Abstract
Similar to patients with Alzheimer’s disease (AD), dogs exhibit age-dependent cognitive decline, amyloid-β (Aβ) pathology, and evidence of cholinergic hypofunction. The present study sought to further investigate the role of cholinergic hypofunction in the canine model by examining the effect of the cholinesterase inhibitors phenserine and donepezil on performance of two tasks, a delayed non-matching-to-position task (DNMP) designed to assess working memory, and an oddity discrimination learning task designed to assess complex learning, in aged dogs. Phenserine (0.5 mg/kg; PO) significantly improved performance on the DNMP at the longest delay compared to wash-out and partially attenuated scopolamine-induced deficits (15 μg/kg; SC). Phenserine also improved learning on a difficult version of an oddity discrimination task compared to placebo, but had no effect on an easier version. We also examined the effects of three doses of donepezil (0.75, 1.5, and 6 mg/kg; PO) on performance of the DNMP. Similar to the results with phenserine, 1.5 mg/kg of donepezil improved performance at the longest delay compared to baseline and wash-out, indicative of memory enhancement. These results further extend the findings of cholinergic hypofunction in aged dogs and provide pharmacological validation of the canine model with a cholinesterase inhibitor approved for use in AD. Collectively, these studies support utilizing the aged dog in future screening of therapeutics for AD, as well as for investigating the links among cholinergic function, Aβ pathology, and cognitive decline.
Keywords: Alzheimer’s disease, cholinesterase inhibitor, dog, donepezil, learning, memory, phenserine
INTRODUCTION
The aged dog is particularly well suited for studying the effects of putative therapeutics for Alzheimer’s disease (AD) [1]. Aged dogs spontaneously develop AD like neuropathology, which includes the cortical deposition of amyloid-β (Aβ) [2–5], cerebral atrophy [6, 7], neuronal death [8], reduced neurogenesis [9], increased markers of oxidative stress [10], and amyloid angiopathy [11]. Unlike AD patients, aged dogs do not develop neurofibrillary tangle pathology, but they do show hypersphosphorylated tau possibly representing pre-tangle pathology [12].
Consistent with pathological brain aging, dogs also exhibit age-related cognitive impairment paralleling the behavioral symptomology of AD. The earliest impairments are most evident in performance on tasks involving complex learning and working memory [13–15]. Additionally, executive function, visuospatial ability and complex learning become impaired earlier than impairments in simple discrimination learning [16–20]. With increasing age, dogs can present with global dementia including impairments in discrimination learning [19] as well as behavioral changes similar to those seen in some dementia patients [21, 22]. Like humans, aged dogs show individual differences in cognitive decline, which permits classification into distinct groups of demented, mildly impaired and successful agers [23]. Collectively, this evidence suggests that the aging dog models the progression of human cognitive decline from age associated memory impairment to early AD and supports the use of the model in screening therapeutics for AD [24–26].
The aged dog also provides a model for studying possible links between age-dependent neurochemical changes and cognitive decline, but to date, little work has been done in this area [1]. In AD, reduction of cholinergic function is linked to cognitive impairment [27]. Patients with AD show deterioration of cholinergic markers including reduced levels of choline acetyltransferase [28–30], which is correlated with intellectual impairment [31], atrophy of the cholinergic basal forebrain [32, 33], and reductions in both muscarinic and nicotinic cholinergic receptors [34–40]. The cholinergic hypothesis is also supported by pharmacological evidence that muscarinic blockade with scopolamine impairs cognitive function in a similar manner to that seen in AD, including short-term working memory deficits [41–44], that AD patients show hypersensitivity to scopolamine [45–47], and, perhaps more importantly, that cholinesterase inhibitors are used therapeutically to treat cognitive dysfunction in AD patients [48].
Previously we provided evidence of cholinergic involvement in canine cognition by examining the effect of scopolamine on performance of a delayed non-matching to position task (DNMP) and found working memory performance in aged dogs was disrupted by a 15 μg/kg dose of scopolamine, which produced no obvious behavioral effects [49]. By contrast, young dogs were not impaired by the same scopolamine dose [50] and showed higher muscarinic receptor density than aged dogs [51]. The current studies sought to extend this work by investigating the effects of two cholinesterase inhibitors, donepezil and (−)-phenserine (phenserine), on measures of complex learning and memory performance in aged Beagle dogs.
Donepezil, (R,S)-1-benzyl-4[(5,6 dimethoxy-1-indanon)-2-yl]-methyl piperidine hydrochloride is a piperidine analog approved by the FDA for use in AD in 1997. Phenserine, by contrast, is a phenylcarbamate of (−)-physostigmine that proved to be well tolerated and was shown to improve short-term memory in clinical trials [52–56]. Both show high selectivity for inhibition of acetylcholinesterase over butrylcholinesterase, as well as favorable pharmacokinetic and pharmacodynamic profiles [48, 52, 57]. Both also improve working memory performance in aged rats and reverse scopolamine induced working memory deficits [58–66].
We first examined the effect of phenserine on relearning ability and working memory performance in aged Beagle dogs. Dogs previously trained on the DNMP were re-trained under either phenserine or placebo, then tested for the effects of their respective treatment on memory performance and on counteracting scopolamine induced impairment, all of which are altered by age in dogs [15, 49, 50, 67]. Next, we examined the effects of phenserine on learning a complex oddity discrimination task, which likewise is age-sensitive depending on the difficulty of the problem [18]. A second set of studies aimed at further determining the effects of cholinesterase inhibition on memory examined the effects of three doses of donepezil on DNMP performance. While we previously reported positive results of phenserine in a review as evidence for the use of the dog to screen AD therapeutics [26], the current article provides sufficient details to permit comparisons between the two cholinesterase inhibitors that substantiate both the hypothesis that cholinergic dysfunction contributes to age-dependent cognitive impairment in dogs and the use of the aged dog model for screening AD therapeutics.
MATERIALS AND METHODS
Subjects
Cognitively sophisticated, random source beagle dogs of both genders served as subjects in all experiments. The dogs for the phenserine experiments were housed alone or in pairs at the Division of Comparative Medicine, University of Toronto, in pens measuring approximately 1.07 × 1.22 m. For the donepezil experiment, the dogs were part of the CanCog Technologies colony, and were housed in quadruplicate, if compatible, in pens measuring 2.27 × 7.27 m. Feeding (Purina Agribrands Laboratory Canine Chow at the University of Toronto and Purina Proplan Chicken and Rice at the CanCog facility) occurred once daily following cognitive testing and water was available ad libitum. The animals also received regular veterinary examinations and were not found to suffer from any health problems, neurological or sensory deficits that could interfere with cognitive testing. All procedures were conducted in accordance with the guidelines of the Canadian Council on Animal Care and the “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1996) and procedures that caused pain or discomfort were avoided.
Test apparatus
The test apparatus [17] consisted of a box measuring approximately 0.61 × 1.15 × 1.08 m. After entering the rear of the box, the dog was able to access the test stimuli and food rewards through openings at the front of the box made by adjusting the height of vertical steel bars as to allow the dog to access the stimuli and rewards with only its head. The test stimuli were presented to the dog on a sliding Plexiglas tray that contained three food wells. The test stimuli were presented over the food wells and, after displacing the correct stimulus, the dog was allowed to retrieve the food in the well below. The incorrect stimulus or stimuli was baited with an unobtainable reward to prevent the dog from solving the task using olfactory cues. A partition with a hinged door was used to prevent the dog from observing the placement of test stimuli and rewards between trials or during delays. The tester raised the hinged door and presented the Plexiglas tray to the dog when the inter-trial interval ended.
