Abstract
Small-diameter vascular grafts developed from vascular extracellular matrix (ECM) can potentially be used for bypass surgeries and other vascular reconstruction and repair procedures. The addition of heparin to the ECM improves graft hemocompatibility but often involves chemical crosslinking, which increases ECM mechanical stiffness compared to native arteries. Herein, we demonstrate the importance of maintaining ECM mechanocompatibility, and describe a mechanocompatible strategy to immobilize heparin onto the ECM via a biodegradable elastomer. Specifically, poly (1,8-octamethylene citrate)-co-cysteine (POC-Cys) was hybridized to the ECM, forming a polymer-ECM composite that allows for heparin immobilization via maleimide-thiol “click” chemistry. Heparinized composites reduced platelet adhesion by >60% in vitro, without altering the elastic modulus of the ECM. In a rat abdominal aortic interposition model, intimal hyperplasia in heparinized mechanocompatible grafts was 65% lower when compared to ECM-only control grafts at 4 weeks. In contrast, grafts that were heparinized with carbodiimide chemistry exhibited increased intimal hyperplasia (4.2 fold) and increased macrophage infiltration (3.5 fold) compared to ECM-only control grafts. All grafts showed similar, partial endothelial cell coverage and little to no ECM remodeling. Overall, we describe a mechanocompatible strategy to improve ECM thromboresistance and highlight the importance of ECM mechanical properties for proper in vivo graft performance.
Keywords: Vascular graft; Extracellular matrix (ECM); Composite; Heparin; Poly (1,8-octamethylene citrate) (POC)
1. Introduction
Small-diameter prosthetic vascular grafts for the treatment of peripheral arterial disease (PAD) have faced great challenges in the clinical setting due to poor long-term patency.[1] Alternatively, biological grafts using acelluar allogenic[2] or xenogenic[3] vascular extracellular matrices (ECM) exhibit excellent mechanical strength and stability in vitro, and maintain the capability to remodel in vivo.[4] However, absence of a functional, intact endothelium leading to exposure of the vascular ECM to flowing blood may precipitate thrombosis and intimal hyperplasia, which are major challenges for ECM-based small-diameter vascular grafts.[2] Heparin has been added to the ECM by our group[5] and others[6] to provide thromboresistant activity via surface immobilization techniques. However, immobilization of heparin onto the ECM often involves chemical crosslinking with carbodiimide chemistry,[7] which increases the mechanical stiffness and changes the ultrastructure of the ECM, leading to loss of biological functions of ECM proteins and reduced scaffold biocompatibility.[8] Therefore, strategies that aim to improve graft thromboresistance should not alter the native ECM mechanical properties in order to maintain proper graft function.
Poly(1,8-octamethylene citrate) (POC) is a biodegradable elastomer with hemocompatible[9] and antioxidant[10] properties. POC-coated vascular grafts decrease formation of intracellular reactive oxygen species (ROS), attenuating oxidative stress within engineered endothelium.[11] We have previously described the use of POC to successfully heparinize ePTFE vascular grafts to provide additional thromboresistant properties.[12] We have also shown that POC may be used to create a heparinized polymer-ECM composite by hybridizing POC onto arterial ECM followed by addition of heparin via carbodiimide chemistry.[5] The heparinized polymer-ECM composite led to improved thromboresistance (decreased platelet adhesion and clot formation) in vitro relative to ECM control. However, carbodiimide chemistry—as with many other heparin immobilization strategies[6]—crosslinks carboxyl and amine groups within the ECM, potentially affecting the mechanical properties of the resulting vascular graft. Therefore, the objective of this study is to develop a strategy to improve ECM hemocompatiblity without affecting its mechanical properties, and evaluate the performance of the resulting heparinized grafts in vivo. We report the use of thiolated POC hybridized to the ECM to fabricate mechanocompatible polymer-ECM vascular grafts that are amenable to heparin immobilization via maleimide-thiol “click” chemistry. We also report the in vivo responses of heparinized polymer-ECM composites, specifically intimal hyperplasia formation, inflammatory response and endothelial cell coverage.
2. Results and Discussion
2.1 Heparin Functionalization and Polymer-ECM Composite Preparation
Decellularized rat descending aortas were used as vascular ECM scaffolds, and heparin was immobilized onto the ECM using the following strategy (Figure 1): (A) conjugating (N-[β-maleimidopropionic acid] hydrazide) (BMPH) to heparin to add maleimide groups onto heparin; (B) hybridizing the decellularized aorta ECM with poly(1,8-octamethylene)-co-cysteine (POC-Cys) to add thiol groups to the ECM; (C) reacting heparin-BMPH with polymer-ECM composites via “click” chemistry to ultimately link heparin to the ECM. We choose to use ECM from decellularized arteries as the foundation for the vascular graft, instead of synthetic materials, because of ECM’s natural three-dimensional ultrastructure and diverse composition of structural and functional proteins, which are essential for cell adhesion, migration, proliferation, and differentiation.[13] [14] [15]
Figure 1.
