicotine: a review of problems and progress. Ann Behav Med. 1989;11:158–163. [Google Scholar]
- 19. Benowitz NL, Hall SM, Herning RI, Jacob P, Jones RT, Osman A-L. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309:139–142. doi:10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- 20. St. Charles FK, Kabbani AA, Borderding MF. Estimating tar and nicotine exposure: human smoking versus machine generated smoke yields. Reg Tox Pharmacol. 2010;56:100–110. doi:10.1016/j.yrtph.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21. Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epid Biomarkers Prev. 2009;18:3305–3320. doi:10.1158/1055-9965.EPI-09-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82:320–329. doi:10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann D, Hoffman I. The changing cigarette, 1950–1995. J Toxicol Envir Health. 1997;50:307–364. doi:10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann AC, Evans SE. Abuse potential of non-nicotine tobacco smoke constituents: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi:10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- 25. Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacol. 2006;184:274–285. doi:10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 26. Kallman WM, Kallman MJ, Harry GJ, Woodson PP, Rosecrans JA. Nicotine as a discriminative stimulus in human subjects. In: Colpaert FC, Slangen JL, eds. Drug Discrimination: Applications in CNS Pharmacology. Amsterdam, the Netherlands: Elsevier Biomedical Press; 1982:211–218. [Google Scholar]
- 27. Rose JE. Discriminability of nicotine in tobacco smoke: implications for titration. Addict Behav. 1984;9:189–193. doi:10.1016/0306-4603(84)90056-X. [DOI] [PubMed] [Google Scholar]
- 28. Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44:23–28. doi:10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 29. Duke AN, Johson MW, Reissig CJ, Griffiths RR. Nicotine reinforcement in never-smokers. Psychopharmacol. 2015;232:4243–4252. doi:10.1007/s00213-015-4053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet. 2001;40:661–684. doi:10.2165/00003088-200140090-00003. [DOI] [PubMed] [Google Scholar]
- 31. Perkins KA, Epstein LH, Stiller R, Jennings JR, Christiansen C, McCarthy T. An aerosol spray alternative to cigarette smoking in the study of the behavioral and physiological effects of nicotine. Behav Res Meth Instr Comput. 1986;18:420–426. [Google Scholar]
- 32. Perkins KA. Discriminative stimulus effects of nicotine in humans. In: Henningfield JE, London E, Pogun S, eds. Nicotine Psychopharmacology. New York, NY: Springer-Verlag; 2009:369–400. [DOI] [PubMed] [Google Scholar]
- 33. Perkins KA. Chapter 15: Nicotine discrimination in humans. In: Glennon RA, Young R, eds. Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York, NY: John Wiley & Sons; 2011:463–481. [Google Scholar]
- 34. Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of Spectrum Research Cigarettes. Nicotine Tob Res. 2013;15:1113–1121. doi:10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epid Biomarkers Prev. 2013;22:382–389. doi:10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Psychiatric Association (APA). Diagnostic and Statistical Manual-V. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 37. Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi:10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 38. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 39. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373:1340–1349. doi:10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Byrd GD, Davis RA, Caldwell WS, Robinson JH, deBethizy JD. A further study of FTC yield and nicotine absorption in smokers. Psychopharmacol. 1998;139:291–299. doi:10.1007/s002130050720. [DOI] [PubMed] [Google Scholar]
- 41. U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [Google Scholar]
- 42. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi:10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. doi:10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gu X, Lohrenz T, Salas R, et al. Belief about nicotine selectively modulates value and reward prediction error signals in smokers. Proc Nat Acad Sci. 2015;112:2539–2544. doi:10.1073/pnas.1416639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasenfratz M, Jacober A, Battig K. Smoking-related subjective and physiological changes: pre- to postpuff and pre- to postcigarette. Pharmacol Biochem Behav. 1993;46:527–534. doi:10.1016/0091-3057(93)90540-A. [DOI] [PubMed] [Google Scholar]
- 46. Herskovic JE, Rose JE, Jarvik ME. Cigarette desirability and nicotine preference in smokers. Pharmacol Biochem Behav. 1986;24:171–175. doi:10.1016/0091-3057(86)90333-3. [DOI] [PubMed] [Google Scholar]
- 47. Chait LE, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. The discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacol. 1988;94:206–212. doi:10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- 48. SRNT Subcommittee. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 49. Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A. Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav. 1996;55:257–263. doi:10.1016/S0091-3057(96)00079-2. [DOI] [PubMed] [Google Scholar]
- 50. Jaffe AJ, Glaros AG. Taste dimensions in cigarette discrimination: a multidimensional scaling approach. Addict Behav. 1986;11:407–413. doi:10.1016/0306-4603(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 51. Rees VW, Kreslake JM, Wayne GF, O’Connor RJ, Cummings KM, Connolly GN. Role of cigarette sensory cues in modifying puffing topography. Drug Alc Depend. 2012;124:1–10. doi:10.1016/j.drugalcdep.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. King BA, Dube SR, Tynan MA. Current tobacco use among adults in the United States: findings from the National Adult Tobacco Survey. Amer J Public Health. 2012;102:e93–e100. doi:10.2105/AJPH.2012.301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. New Engl J Med. 1994;331:123–125. doi:10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 54. Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller MR. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013;15:1003–1013. doi:10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]