Drug preparation and administration
Phenserine tartrate ((−)-phenylcarbamoyl eseroline L-tartrate) was synthesized at the National Institute on Aging to in excess of 99.9% chemical and 100% chiral purity, and was administered to the dogs PO in gelatine capsules placed in moist dog food (Hill’s P/D). A dose of 0.5 mg/kg (as a dry tartrate salt) was selected based on preclinical data in Beagle dogs. The capsules were prepared according to individual subject weights and were administered one hour prior to cognitive testing. For a placebo control comparison, an empty gelatin capsule was administered in moist dog food. For both phenserine experiments, a five-day treatment wash-in occurred immediately prior to the initiation of cognitive testing, and phenserine was administered once daily for the duration of the study or until wash-out.
Scopolamine hydrobromide was obtained from Sigma-Aldrich Inc. Scopolamine hydrobromide was dissolved in normal saline to achieve a final concentration of 0.2 mg/ml (free base). In all cases, scopolamine was administered by SC injection 45-min prior to testing at a dose of 15 μg/kg. The dose was selected based on previous work demonstrating selective impairments on the DNMP without disruption of discrimination performance or non-cognitive behaviors in aged dogs [49]. For vehicle injections, a volume of normal saline equivalent to the volume of the scopolamine solution was administered in an identical manner.
Donepezil hydrochloride was provided by Astra Zenica R&D as a powder and was administered to the dogs PO in gelatin capsules. The capsules were prepared according to individual subject weights (hydrochloride salt) and were administered prior to cognitive testing once daily by personnel not involved in data collection. For placebo (0 mg/kg dose) control, capsules containing methylcellulose were used with the standardized dose of 0.5 mg/kg.
Delayed non-matching to position (DNMP)
Prior to initiating the study, all dogs had been trained on a 3-component DNMP task, which assesses visuospatial memory [13]. Each trial on the DNMP task consisted of two phases. During the first phase, a red block was presented to the animal over one of the three food wells. Once the dog displaced the block and obtained the hidden food reward, the tray was withdrawn and a delay initiated. Following the delay, two identical red blocks were presented to the subject in the second phase; one block was placed in the same position as in the first phase and the second block covered one of the remaining two food wells. The dog was rewarded for displacing the block in the new, or non-match, position. Dogs were tested on 12 trials daily each separated by a 60-s inter-trial interval.
In the phenserine study, five male and two female geriatric dogs (M ± S.D. = 14.60 ± 1.46 years) were tested on both relearning and variable-delay paradigms. The dogs were divided into two groups matched for most previous performance. One group was randomly assigned to receive phenserine (n = 4) and the second group was randomly assigned to placebo control (n = 3). After a five day wash-in onto their respective treatments, the dogs were tested at a 5-s delay until they passed a two-stage criterion. The first stage required a subject to achieve a minimum score of 90% correct on one day, or 80% correct over two or three consecutive days. To pass the second stage, the subject was required to obtain a minimum score of 70% over 3 test sessions subsequent to passing the first stage.
Following relearning, dogs were tested on a variable-delay paradigm in which delays of 20 and 80 s occurred equally amongst the 12 trials in a daily test session. The study schedule is indicated in Table 1. All subjects received two practice days, and then mean performance on the three subsequent days (stabilization) was examined to determine if there were differences between the placebo and phenserine groups on basal performance. Following these five sessions, the dogs then were tested under scopolamine and saline vehicle, in a balanced cross-over design. For the balanced cross-over design, each treatment group was randomly divided into two groups, both of which received either scopolamine or saline on the first test day; the treatment was reversed on the second test day. A day without testing separated the saline and scopolamine challenges. Immediately thereafter, the dogs were removed from phenserine and placebo treatments for five days. They then were re-tested on the variable delay DNMP in an identical manner to the treatment phase. This was an exploratory non-blinded study.
Table 1.
Study schedule for phenserine DNMP experiment
| Test day | Time-point | Phenserine group
|
Placebo group
|
||
|---|---|---|---|---|---|
| Order 1 (n = 2) | Order 2 (n = 2) | Order 1 (n = 1) | Order 2 (n = 2) | ||
| 1 | Phenserine/Placebo daily treatment continued | ||||
| 1–2 | DNMP practice days | ||||
| 3–5 | Treatment | Stabilization
|
|||
| 6 | Scopolamine | Saline | Scopolamine | Saline | |
|
|
|||||
| 7 | No testing
|
||||
| 8 | Saline | Scopolamine | Saline | Scopolamine | |
|
| |||||
| 9 | Washout – Phenserine/Placebo discontinued | ||||
| 14–15 | DNMP practice days | ||||
| 16–18 | Wash-out | Stabilization
|
|||
| 19 | Scopolamine | Saline | Scopolamine | Saline | |
|
|
|||||
| 20 | No testing
|
||||
| 21 | Saline | Scopolamine | Saline | Scopolamine | |
For the donepezil study, 46 aged dogs (M ± S.D. = 11.31 ± 2.08 years) from the CanCog Technologies colony were used. The DNMP testing consisted of a variable-delay paradigm using 20 and 90 s delays, which were equally divided between 12 trials per test session. Initially, the dogs were tested over five baseline days, and were then divided into 4 groups matched for baseline performance and randomly assigned to a dose (0, 0.75, 1.5, or 6 mg/kg). Following a five day wash-in period on which no testing occurred, the dogs were tested over the subsequent 9 days on the variable-delay DNMP at either 1, 3, or 5 h after treatment administration using a quasi Latin square design in which the baseline performance of dogs in each test order were balanced to the extent possible. A five day wash-out phase, in which no treatment was administered and no testing was conducted, separated the nine day treatment testing and 5 day wash-out testing. Mean performance on trials completed (there were several non-responses in the high dose donepezil group) was calculated for each of the time-points (i.e., baseline, 1 h, 3 h, 5 h and wash-out) and was used for statistical analysis. All staff performing cognitive testing and recording daily observations were blinded to treatment condition.
Oddity discrimination
The oddity discrimination task is a complex learning task that requires the subject to displace the dissimilar (odd) object amongst three presented [18]. Prior to each oddity test, the dogs were given a preference test in which they were required to choose one of two objects. The object chosen most often during the 10 trial preference test was considered the preferred object, was never rewarded, and appeared in duplicate over all subsequent trials. The object chosen least during the preference test was always rewarded. Thus, each trial consisted of a presentation of three objects; two identical preferred objects, and the rewarded, non-preferred, object. The subjects were tested until they passed a two-stage learning criterion, similar to that in the DNMP relearning, or were tested on 480 trials, whichever occurred first. All animals were initially tested on the first oddity discrimination problem (ODD1) that we presumed would be easier based on greater differences between the correct and incorrect objects. This was followed by a second oddity problem (ODD2) using the same protocol, but with different and more similar objects [18].
In the oddity study, eleven aged beagle dogs (M ± S.D. = 8.76 ± 0.96 years) served as subjects. Animals were divided into two groups matched for previous performance on discrimination tests. For ODD1, one group was randomly assigned to receive phenserine and the other placebo. For ODD2, the treatments were crossed-over such that the group receiving phenserine on ODD1 received the placebo on ODD2 and vice versa. This was an exploratory non-blinded study.