Schematic representation of the process used to fabricate a heparinized mechanocompatible polymer-ECM composite. (A) Heparin was conjugated with BMPH (containing maleimide group) using carbodiimide chemistry. (B) POC-Cys prepolymer was synthesized with 1, 8 octanediol, citric acid and cysteine (1:1:0.2 molar ratio) and hybridized onto ECM to form polymer-ECM composites with additional thiol groups. (C) Maleimide-thiol “click” chemistry between heparin-BMPH and polymer-ECM composite to immobilize heparin onto ECM.
2.2.1. Addition of maleimide containing side chains to heparin does not impair heparin bioactivity
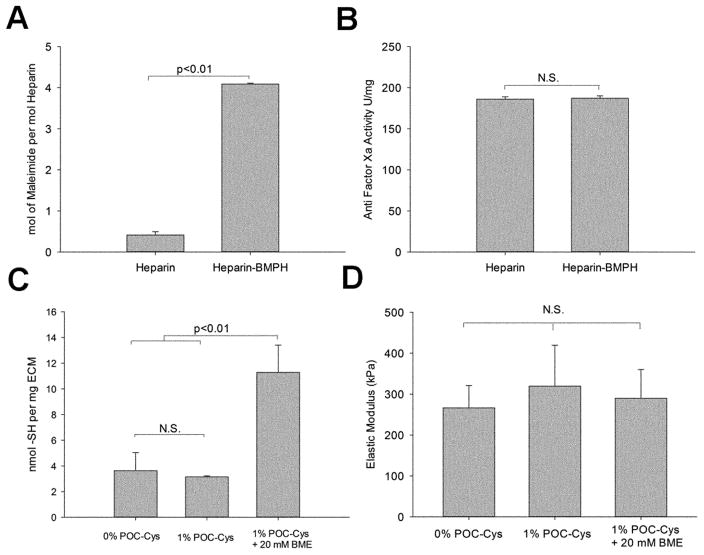
Heparin was modified with maleimide functional groups to permit immobilization onto ECM that had been thiolated. The maleimide-containing coupling reagent BMPH was conjugated to heparin at a 10: 1 molar ratio during reaction, resulting in a yield of 4.08 ± 0.02 mol of maleimide per mol of heparin (Figure 2. A). Importantly, modification of heparin with BMPH did not alter its anticoagulant ability by assessing anti-Factor Xa activity before and after modification (186.1 ± 1.9 U mg−1 heparin vs. 187.3 ± 3.0 U mg−1 heparin-BMPH, p=0.639, Figure 2.B). Factor Xa plays a central role in the coagulation cascade, and heparin binds to antithrombin to inactivate factor Xa, providing anticoagulant activity.[16]
Figure 2.
Characterization of heparin-BMPH (A, B) and polymer-ECM composites (C, D). (A) Quantification of maleimide groups shows a significantly higher degree of maleimides in heparin-BMPH conjugate compared to heparin control. (B) Factor Xa assay results show that the anticoagulant activity of heparin is not altered by the conjugation of BMPH to its carboxyl groups. (C) Quantification of thiol groups in the polymer-ECM composite confirms an increase in free thiols after treatment with the reducing agent BME. (D) Tensile test shows no significant difference in ECM elastic modulus with or without POC-Cys or BME. (n=3 for each study)
2.1.2. Vascular ECM can be thiolated via POC-Cys without changing elastic moduli
We hypothesized that the versatility of polydiol-citrate chemistry can modify the chemical and biological properties of the ECM without altering the elastic modulus. For example, cysteine- and serine-modified POC copolymers have tunable fluorescence emission [17], allowing fluorescence imaging of polymer-ECM scaffolds.[5] Moreover, POC bearing specific amino acids adds additional functional groups to the ECM, such as thiol groups in cysteine and primary amine groups in lysine. In this study, POC-Cys prepolymer was hybridized onto ECM to form a polymer-ECM composite in order to provide additional thiol groups throughout the ECM to enable the “click” reaction with maleimide groups of heparin-BMPH. Post-polymerization of the POC-Cys prepolymer onto the ECM (hybridization process) involves both covalent and non-covalent interactions.[5, 18] The polymer-ECM composite was subsequently treated with 2-β Mercaptoethanol (BME) for one hour to break disulfide bonds and expose thiol groups to allow subsequent reaction with heparin-BMPH to proceed. BME treatment resulted in a significant increase in thiol concentration (11.28 ± 2.12 nmol mg−1 after BME treatment vs. 3.15 ± 0.07 nmol mg−1 before BME treatment, p<0.01) (Figure 2.C).
Hybridization of the ECM with 1% POC-Cys and subsequent BME treatment did not significantly change ECM mechanical stiffness (elastic modulus for ECM with 0% POC-Cys: 266.5 ± 54.5 kPa; 1% POC-Cys: 319.7 ± 99.7 kPa; and 1% POC-Cys followed by 20 mM BME: 290.0 ± 70.1 kPa, p=0.824) (Figure 2.D). Therefore, polymer-ECM composites provide additional thiol groups for further modification with maleimide-thiol “click” chemistry for subsequent addition of heparin without significantly altering the ECM’s elastic modulus.