Data analysis
For learning and relearning of the oddity and DNMP, respectively, errors to criterion served as the dependent variable in all analyses. Mean percentage of correct responses at each delay and for each time-point served as the dependent variable for the variable-delay DNMP performance analyses. An independent t-test was used to assess DNMP relearning. To examine the effects of phenserine on oddity learning, a two-way repeated-measures ANOVA was conducted with drug treatment (phenserine versus placebo) serving as a within-subject measure and treatment order between oddity levels as a between-subject variable. All other measures were analyzed with a “repeated-measures” ANOVA, with dose as a “between-subject” measure and time-point and delay serving as a “within-subject” measure, if applicable. Post hoc analyses consisted of repeated-measures ANOVA when examining performance by delay effects and post hoc Fisher’s test in all other cases. Statistical analysis was conducted with the Statistica 6.0 (Statsoft Inc.) software package with the significance level set to p = 0.05. Effects of gender were not considered because we have not previously detected sex differences in cognitive performance of dogs [13, 68].
RESULTS
Effect of phenserine on DNMP relearning, performance and scopolamine-induced impairment in geriatric dogs
Senior dogs were divided into placebo (n = 3) and phenserine (n = 4) treated groups balanced for most previous DNMP learning and were tested for relearning the DNMP at 5 s. Number of errors to reach criterion was analyzed using an independent t-test. The dogs administered phenserine committed fewer mean errors (M ± SEM = 5.25 ± 0.85) than dogs in the placebo group (M ± SEM = 14.33 ± 6.98); however, this difference did not reach significance.
Subsequently, all dogs were tested on a variable delay paradigm of the DNMP using delays of 20 and 80 s. Following two practice days, they were tested for three consecutive (stabilization) days followed by both saline and scopolamine challenges while under either phenserine or control treatment. The treatments were then washed-out and the dogs were re-tested in an identical manner as during treatment.
Mean performance on the variable delay DNMP was initially analyzed using a repeated measures ANOVA with treatment group (phenserine and placebo) serving as a between subject variable and with time-point (treatment and wash-out testing), challenge condition (stabilization, saline and scopolamine), and delay (20 and 80 s) serving as within subject variables. Significant main effects of treatment [F(1,5) = 11.08; p = 0.021] and delay [F(1,5) = 50.28; p = 0.00086] were found. The treatment effect was due to overall higher performance levels in the phenserine treated subjects compared to placebo, and the delay effect represented lower performance levels at the 80 s delay compared to the 20 s delay. Additionally, significant interactions between treatment and delay [F(1,5) = 12.86; p = 0.016] and among challenge, delay and treatment [F(2,10) = 6.52; p = 0.015] were found.
Independent repeated measures ANOVAs for performance at both the 20 and 80 s delays were conducted to elucidate the nature of the interactions. At 20 s, there were significant effects of treatment [F(1,5) = 58.23; p = 0.00062], challenge condition [F(2,10) = 7.98; p = 0.0085], and an interaction between the two [F(2,10) = 6.71; p = 0.014]. Post hoc Fisher’s indicated that scopolamine significantly reduced performance at 20 s in the placebo group compared to both stabilization and saline [p < 0.05 in all cases; Fig. 1A]; however, no differences were seen amongst any conditions in the phenserine group indicative of an attenuation of scopolamine deficits (Fig. 1B). No other significant differences between phenserine and placebo were found.
Fig. 1.
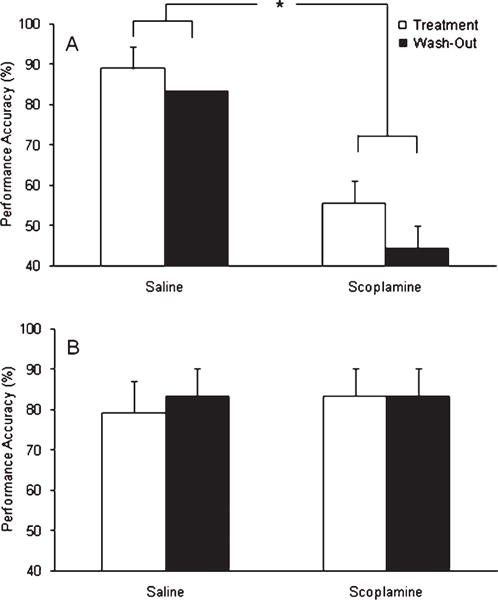
Mean performance accuracy on the DNMP at the 20 s delay in (A) placebo treated dogs and (B) phenserine treated dogs. A three-way ANOVA revealed a significant interaction between treatment and challenge condition, which was further examined using Fisher’s post-hoc test. Post-hoc Fisher’s indicated that scopolamine significantly impaired performance of the placebo treated dogs compared to saline performance. By contrast, scopolamine impairment was not evident in the phenserine treated dogs. Significant differences determined using post-hoc Fisher’s test are depicted by *. Error bars represent S.E.M.
At the 80 s delay, a significant interaction among time-point, challenge condition and treatment was found [F(2,10) = 5.91; p = 0.020], which was investigated using post hoc Fisher’s test. In the dogs receiving placebo, performance at 80 s did not differ significantly between time-points or in response to scopolamine, with the exception of a difference between the saline and scopolamine treatment during wash-out [p = 0.032], suggesting performance at this delay was generally too low to reveal scopolamine induced performance deficits (Fig. 2A). By contrast, scopolamine significantly [p < 0.05 in both cases] impaired performance, compared to the stabilization and saline challenge, while the subjects were receiving phenserine (Fig. 2B). Following wash-out, however, performance between challenge conditions did not differ within the phenserine group due to lower performance levels during stabilization and saline challenge. Specifically, performance of phenserine treated subjects under saline challenge after phenserine wash-out was significantly [p < 0.05 in both cases] lower than the stabilization and saline conditions while they received phenserine. Moreover, reduced performance under the stabilization after phenserine wash-out approached significance [p < 0.08 in both cases] compared to both stabilization and saline performance during phenserine administration. The latter two findings suggest phenserine improved memory performance, and that this effect was no longer present upon wash-out. Similarly, stabilization and saline performance at 80 s in the phenserine treated dogs was consistently higher than in animals receiving placebo; however, this improvement approached significance [p < 0.08 in both cases] only when compared to the saline condition in placebo dogs (Fig. 3).
Fig. 2.
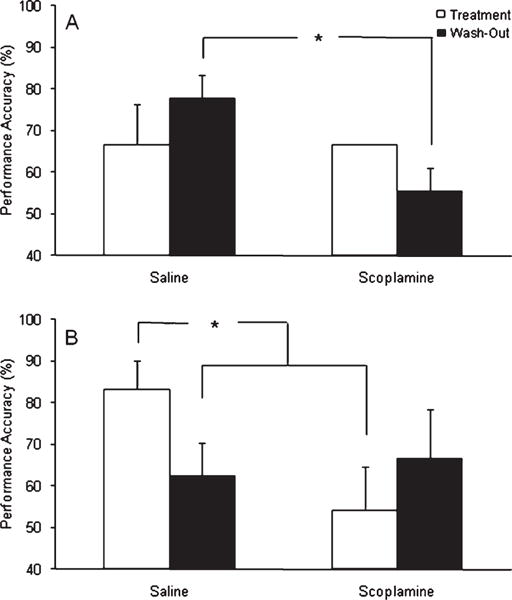
Mean performance accuracy on the DNMP at the 80 s delay in (A) placebo treated dogs and (B) phenserine treated dogs. A three-way ANOVA revealed a significant interaction among treatment, time-point and challenge condition, which was further examined using Fisher’s post-hoc test. In placebo treated animals, a significant scopolamine-induced impairment was found compared to saline performance during the wash-out phase. By contrast, post-hoc Fisher’s test revealed that scopolamine challenge significantly impaired performance at 80 s compared to saline while the dogs were treated with phenserine. Moreover, post-hoc Fisher’s also revealed that performance under saline was significantly better under phenserine compared to after phenserine was discontinued (i.e., wash-out) for at least 10 days. No scopolamine effect was evident during wash-out, partially due to lower levels of performance at the 80 s delay after phenserine was discontinued. Significant differences determined using post-hoc Fisher’s test are depicted by *. Error bars represent S.E.M.