2.2. In vitro Characterization of Heparinized Polymer-ECM Composites
2.2.1. Heparinized polymer-ECM composites are thromboresistant
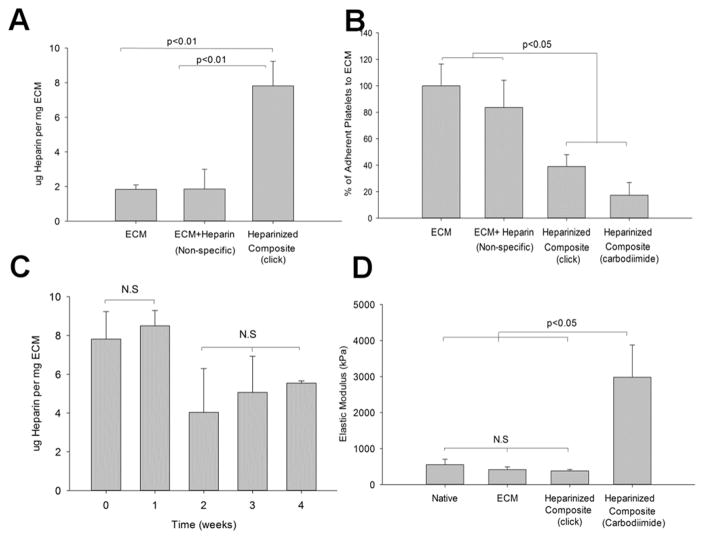
Heparinized polymer-ECM composites have a significantly higher degree of glycosaminoglycans (GAG) (7.82 ± 1.41 μg mg−1 ECM) compared to ECM control (1.83 ± 0.25 μg mg−1 ECM, p<0.01) or non-specific binding between heparin and ECM (1.86 ± 1.14 μg mg−1 ECM) (Figure 3. A), indicating heparin was incorporated in modified grafts. Platelet activation and adhesion to the ECM is an early initiator of thrombosis and plaque formation.[19] Heparinized polymer-ECM composites significantly reduced platelet adhesion by at least 60% compared to polymer-ECM treated with PBS alone or heparin without the BMPH linker, confirming improved thromboresistance due to the specific addition of heparin (Figure 3. B). This finding is comparable to what we found in our previous study, where carbodiimide chemistry was used to immobilize heparin onto polymer-ECM composites and resulted in a 85% decrease in platelet binding (Figure 3. B) with stable heparin retention for at least 4 weeks.[5]
Figure 3.
Characterization of mechanocompatible polymer-ECM composites after “click” chemistry. (A) Heparin quantification shows significantly higher amount of heparin present in the heparinized polymer-ECM composites relative to the ECM control and the ECM that was exposed to heparin through non-specific binding interactions. (B) Platelet adhesion is significantly reduced on heparinized polymer-ECM composites with both “click” chemistry and carbodiimide chemistry when compared to the ECM only and non-specific binding between ECM and heparin. (C) The immobilized heparin is stable at 1 week and over half of the heparin could be detected on the ECM at 4 weeks of incubation in PBS. (D) The elastic modulus of the heparinized polymer-ECM composites prepared using “click” chemistry is comparable to that of the native artery and artery ECM, as opposed to heparinized polymer-ECM composites prepared via carbodiimide chemistry. (n=3 for each study)
The amount of heparin immobilized using “click” chemistry was stable within the first week with no significant change (7.82 ± 1.41 μg mg−1 ECM at week 0 vs. 8.50 ± 0.79 μg mg−1 ECM at week 1), but decreased ~50% at week 2, remaining stable thereafter until the study was terminated at 4 weeks (5.54 ± 1.28 μg mg−1 ECM at week 4). Nonetheless, the remaining heparin at 4 weeks was higher than the amount measured in the ECM control (1.83 ± 0.25 μg mg−1 ECM, p<0.05, Figure 3.C). POC-Cys polymer is biodegradable by hydrolysis, which may explain the 50% loss of heparin during the first 2 weeks. An early thromboresistance to the ECM during the first weeks is more important as it prevents thrombosis early on until endothelial cells are recruited and cover the lumen of the ECM in vivo.
2.2.2. Polymer-ECM composites heparinized via “click” chemistry are mechanocompatible
No significant difference in elastic modulus was found before and after decellularization (416.5 ± 72.6 kPa before decellularization vs. 379.5 ± 38.8 kPa after decellularization, p=0.2390). Despite similar thromboresistant properties, a major difference in the mechanical properties was found between the two heparinized polymer-ECM composites (“click” vs. carbodiimide). Unlike immobilization of heparin via carbodiimide chemistry, there was no significant change in elastic modulus of the “click” chemistry modified polymer-ECM composites, compared to the native artery or ECM control (Figure 3.D), rendering it mechanocompatible. Heparinized polymer-ECM composites developed using carbodiimide chemistry led to ECM crosslinking and resulted in a significant increase (p<0.05) in ECM stiffness (6-fold increase in elastic modulus) (Figure 3.D). Representative stress-strain curves for two heparinized polymer-ECM composites (“click” vs carbodiimide) are shown in Supplementary Figure 1.