Fig. 3.
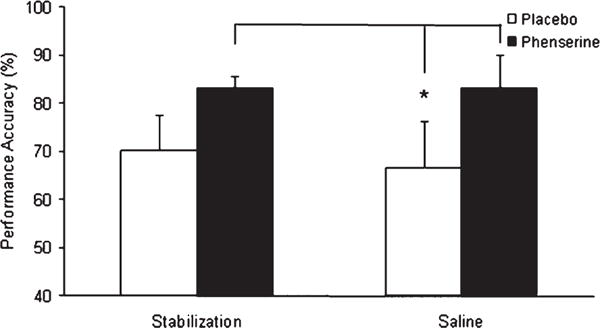
Mean performance accuracy of placebo and phenserine treated dogs on the DNMP at the 80 s delay during the treatment phase. Post-hoc Fisher’s indicated that phenserine marginally (p < 0.08) enhanced performance during stabilization (i.e., 3 control test days) and saline challenge compared to saline challenge under the placebo. Marginally significant differences determined using post-hoc Fisher’s test are depicted by *. Error bars represent S.E.M.
Effect of phenserine on oddity discrimination learning
In this experiment, 14 aged Beagle dogs were divided into two groups balanced for previous learning experience. The groups were randomly assigned to either receive placebo or phenserine during the first oddity problem, and the treatments were crossed over for the second oddity problem. Errors to learn each problem were analyzed using a two-way ANOVA with treatment order across oddity problems (placebo first and phenserine first) serving as a between subject measure and with drug treatment (placebo and phenserine) serving as a within subject measure. A significant interaction between drug treatment and treatment order [F(1,9) = 14.20; p = 0.0044] was found. Post-hoc analysis indicated that more errors were committed on ODD2 than ODD1 under the placebo condition [p = 0.039; Fig. 4]. No difference between phenserine and placebo treatment was found on ODD1; however, errors on ODD2 were significantly lower under phenserine compared to placebo [p = 0.044].
Fig. 4.
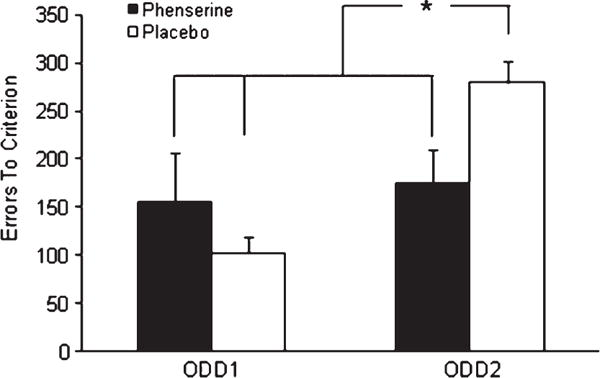
Mean errors to criterion on both ODD1 and ODD2. A two-way repeated measures ANOVA revealed a significant interaction between drug treatment and treatment order, which was further analyzed using post-hoc Fisher’s test. No differences were found between phenserine and placebo on the more simple oddity problem (ODD1); however, phenserine significantly reduced learning errors on the more difficult problem (ODD2) compared to placebo. The placebo groups committed significantly more errors on ODD2 than either treatment group on ODD1, which confirmed ODD2 was more difficult than ODD1. Significant differences determined using post-hoc Fisher’s tests are depicted by *. Error bars represent SEM.
Effect of donepezil on DNMP performance
Groups were initially equated for mean baseline performance over five DNMP test sessions. The effect of donepezil on mean DNMP performance was initially analyzed using a three-way repeated measures ANOVA with dose level (0, 0.75, 1.5, and 6 mg/kg) serving as a between subject variable and with time-point (baseline, 1 h, 3 h, 5 h, and wash-out) and delay (20 and 90 s) serving as within subject variables. Performance at the 90 s delay was significantly impaired compared to performance at the 20 s delay [F(1,41) = 13.060; p = 0.00082]. A significant effect of time-point was also found [F(4,164) = 4.19; p = 0.0029], which was due to significantly [p < 0.05] impaired performance at the 1 hour time-point compared to all other time-points except baseline. There was also a significant interaction [F(4,164) = 2.95; p = 0.022] between time-point and delay, which reflected reduced accuracy at the 90 s delay at the 1 h time-point compared to the 20 s delay. On the other hand, accuracy at the 90 s delay was significantly higher than baseline at the 3 h time-point, collectively suggesting that the effect of donepezil varied as a function of post-dose time.
Although no main effects of dose were found, the analysis indicated a marginally significant time-point by dose interaction [F(12,164) = 1.64; p = 0.086]. Because of the time-point by delay interaction, we used a repeated measures ANOVA to separately analyze the data at each delay, with dose as a between subject variable and time-point as a within subject variable. There were no dose effects found at 20 s delay (Fig. 5A), but the analysis of the 90 s delay (Fig. 5B) revealed a significant dose by time-point interaction [F(12,164) = 1.89; p = 0.040]. Post hoc Fisher’s revealed no change from baseline in both the placebo and 0.75 mg/kg donepezil groups at any time-point. Donepezil at 6 mg/kg significantly impaired performance at 3 h compared to baseline [p = 0.027]. By contrast, 1.5 mg/kg significantly improved performance compared to baseline at both 3 [p = 0.018] and 5 hours [p = 0.019]. No differences from baseline were seen at wash-out for any dose group.
Fig. 5.
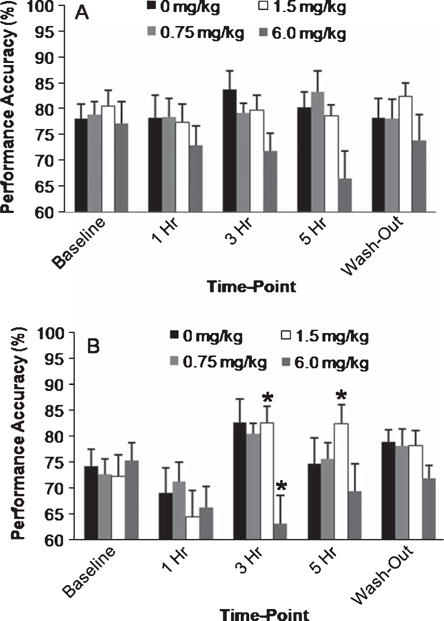
Mean performance accuracy on the DNMP at the (A) 20 and (B) 90 s delays by donepezil dose. For each delay, performance under each dose across time-points (i.e., baseline, 1 h, 3 h, 5 h, and following a 5 day wash-out) was analyzed using a 2-way repeated measures ANOVA. No significant effects of dose or time-point were found at 20 s. At the 90 s delay, a significant interaction between dose and time-point was found. Post-hoc Fisher’s revealed a significant improvement in performance compared to baseline at 3 and 5 h following the 1.5 mg/kg donepezil dose. By contrast, the 6 mg/kg dose significantly impaired performance at the 90 s delay compared to baseline three hours following dosing. No differences from baseline were found at any other dose and wash-out performance did not differ from baseline in any dose group. Significant differences from baseline are depicted by * as determined using post-hoc Fisher’s test. Error bars represent S.E.M.
We also obtained daily observations of the animals performed by animal care staff blinded to treatment conditions. Dogs receiving 6 mg/kg of donepezil showed signs consistent with cholinesterase inhibition (85 individual events), including tremors, vomiting, and hypersalivation more frequently than placebo (15 individual events) or the 1.5 mg/kg group (8 individual events). Likely related to these cholinergic side effects, the high dose group also showed increased non-responses (M ± S.D. = 0.83 ± 1.52) compared to placebo (M ± S.D. = 0.00 ± 0.00), low dose (M ± S.D. = 0.00 ± 0.00), and mid dose (M ± S.D. = 0.24 ± 0.59) donepezil; therefore, only trials in which responses occurred were used in the analysis of DNMP performance.
DISCUSSION
The current studies were designed to further assess the hypothesis that cholinergic hypofunction contributes to age-dependent cognitive decline in dogs by examining the effect of cholinesterase inhibitors on measures of cognitive performance in aged dogs. Initially, we examined the effectiveness of phenserine in improving performance on cognitive tasks that are sensitive to aging and in counteracting scopolamine-induced deficits on DNMP performance; scopolamine has been used to mimic some aspects of AD dementia [69] and has been effective in identifying cholinesterase inhibitors [70–72]. Consistent with previous reports [54, 62, 64, 73], phenserine improved measures of learning, memory, and partially attenuated scopolamine-induced working memory deficits. The absence of significant effects on DNMP relearning compared to placebo raises the issue of limited power in this experiment including only 7 dogs.
To further examine the effect of cholinesterase inhibitors in memory in aged dogs, we also examined the effects of donepezil on DNMP performance in a larger group of aged dogs. Similar to the phenserine results, 1.5 mg/kg of donepezil improved performance on the DNMP at 90 s compared to baseline, but not 20 s, which is consistent with the memory enhancing effects reported in other species [58–61, 66]. To the best of our knowledge, these results represent the first time a cholinesterase inhibitor approved for use in AD has been shown to have memory enhancing effects in aged dogs. Consistent with our previous studies [49, 50], these findings suggest that cholinergic deficits contribute to the development of age-associated cognitive impairment in dogs, and more specifically to the memory impairment assessed by the DNMP.
The dogs used in the phenserine DNMP study comprised a subset of animals that had previously been extensively trained on the DNMP, and also were the oldest subjects in the colony; therefore, we were likely to obtain a high proportion of cognitively impaired dogs. Both groups showed rapid relearning of the task when compared to naïve aged dogs [74], and both groups performed poorly at the long delay. The phenserine group committed fewer mean errors to relearn the DNMP than the placebo group, although the group difference did not achieve statistical significance. We also found that phenserine significantly improved performance on the variable delay DNMP task regardless of delay, challenge condition or time-point, suggesting performance enhancing effects. At 20 s, no differences were found compared to the placebo group except that phenserine attenuated the scopolamine deficit regardless of time-point. We have reported previously delay-independent effects of scopolamine on DNMP performance [49] and, therefore, the absence of a time-point effect could represent either a long lasting effect of phenserine or group differences in scopolamine sensitivity. The absence of improvement at the 20 s delay may also represent a ceiling effect in which performance was sufficiently high to preclude detection of drug enhancement. By contrast, performance at the 80 s delay was higher during phenserine administration compared to after phenserine was washed-out. Moreover, this enhanced performance under phenserine approached significance compared to placebo, but declined to levels comparable to the placebo group after wash-out. Collectively, this suggests that the effects of phenserine reflected memory enhancement at the longer delay. The absence of a phenserine effect at the longer delay during the scopolamine challenge contrasted with the attenuation at 20 s. This could represent a floor effect as scopolamine alone did not consistently impair performance in the placebo treated animals at the long delay.
We also examined the effect of phenserine using a larger sample size on an oddity discrimination learning protocol that shows age sensitivity in Beagle dogs [18]. The dogs were tested with successive oddity discrimination problems in which the objects were made progressively more similar. We found no differences between the phenserine and placebo groups on the easier of the 2 oddity discrimination problems. By contrast, phenserine significantly improved learning on the more difficult oddity problem. A similar interaction between oddity task difficulty and the ability to detect therapeutic effects has been reported previously [18], suggesting that the more simple the problem, the more difficult it is to detect an effect.
In addition to cholinesterase activity [52, 53, 73], phenserine shows posttranslational reduction of AβPP with the subsequent reduction of Aβ [75–79]. Both DNMP deficits and executive dysfunction is correlated with cholinergic deficits [49, 50] and Aβ deposition [2, 7] in the dog. Furthermore, several studies both in vitro and in vivo suggest that various Aβ fragments and oligomeric forms directly impair memory, or memory-related mechanisms [80–83]. However, the doses of phenserine required to achieve effects on Aβ processing are greater than those required for acetylcholinesterase inhibition, result in cholinomimetic side effects, and require administration over an extended duration [77]; no cholinergic side-effects were detected in this relatively short study [84]. Therefore it is more likely the effects observed reflected cholinergic modulation rather than modulation of Aβ processing. This suggestion is also consistent with the evidence that phenserine partially reversed the disruptive effects of scopolamine.
While the studies with phenserine support the hypothesis that cholinergic dysfunction contributes to age-related cognitive decline in dogs, several limitations of the phenserine experiments warrant caution of this hypothesis. First, the limited sample size in the DNMP study likely reduced the statistical power to accurately identify treatment differences, and consequently the effects attributed to cholinesterase inhibition may be partially related to group differences. Second, although preclinical and clinical studies with phenserine support memory enhancement [54, 56], phenserine clinical development was ultimately halted following the failure of an initial phase III clinical trial likely related to methodological problems that were later independently verified [85]. Nonetheless, the use of an approved acetylcholinesterase inhibitor would afford even greater validation of the canine model [1]. Last, the dualistic mechanism of action of phenserine was only partially addressed in the current study.
To further evaluate the effect of acetylcholinesterase inhibition, we also tested the effects of donepezil on DNMP performance in a larger number of aged dogs matched for initial performance. Donepezil significantly improved memory performance, but this was highly dependent on delay, dose and post-dose test time-point. Similar to phenserine, no effect of donepezil was evident at the 20 s delay, possibly due to high levels of performance limiting our ability to detect enhancement. At 90 s, the 1.5 mg/kg dose significantly improved performance three and five hours after dosing compared to baseline, which was consistent with memory enhancement. By contrast, the highest dose (6 mg/kg) of donepezil impaired performance compared to baseline three hours after administration and was associated with cholinergic side effects, which were less evident at lower doses. Compared to baseline, there was no change in performance in either the 0.75 mg/kg donepezil or the placebo group at any time-point, and wash-out performance of all groups did not differ from baseline. The dose dependent results on DNMP performance are consistent with the inverted U-shaped curve effect of cholinesterase inhibitors described by others [58, 86].