Crosslinking decellularized scaffolds is a common practice to enhance mechanical strength and stabilize against chemical and enzymatic degradation in vivo.[20] Various crosslinking mechanisms, including EDC/NHS,[21] glutaraldehyde,[22] and genipin[23] have been explored in an attempt to find the optimal chemical stabilizer for ECM based materials while maintaining normal compliance. While it is important to maintain ECM mechanical integrity, the degree of crosslinking is often difficult to control and often results in increased stiffness, cytotoxicity and calcification.[20] Moreover, degradation of the ECM scaffold is an essential component to graft remodeling.[24] Therefore, if the mechanical properties and structural integrity of native tissues are well maintained after decellularization, it may not be necessary to implement exogenous crosslinking methods. In our study, the decellularization process using Triton X-100 and sodium dodecyl sulfate followed with DNAse I treatment largely maintained the ECM integrity without significantly weakening material mechanical properties when compared to original native arteries (Figure 3.D).
2.3 In vivo characterization of heparinized polymer-ECM composites
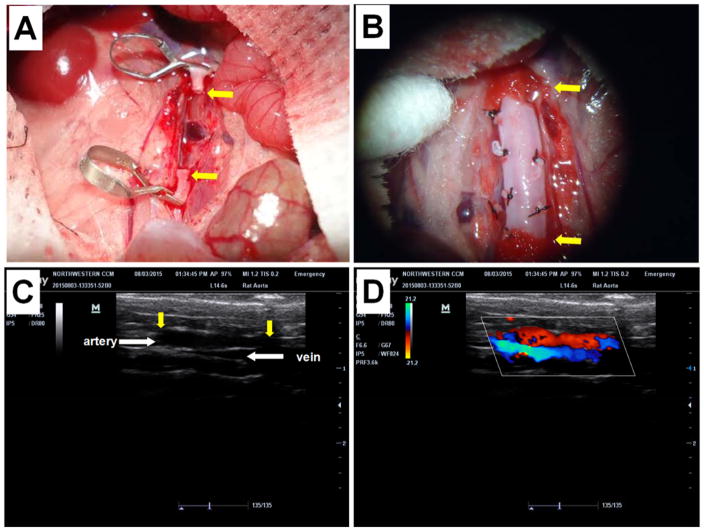
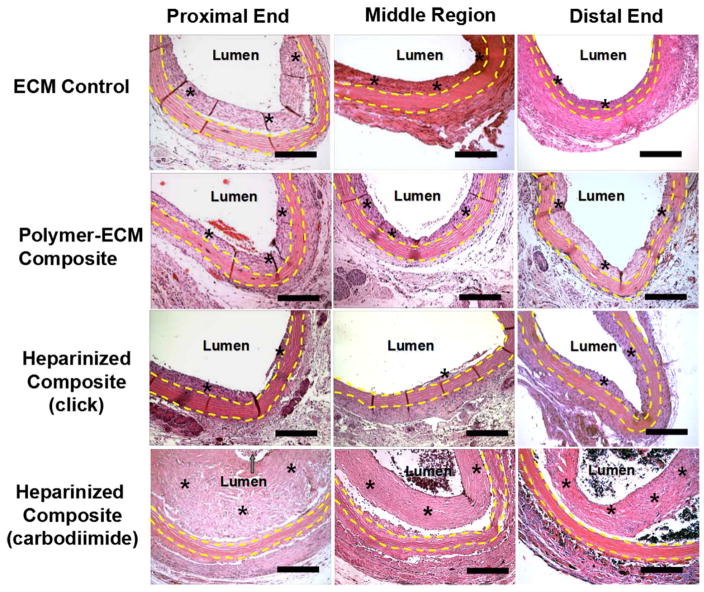
A rat abdominal aorta interposition model [25] was used to evaluate patency and biocompatibility of ECM-based vascular grafts in vivo (Figure 4, A, B). A total of 16 animals, equally divided into 4 groups, received the following vascular grafts (n=4): (1) decellularized donor rat aorta ECM without any modification (ECM Control); (2) POC-modified ECM composites without heparin (Polymer-ECM Composite); (3) heparin-modified polymer-ECM composite grafts prepared via “click” chemistry as described in this study (Heparinized Composite [click]); and (4) heparin-modified polymer-ECM composites prepared via carbodiimide chemistry (Heparinized Composite [carbodiimide]) as previously described by us.[5] All grafts remained patent during the 4-week time frame, as determined by in vivo Doppler ultrasound imaging (Figure 4, C, and D) and histologic assessment when explanted at 4 weeks after surgery. Histology was compared segmentally at cross-sections along the length (proximal, middle, and distal) of the graft (Figure 5 and Figure 6).
Figure 4.
Rat abdominal aorta interposition model used to evaluate rat ECM based vascular grafts. (A) Prior to implantation, the abdominal aorta was clamped in both proximal and distal regions using micro-vessel clips and a segment (1 cm) of native artery was excised between the clips. (B) A segment (1 cm) of ECM graft was connected to the native aorta using an end-to-end anastomosis at each end. Branches of aorta grafts were tied with silk sutures to prevent bleeding. (C) Ultrasound imaging (B mode) was used to monitor the morphology of the vascular graft. Yellow arrows indicate the anastomoses between native aorta and the ECM vascular graft. (D) Doppler ultrasound was used to monitor patency of the graft (aortic position, red) and the adjacent vena cava (blue).
Figure 5.
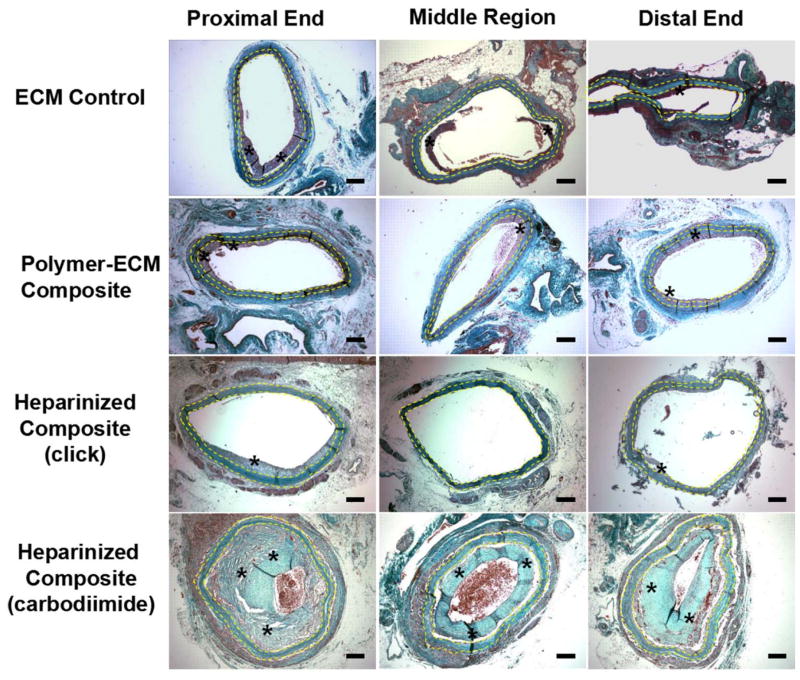
H&E stain of ECM control, polymer-ECM composite, heparinized polymer-ECM composite prepared via “click” chemistry, and heparinized polymer-ECM composite prepared via carbodiimide chemistry at the proximal end, middle region and distal end of the graft. All vascular grafts were recovered after implantation as an aortic interposition graft at 4 weeks after surgery. All microscopic images were taken with a 10x objective. Grafts are outlined between yellow dashed lines, while * indicates the presence of intimal hyperplasia. Scale bar = 200 μm.
Figure 6.
Masson’s Trichrome stain of ECM control, polymer-ECM composite, heparinized polymer-ECM composite prepared via “click” chemistry, and heparinized polymer-ECM composite prepared via carbodiimide chemistry at the proximal end, middle region and distal end of the graft. All vascular grafts were recovered after implantation as an aortic interposition graft at 4 weeks after surgery and all microscopic images were taken with a 4x objective. Grafts are outlined between yellow dashed lines, while * indicates the presence of intimal hyperplasia. Scale bar = 200 μm.
2.3.1. Mechanocompatible vascular grafts exhibit reduced intimal hyperplasia
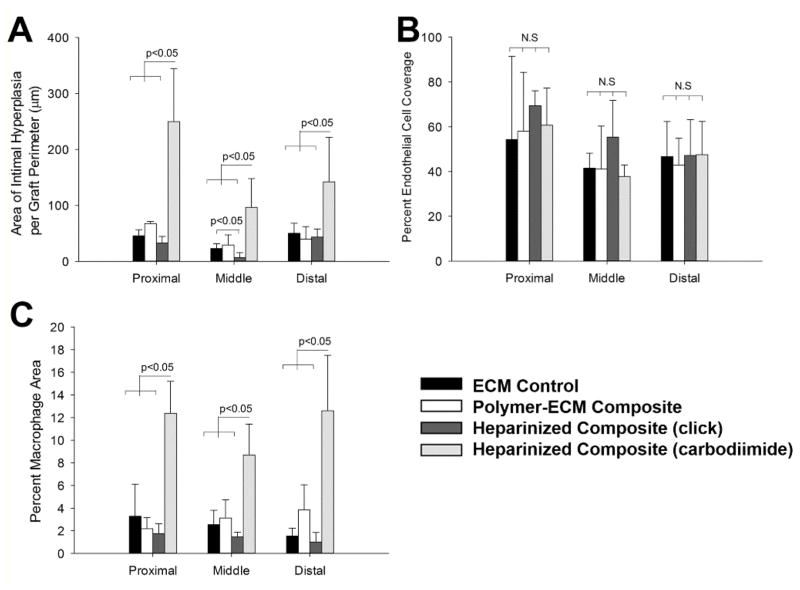
Formation of intimal hyperplasia within ECM-based vascular grafts was confirmed by histology (Figure 5 and Figure 6) and immunofluorescence staining for α-SMA (VSMC marker, Figure 7). A moderate amount of intimal hyperplasia was formed on the luminal side of ECM control (23.1 ± 8.3 μm midgraft) and polymer-ECM composite grafts (29.1 ± 18.0 μm midgraft), with no significant difference (p=0.614) (Figure 8.A). Heparinized polymer-ECM composites (click) resulted in the least amount of intimal hyperplasia (6.7 ± 6.9 μm midgraft, p<0.05 compared to ECM control), while heparinized polymer-ECM composites (carbodiimide) resulted in the highest amount of intimal hyperplasia (96.5 ± 51.4 μm midgraft, p<0.05 compared to ECM control).