The memory enhancing effects in dogs obtained with donepezil were similar to those obtained with phenserine; specifically, performance at the longer delay was increased compared to baseline, and this enhancement was no longer evident after the drugs were discontinued. In both cases, the effect size was small, which is consistent with results obtained in other species. Thus, donepezil does not consistently reverse scopolamine- or age-related deficits on memory tasks in mice and rats, which may be related to the absence of test standardization and to variation in the experimental protocols used in different laboratories [87]. In non-human primates, donepezil, and other cholinesterase inhibitors, also show memory enhancement [59, 60, 66], but this result is most apparent with the use of the “Best Dose” procedure, which systematically biases the results toward finding positive effects. Coupled with the inconsistent findings in rodents, this may reflect the limited effect size of cholinesterase inhibitors in general, and/or the variability of individual response [86], both of which are factors that may impact the clinical utility of donepezil [48, 88, 89]. While significant improvements compared to placebo are found in clinical trials for AD that typically employ a parallel group design, the current studies were much shorter in length.
Previously, we suggested the canine model may better predict clinical outcomes than rodent and non-human primate models particularly in detecting false positives, i.e. drugs positive in animal models that subsequently fail in the clinic [26]. The results of the donepezil study substantiates that positive outcomes are also detectable using the aged canine model and that the outcomes are comparable to those seen in humans. Given the occurrence of neuropathological changes similar to those seen in AD [90], these results supports the use of the aged dog in examining the interaction among cholinergic dysfunction, Aβ pathology and cognitive function, suggested by others [91–93], as well as for comparing the effectiveness of therapeutics targeting Aβ with currently approved therapies.
In addition to AD research and drug development, the findings of the present study may have applications in the field of veterinary medicine. Some aged dogs demonstrate behavioral changes attributable to brain aging and cognitive dysfunction, which is diagnosed as canine cognitive dysfunction syndrome [94, 95]. Given the cognitive benefits observed in the current studies, cholinesterase inhibitors may prove to be an effective therapeutic for canine cognitive dysfunction. The differential effects of dose and time-point between the two drugs in the current study highlight the need for extensive pharmacokinetic and pharmacodynamic profiling in the dog before consideration for veterinary use. While phenserine enhanced memory one hour after administration, no tested dose of donepezil was effective at the one hour time-point. Further, the effective dose of donepezil (1.5 mg/kg) reduced performance at one hour, although this was not significant. This suggests a narrow therapeutic window for donepezil, which was anticipated in the design of the study because the half-life of donepezil in dogs is approximately 25 times shorter than in humans [96]. Although fewer subjects were used in the phenserine experiment, the differences between phenserine and placebo approached significance, which suggests phenserine may be better suited than donepezil for dogs particularly since it has demonstrated a wide therapeutic window in other species [53, 73]. However, additional studies in a larger sample size examining the time course of effects are required.
The goal of the present study was to examine the effects of the cholinesterase inhibitors on cognitive function in aged dogs. Phenserine improved memory, partially attenuated scopolamine-induced DNMP deficits, and improved learning of sufficiently difficult problems. Donepezil showed a dose- and time-dependent enhancement of memory, similar to that seen with phenserine. Collectively, these studies support the hypothesis that age-related cognitive decline, particularly in DNMP performance, in dogs is partially attributable to cholinergic dysfunction and that the canine model can predict the effects of an approved AD therapeutic. This further validates the canine model and augments its value as an animal model for studying hypotheses and potential therapeutics for AD.
Acknowledgments
The Natural Sciences and Engineering Research Council of Canada supported this work with Grant A7659 to NWM, and postgraduate scholarships to J.A.A. Special thanks are extended to the University of Toronto’s Division of Comparative Medicine’s staff. This work was also supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA and research and development funding from CanCog Technologies, Inc. JAA, CD and NWM served as shareholders and/or employees of CanCog. JAA is an employee of Inter-Vivo Solutions.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=833).
References
- 1.Woodruff-Pak DS. Animal models of Alzheimer’s disease: therapeutic implications. J Alzheimers Dis. 2008;15:507–521. doi: 10.3233/jad-2008-15401. [DOI] [PubMed] [Google Scholar]
- 2.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996;66:11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 3.Cummings BJ, Satou T, Head E, Milgram NW, Cole GM, Savage MJ, Podlisny MB, Selkoe DJ, Siman R, Greenberg BD, Cotman CW. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: evidence from cats and dogs. Neurobiol Aging. 1996;17:653–659. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 4.Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 5.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 6.Tapp PD, Head K, Head E, Milgram NW, Muggenburg BA, Su MY. Application of an automated voxel-based morphometry technique to assess regional gray and white matter brain atrophy in a canine model of aging. Neuroimage. 2006;29:234–244. doi: 10.1016/j.neuroimage.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 7.Tapp PD, Siwak CT, Gao FQ, Chiou JY, Black SE, Head E, Muggenburg BA, Cotman CW, Milgram NW, Su MY. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J Neurosci. 2004;24:8205–8213. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29:39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- 11.Su MY, Head E, Brooks WM, Wang Z, Muggenburg BA, Adam GE, Sutherland R, Cotman CW, Nalcioglu O. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging. 1998;19:479–485. doi: 10.1016/s0197-4580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese M, Mascort J, Mahy N, Ferrer I. Diffuse beta-amyloid plaques and hyperphosphorylated tau are unrelated processes in aged dogs with behavioral deficits. Acta Neuropathol. 2006;112:175–183. doi: 10.1007/s00401-006-0087-3. [DOI] [PubMed] [Google Scholar]
- 13.Chan AD, Nippak PM, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behav Neurosci. 2002;116:443–454. [PubMed] [Google Scholar]
- 14.Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman CW, Ruehl WW, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- 15.Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. Visuospatial function in the beagle dog: an early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem. 2006;86:197–204. doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Milgram NW, Adams B, Callahan H, Head E, Mackay B, Thirlwell C, Cotman CW. Landmark discrimination learning in the dog. Learn Mem. 1999;6:54–61. [PMC free article] [PubMed] [Google Scholar]