Figure 7.
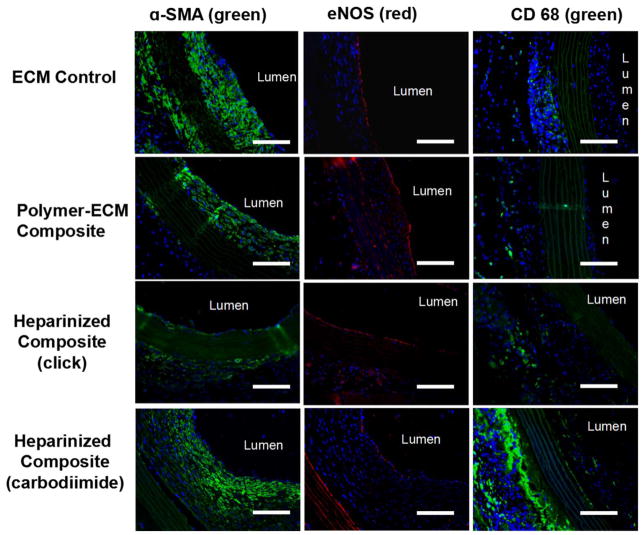
Immunofluorescence imaging for α-SMA (green), eNOS (red) and CD 68 (green) within ECM control, polymer-ECM composite, heparinized polymer-ECM composite prepared via “click” chemistry, and heparinized polymer-ECM composite prepared via carbodiimide chemistry at midgraft. All vascular grafts were recovered after implantation as an aortic interposition graft at 4 weeks after surgery. Scale bar = 100 μm.
Figure 8.
Quantitative analysis of intimal hyperplasia (A), endothelial cell coverage (B), and macrophage density (C) for the ECM control, polymer-ECM composite, heparinized polymer-ECM composite prepared via “click” chemistry, and heparinized polymer-ECM composite prepared via carbodiimide chemistry at proximal end, middle region and distal end of the graft. (n=4 for each study).
Intimal hyperplasia is the over-proliferation of vascular smooth muscle cells (VSMCs), which is a common cause for vascular graft failure.[26] Mismatch in compliance and elastic properties between vascular grafts and native vessels is considered an important, if not primary contributing factor to intimal hyperplasia formation.[27] Compliance mismatch between vascular grafts and native vessels leads to changes in hemodynamic flow, causing turbulence that injures endothelial cells,[28] and stimulates VSMCs to transform from a fully differentiated, quiescence phenotype to highly synthetic and migrating phenotypes.[29] Therefore, the ideal vascular graft should mimic the mechanical properties of the target native artery as closely as possible to minimize intimal hyperplasia formation and maximize graft patency, i.e. the concept of mechanocompatibility.
We found that heparinized composite (carbodiimide) led to severe intimal hyperplasia within 4 weeks, which is believed to be largely related to the increase in stiffness caused by tissue crosslinking, leading to compliance mismatch exhibited between the grafts and native vessels. On the other hand, mechanocompatible heparinized composites (click) exhibited decreased intimal hyperplasia formation, likely due to the inhibition of VSMC proliferation[30] and migration[31] by heparin while maintaining ECM mechanical properties compared to native arteries. The dramatic difference between all ECM graft groups with or without crosslinking provides strong evidence for the importance of maintaining mechanocompatibility of ECM-based vascular grafts.
In this study we use non-cellularized grafts; however, there are a number of possible routes for VSMCs to enter the graft and cause intimal hyperplasia: (1) migration of VSMCs through the graft wall, which is unlikely in our case, given few to no cells were present within the ECM wall; (2) migration of VSMCs from the media of the native artery at the anastomosis to the ECM graft lumen side, which is highly likely since a higher amount of intimal hyperplasia was observed in the proximal and distal ends compared to the middle region in all groups (Figure 5 and 6); (3) differentiation of circulating progenitor and mesenchymal stem cells (MSCs) into VSMCs at the blood/ECM interface,[32] which is also a possible mechanism that accounts for this observation.
2.3.2. Polymer-ECM composites support endothelialization
Despite decellularization and the lack of cellular reconstitution of the grafts prior to implantation, we found functional endothelial cells (ECs) along the luminal side of all grafts by staining for endothelial nitric oxide synthase (eNOS) (Figure 7). We chose to stain for eNOS because it is a specific EC marker that together with the localization of the staining is strongly indicative of an endothelium. Furthermore, this enzyme is responsible for nitric oxide (NO) production, which is one of the endothelial cell functions that contribute to its antithrombotic properties.[33] Specifically, we found partial EC coverage for all grafts with no significant difference among groups (Figure 8. B). Since no ECs were pre-seeded within ECM-based vascular grafts, all ECs present on the lumen were recruited from the recipient animal during the 4 weeks of implantation, either through migration of ECs from the native artery, or via deposition and differentiation of circulating endothelial progenitor cells (EPCs) from blood.
An intact, functional EC lining plays a pivotal role in maintaining vascular function, including selective barrier and filtration, thromboresistance, and inflammation mediation.[11] Vascular grafts that were pre-seeded with ECs prior to implantation have shown improved graft patency[34] and decreased intimal hyperplasia formation.[35] Therefore, it is important to have a functional EC coverage for all vascular grafts to support their long-term success. However, for patients with cardiovascular diseases, it is difficult to obtain sufficient number of autologous, functional ECs for complete seeding onto vascular grafts prior to implantation. Although new research to obtain autologous ECs from novel, renewable sources, such as induced pluripotent stem cells (iPSC) is promising, efficacy and potential clinical risks remain unclear.[36] Alternatively, ECs can be recruited to vascular grafts during implantation in a process called in situ endothelialization that is aided by chemical and physical modification of the graft surfaces, such as growth factor incorporation and topography.[37] In our study, the mechanocompatible heparinized composites (click) did not inhibit in situ endothelialization during the time frame of our study (4 weeks) compared to other groups (ECM control, polymer-ECM composites, and heparinized composites [carbodiimide]), resulting in partial EC coverage. It is likely that EC coverage will improve over a longer implantation time as the EC layer reaches confluence along the graft.
2.3.3 Inflammation is reduced in mechanocompatible vascular grafts
Evaluation of macrophages as a surrogate marker for the inflammatory response to the grafts was assessed via immunofluorescence staining for CD68, a macrophage marker (Figure 7). CD68-positive cells were primarily found on the outer layer of each graft, with a higher amount of CD68-positive cells found in heparinized polymer-ECM composites (carbodiimide) than any other group, including the mechanocompatible heparinized polymer-ECM composite (click) (carbodiimide 8.7 ± 2.7 % vs. click 1.5 ± 0.4 % CD68+ at midgraft, p<0.01) (Figure 8. C). In addition, a small number of CD68-positive cells were also observed within the thick layer of intimal hyperplasia of heparinized polymer-ECM composites (carbodiimide). These results suggest a severe inflammatory response to the grafts crosslinked with the carbodiimide chemistry. Inflammation has often been associated with an increase in tissue stiffness in numerous pathological conditions, such as cancer,[38] liver fibrosis,[39] atherosclerosis,[40] and osteoarthritis,[41] though the detailed mechanisms are still not fully understood. Therefore, mechanocompatibility of ECM-based vascular grafts and other biomaterial scaffolds for vascular tissue engineering must be taken into account as important design criteria, eliminating unnecessary crosslinking that may give rise to undesirable inflammation.
Calcification within the vascular graft wall, possibly as a result of inflammation,[42] was observed in 1 out of 4 ECM control grafts (Supplemental Figure 2. A), and 2 out of 4 heparinized polymer-ECM composite (carbodiimide) grafts (Supplemental Figure 2.D). Calcification was not observed within the vascular wall of polymer-ECM composite or heparinized polymer-ECM composite (click) grafts, but was found at the anastomosis adjacent to sutures in 1 out 4 animals for each group (Supplemental Figure 1. B and C), possibly due to increased inflammatory responses at the anastomoses. Calcification is a known problem for many ECM-based cardiovascular prostheses, including acellular heart valves[43] and vascular grafts[44]. It is believed to be caused by osteoblast-like cells, derived from stem cells (circulating or within the vessel wall) or transdifferentiation of existing cells, such as VSMCs.[45] However, the detailed mechanisms triggering the onset of cell differentiation into osteogenic phenotype is still unclear. A number of molecules are considered inhibitors for vascular calcification, including inorganic pyrophosphate,[46] Matrix Gla-protein (MGP)[47] and fetuin [48]. If the problem of vascular calcification becomes more severe in the long term, calcification inhibitors could potentially be incorporated into the polymer-ECM composites via surface modification and/or sustained release to prevent the process, until the grafts are completely remodeled into functional vascular tissue.
2.4 Prospects
Minimal cell invasion within the ECM wall was noted in all groups at 4 weeks, suggesting little ECM remodeling or degradation within the 4-week time frame of our study. A study of long-term (3 month and longer) examination for ECM based vascular grafts will be performed next to further evaluate the long-term in vivo responses to ECM-based vascular grafts, including remodeling.