- 17.Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 19.Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapp PD, Siwak CT, Estrada J, Holowachuk D, Milgram NW. Effects of age on measures of complex working memory span in the beagle dog (Canis familiaris) using two versions of a spatial list learning paradigm. Learn Mem. 2003;10:148–160. doi: 10.1101/lm.56503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siwak CT, Tapp PD, Milgram NW. Effect of age and level of cognitive function on spontaneous and exploratory behaviors in the beagle dog. Learn Mem. 2001;8:317–325. doi: 10.1101/lm.41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siwak CT, Tapp PD, Zicker SC, Murphey HL, Muggenburg BA, Head E, Cotman CW, Milgram NW. Locomotor activity rhythms in dogs vary with age and cognitive status. Behav Neurosci. 2003;117:813–824. doi: 10.1037/0735-7044.117.4.813. [DOI] [PubMed] [Google Scholar]
- 23.Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:675–692. doi: 10.1016/s0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 24.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 25.Head E, Pop V, Vasilevko V, Hill M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studzinski CM, Araujo JA, Milgram NW. The canine model of human cognitive aging and dementia: pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:489–498. doi: 10.1016/j.pnpbp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 28.Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci. 1977;34:247–265. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 29.Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- 30.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978;4:273–277. doi: 10.1111/j.1365-2990.1978.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 31.Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE. Pathological changes in the nucleus of Meynert in Alzheimer’s and Parkinson’s diseases. J Neurol Sci. 1983;59:277–289. doi: 10.1016/0022-510x(83)90045-x. [DOI] [PubMed] [Google Scholar]
- 33.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 34.Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer’s disease. J Neurochem. 1988;50:1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 35.Mulugeta E, Karlsson E, Islam A, Kalaria R, Mangat H, Winblad B, Adem A. Loss of muscarinic M4 receptors in hippocampus of Alzheimer patients. Brain Res. 2003;960:259–262. doi: 10.1016/s0006-8993(02)03542-4. [DOI] [PubMed] [Google Scholar]
- 36.Reinikainen KJ, Riekkinen PJ, Halonen T, Laakso M. Decreased muscarinic receptor binding in cerebral cortex and hippocampus in Alzheimer’s disease. Life Sci. 1987;41:453–461. doi: 10.1016/0024-3205(87)90221-9. [DOI] [PubMed] [Google Scholar]
- 37.Rinne JO, Laakso K, Lonnberg P, Molsa P, Paljarvi L, Rinne JK, Sako E, Rinne UK. Brain muscarinic receptors in senile dementia. Brain Res. 1985;336:19–25. doi: 10.1016/0006-8993(85)90411-1. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Puertas R, Pascual J, Vilaro T, Pazos A. Autoradiographic distribution of M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimer’s disease. Synapse. 1997;26:341–350. doi: 10.1002/(SICI)1098-2396(199708)26:4<341::AID-SYN2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse PJ, Au KS. Cholinergic receptors in aging and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:665–676. doi: 10.1016/0278-5846(86)90035-7. [DOI] [PubMed] [Google Scholar]
- 40.Whitehouse PJ, Kellar KJ. Nicotinic and muscarinic cholinergic receptors in Alzheimer’s disease and related disorders. J Neural Transm Suppl. 1987;24:175–182. [PubMed] [Google Scholar]
- 41.Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 42.Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- 43.Sunderland T, Tariot P, Murphy DL, Weingartner H, Mueller EA, Cohen RM. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology. 1985;87:247–249. doi: 10.1007/BF00431817. [DOI] [PubMed] [Google Scholar]
- 44.Sunderland T, Tariot PN, Weingartner H, Murphy DL, New-house PA, Mueller EA, Cohen RM. Pharmacologic modelling of Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 45.Ray PG, Meador KJ, Loring DW, Zamrini EW, Yang XH, Buccafusco JJ. Central anticholinergic hypersensitivity in aging. J Geriatr Psychiatry Neurol. 1992;5:72–77. doi: 10.1177/002383099200500203. [DOI] [PubMed] [Google Scholar]
- 46.Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 47.Sunderland T, Tariot PN, Mueller EA, Murphy DL, Weingartner H, Cohen RM. Cognitive and behavioral sensitivity to scopolamine in Alzheimer patients and controls. Psychopharmacol Bull. 1985;21:676–679. [PubMed] [Google Scholar]
- 48.Doody RS. Current treatments for Alzheimer’s disease: cholinesterase inhibitors. J Clin Psychiatry. 2003;64:S7. [PubMed] [Google Scholar]
- 49.Araujo JA, Chan AD, Winka LL, Seymour PA, Milgram NW. Dose-specific effects of scopolamine on canine cognition: impairment of visuospatial memory, but not visuospatial discrimination. Psychopharmacology. 2004;175:92–98. doi: 10.1007/s00213-004-1777-y. [DOI] [PubMed] [Google Scholar]
- 50.Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Araujo JA, Nobrega JN, Raymond R, Milgram NW. Aged dogs demonstrate both increased sensitivity to scopolamine impairment and decreased muscarinic receptor density. Pharmacol Biochem Behav. 2011;98:203–209. doi: 10.1016/j.pbb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Greig NH, Ruckle J, Comer P, Brownell L, Holloway HW, Flanagan DR, Canfield CJ, Burford RG. Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects. Curr Alzheimer Res. 2005;2:483–492. doi: 10.2174/156720505774330564. [DOI] [PubMed] [Google Scholar]
- 53.Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:281–290. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- 54.Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, Blennow K, Langstrom B, Nordberg A. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann Neurol. 2008;63:621–631. doi: 10.1002/ana.21345. [DOI] [PubMed] [Google Scholar]
- 55.Klein J. Phenserine. Expert Opin Investig Drugs. 2007;16:1087–1097. doi: 10.1517/13543784.16.7.1087. [DOI] [PubMed] [Google Scholar]
- 56.Winblad B, Giacobini E, Frölich L, Bruinsma G, Becker RE, Greig NH. Phenserine efficacy in Alzheimer’s disease. J Alzheimers Dis. 2010;22:1201–1208. doi: 10.3233/JAD-2010-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greig NH, De ME, Holloway HW, Yu QS, Utsuki T, Perry TA, Brossi A, Ingram DK, Deutsch J, Lahiri DK, Soncrant TT. The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics. Acta Neurol Scand. 2000;(Suppl):176–184. doi: 10.1034/j.1600-0404.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 58.Braida D, Paladini E, Griffini P, Lamperti M, Maggi A, Sala M. An inverted U-shaped curve for heptylphysostigmine on radial maze performance in rats: comparison with other cholinesterase inhibitors. Eur J Pharmacol. 1996;302:13–20. doi: 10.1016/0014-2999(96)00072-6. [DOI] [PubMed] [Google Scholar]
- 59.Buccafusco JJ, Terry AV. Donepezil-induced improvement in delayed matching accuracy by young and old rhesus monkeys. J Mol Neurosci. 2004;24:85–91. doi: 10.1385/JMN:24:1:085. [DOI] [PubMed] [Google Scholar]
- 60.Buccafusco JJ, Terry AV, Webster SJ, Martin D, Hohnadel EJ, Bouchard KA, Warner SE. The scopolamine-reversal paradigm in rats and monkeys: the importance of computer-assisted operant-conditioning memory tasks for screening drug candidates. Psychopharmacology. 2008;199:481–494. doi: 10.1007/s00213-007-0887-8. [DOI] [PubMed] [Google Scholar]
- 61.Higgins GA, Enderlin M, Fimbel R, Haman M, Grottick AJ, Soriano M, Richards JG, Kemp JA, Gill R. Donepezil reverses a mnemonic deficit produced by scopolamine but not by perforant path lesion or transient cerebral ischaemia. Eur J Neurosci. 2002;15:1827–1840. doi: 10.1046/j.1460-9568.2002.02018.x. [DOI] [PubMed] [Google Scholar]
- 62.Ikari H, Spangler EL, Greig NH, Pei XF, Brossi A, Speer D, Patel N, Ingram DK. Maze learning in aged rats is enhanced by phenserine, a novel anticholinesterase. Neuroreport. 1995;6:481–484. doi: 10.1097/00001756-199502000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Ingram DK, Spangler EL, Iijima S, Ikari H, Kuo H, Greig NH, London ED. Rodent models of memory dysfunction in Alzheimer’s disease and normal aging: moving beyond the cholinergic hypothesis. Life Sci. 1994;55:2037–2049. doi: 10.1016/0024-3205(94)00384-x. [DOI] [PubMed] [Google Scholar]
- 64.Janas AM, Cunningham SC, Duffy KB, Devan BD, Greig NH, Holloway HW, Yu QS, Markowska AL, Ingram DK, Spangler EL. The cholinesterase inhibitor, phenserine, improves Morris water maze performance of scopolamine-treated rats. Life Sci. 2005;76:1073–1081. doi: 10.1016/j.lfs.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 65.Patel N, Spangler EL, Greig NH, Yu QS, Ingram DK, Meyer RC. Phenserine, a novel acetylcholinesterase inhibitor, attenuates impaired learning of rats in a 14-unit T-maze induced by blockade of the N-methyl-D-aspartate receptor. Neuroreport. 1998;9:171–176. doi: 10.1097/00001756-199801050-00035. [DOI] [PubMed] [Google Scholar]
- 66.Rupniak NM, Tye SJ, Field MJ. Enhanced performance of spatial and visual recognition memory tasks by the selective acetylcholinesterase inhibitor E2020 in rhesus monkeys. Psychopharmacology. 1997;131:406–410. doi: 10.1007/s002130050310. [DOI] [PubMed] [Google Scholar]
- 67.Araujo JA, Studzinski CM, Head E, Cotman CW, Milgram NW. Assessment of nutritional interventions for modification of age-associated cognitive decline using a canine model of human aging. Age. 2005;27:27–37. doi: 10.1007/s11357-005-4001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenberg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Ebert U, Kirch W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 70.Dawson GR, Iverson SD. The effect of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behav Brain Res. 1993;57:143–153. doi: 10.1016/0166-4328(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 71.Kirkby DL, Jones DN, Barnes JC, Higgins GA. Effects of anticholinesterase drugs tacrine and E2020, the 5-HT(3) antagonist ondansetron, and the H(3) antagonist thioperamide, in models of cognition and cholinergic function. Behav Pharmacol. 1996;7:513–525. [PubMed] [Google Scholar]
- 72.Poorheidari G, Stanhope KJ, Pratt JA. Effects of the potassium channel blockers, apamin and 4-aminopyridine, on scopolamine-induced deficits in the delayed matching to position task in rats: a comparison with the cholinesterase inhibitor E2020. Psychopharmacology. 1998;135:242–255. doi: 10.1007/s002130050506. [DOI] [PubMed] [Google Scholar]
- 73.Iijima S, Greig NH, Garofalo P, Spangler EL, Heller B, Brossi A, Ingram DK. Phenserine: a physostigmine derivative that is a long-acting inhibitor of cholinesterase and demonstrates a wide dose range for attenuating a scopolamine-induced learning impairment of rats in a 14-unit T-maze. Psychopharmacology. 1993;112:415–420. doi: 10.1007/BF02244888. [DOI] [PubMed] [Google Scholar]
- 74.Milgram NW, Siwak-Tapp CT, Araujo J, Head E. Neuroprotective effects of cognitive enrichment. Ageing Res Rev. 2006;5:354–369. doi: 10.1016/j.arr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Haroutunian V, Greig N, Pei XF, Utsuki T, Gluck R, Acevedo LD, Davis KL, Wallace WC. Pharmacological modulation of Alzheimer’s beta-amyloid precursor protein levels in the CSF of rats with forebrain cholinergic system lesions. Brain Res Mol Brain Res. 1997;46:161–168. doi: 10.1016/s0169-328x(96)00297-5. [DOI] [PubMed] [Google Scholar]
- 76.Lahiri DK, Farlow MR, Hintz N, Utsuki T, Greig NH. Cholinesterase inhibitors, beta-amyloid precursor protein and amyloid beta-peptides in Alzheimer’s disease. Acta Neurol Scand. 2000;(Suppl):176–177. doi: 10.1034/j.1600-0404.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 77.Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer’s disease drug posiphen [(+)-phenserine]. lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320:386–396. doi: 10.1124/jpet.106.112102. [DOI] [PubMed] [Google Scholar]
- 78.Shaw KT, Utsuki T, Rogers J, Yu QS, Sambamurti K, Brossi A, Ge YW, Lahiri DK, Greig NH. Phenserine regulates translation of beta-amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci U S A. 2001;98:7605–7610. doi: 10.1073/pnas.131152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venti A, Giordano T, Eder P, Bush AI, Lahiri DK, Greig NH, Rogers JT. The integrated role of desferrioxamine and phenserine targeted to an iron-responsive element in the APP-mRNA 5′-untranslated region. Ann N Y Acad Sci. 2004;1035:34–48. doi: 10.1196/annals.1332.003. [DOI] [PubMed] [Google Scholar]
- 80.Dodart JC, Mathis C, Ungerer A. The beta-amyloid precursor protein and its derivatives: from biology to learning and memory processes. Rev Neurosci. 2000;11:75–93. doi: 10.1515/revneuro.2000.11.2-3.75. [DOI] [PubMed] [Google Scholar]
- 81.Holscher C, Gengler S, Gault VA, Harriott P, Mallot HA. Soluble beta-amyloid [25–35]. reversibly impairs hippocampal synaptic plasticity and spatial learning. Eur J Pharmacol. 2007;561:85–90. doi: 10.1016/j.ejphar.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 82.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 83.Yamaguchi Y, Kawashima S. Effects of amyloid-beta-(25–35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur J Pharmacol. 2001;412:265–272. doi: 10.1016/s0014-2999(01)00730-0. [DOI] [PubMed] [Google Scholar]
- 84.Utsuki T, Yu QS, Davidson D, Chen D, Holloway HW, Brossi A, Sambamurti K, Lahiri DK, Greig NH, Giordano T. Identification of novel small molecule inhibitors of amyloid precursor protein synthesis as a route to lower Alzheimer’s disease amyloid-beta peptide. J Pharmacol Exp Ther. 2006;318:855–862. doi: 10.1124/jpet.106.103309. [DOI] [PubMed] [Google Scholar]
- 85.Becker RE, Greig NH. Why so few drugs for Alzheimer’s disease? Are methods failing drugs? Curr Alzheimer Res. 2010;7:642–651. doi: 10.2174/156720510793499075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buccafusco JJ. Estimation of working memory in macaques for studying drugs for the treatment of cognitive disorders. J Alzheimers Dis. 2008;15:709–720. doi: 10.3233/jad-2008-15414. [DOI] [PubMed] [Google Scholar]
- 87.Yuede CM, Dong H, Csernansky JG. Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol. 2007;18:347–363. doi: 10.1097/FBP.0b013e3282da278d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster RH, Plosker GL. Donepezil. Pharmacoeconomic implications of therapy. Pharmacoeconomics. 1999;16:99–114. doi: 10.2165/00019053-199916010-00009. [DOI] [PubMed] [Google Scholar]
- 89.Weinstock M. Possible role of the cholinergic system and disease models. J Neural Transm. 1997;(Suppl):49–102. doi: 10.1007/978-3-7091-6844-8_10. [DOI] [PubMed] [Google Scholar]
- 90.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- 91.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics. 2008;5:433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pakaski M, Kalman J. Interactions between the amyloid and cholinergic mechanisms in Alzheimer’s disease. Neurochem Int. 2008;53:103–111. doi: 10.1016/j.neuint.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 93.Pakaski M, Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. Curr Drug Targets CNS Neurol Disord. 2003;2:163–171. doi: 10.2174/1568007033482869. [DOI] [PubMed] [Google Scholar]
- 94.Landsberg G, Araujo JA. Behavior problems in geriatric pets. Vet Clin North Am Small Anim Prac. 2005;35:675–698. doi: 10.1016/j.cvsm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Landsberg G. Therapeutic options for cognitive decline in senior pets. J Am Anim Hosp Assoc. 2006;42:407–413. doi: 10.5326/0420407. [DOI] [PubMed] [Google Scholar]
- 96.Matsui K, Taniguchi S, Yoshimura T. Correlation of the intrinsic clearance of donepezil (Aricept) between in vivo and in vitro studies in rat, dog and human. Xenobiotica. 1999;29:1059–1072. doi: 10.1080/004982599237958. [DOI] [PubMed] [Google Scholar]