The strategy of using maleimide-thiol “click” chemistry to heparinize polymer-ECM composites may provide a structure to link other bioactive molecules onto ECM based scaffolds without crosslinking for various tissue engineering applications. For example, growth factors or cytokines such as vascular endothelial growth factor (VEGF) could be modified with BMPH and linked to POC-Cys coated arterial ECM to improve EC recruitment and enhance endothelialization of vascular grafts. To prevent calcification of acellular vascular grafts and heart valves, calcification inhibitors such as MGP could also be immobilized onto the polymer-ECM composite scaffolds using “click” chemistry, without chemical crosslinking which may intensify calcification. This strategy could also be applied to regenerative medicine, where the fate of stem cells seeded onto tissue-specific ECM scaffold could be controlled by spatially presenting various signaling proteins on tissue-specific ECM. A similar strategy may be applied to whole organ engineering to modify the ECM of intraparenchymal vessels in organs such as the heart, kidney and liver, which may also benefit from this strategy to enhance thromboresistance and recellularization without altering mechanical properties.
3. Conclusion
In this study, we describe a method to immobilize heparin onto the ECM via polymer-ECM hybridization followed by maleimide-thiol “click” chemistry. This novel strategy allows the immobilization of active heparin onto the ECM without crosslinking it, therefore reducing platelets adhesion in vitro while maintaining the ECM’s mechanical properties. Evaluated in a rodent aorta interposition model, mechanocompatible heparinized polymer-ECM composite grafts decreased intimal hyperplasia formation in the mid-graft and did not increase inflammation, which is in direct contrast to carbodiimide crosslinked polymer-ECM grafts. The methods used to fabricate the mechanocompatible heparinized polymer-ECM composites described herein offer a novel approach to create scaffolds for tissue engineering where immobilization of bioactive molecules is desirable.
4. Experimental Section
4.1. Animals
Male Sprague Dawley rats (200–250 g) were used as donors and recipients for abdominal aorta recovery and implantation. Animal care was performed in accordance with the NIH Guide for Care and Use of Laboratory Animals, and all experiments using animals were approved by the Animal Care and Use Committee of Northwestern University.
4.2. Heparinization of polymer-ECM composites
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise.
4.2.1 Heparin functionalization with maleimide group (Figure 1. A)
Heparin sodium salt (200 U mg−1, Celsus Laboratories, Cincinnati, OH) was dissolved in MES buffer (pH 6.5) at 1 mM, with 60 mM N-hydroxysuccinimide (NHS) and 120 mM 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). N-β-maleimidopropionic acid hydrazide (BMPH, 10 mM) was reacted with heparin for 2 hours at room temperature to allow conjugation of heparin (-COOH) with BMPH (-NH2). The product was dialyzed against Milli-Q water with MWCO 3500 membrane and lyophilized dry. The amount of maleimide group per mol of heparin was quantified by Ellman’s reagent assay[49] after reacting with excessive L-cysteine solution.
4.2.2 Polymer-ECM composite preparation (Figure 1. B)
Decellularization of rat descending aortas was performed as described before.[5] Briefly, rat aortas were incubated at room temperature with 1% Triton X for 48 h followed by 1.5% SDS for 48 h. Afterwards, the ECM was incubated with 100 U/ml DNase I solution at 37 °C for 4 h to remove residual dna, which resulted in an over 95% decrease in overall DNA content compared to native aortas before decellularization. POC-Cys prepolymer (molar ratio of 1,8-octanediol, citric acid and cysteine 1:1:0.2) was synthesized as described previously.[17] The POC-Cys pre-polymer was diluted with absolute ethanol to 1% (w./w.). Decellularized aorta ECM was firstly dehydrated with ethanol and then incubated in 1% pre-polymer solution for 30 min with continuous stirring. The pre-polymer infused ECM was then post-polymerized at 45°C for 4 days, then incubated with PBS at 37°C for 3 days to remove unbound prepolymer. Prior to “click” chemistry, the polymer-ECM composites were treated with 20 mM 2-Mercaptoethanol (BME) for 1 hour at room temperature to free thiol groups provided by POC-Cys polymer, after which the samples were rinsed extensively with PBS to remove BME. The amount of free thiol groups present on polymer-ECM composites were quantified via Ellman’s reagent assay and normalized to tissue weight.
4.2.3 Maleimide-thiol “click” chemistry (Figure 1. C)
The polymer-ECM composites were incubated in heparin-BMPH solution (1 mg ml−1 in PBS) overnight to allow diffusion of heparin-BMPH throughout the entire polymer-ECM scaffold. Three washes with PBS were performed to remove unbound heparin.
4.3. Evaluation of heparinized polymer-ECM composites in vitro
4.3.1 Heparin quantification
A dimethylmethylene blue (DMMB) based glycosaminoglycan (GAG) assay [50] was used to quantify heparin concentration on ECM. Briefly, ECM samples were weighed first and then treated with Proteinase K (15 U ml−1) at 60 °C overnight to digest the entire ECM scaffold. The digested samples were added to DMMB solution for colorimetric measurement, in comparison with heparin serial dilutions as standard curve. ECM samples without heparin modification served as controls to provide background GAG concentration value.
4.3.2 Platelet adhesion
A platelet adhesion assay previously described[5] was performed on ECM samples to evaluate their anti-thrombogenic properties after heparin modification. Briefly, rat blood was collected in ACD tubes during organ procumbent and the platelet-rich plasma separated by centrifugation and diluted to 2–5 × 108 platelet/ml. Various ECM grafts were incubated in platelet suspension at 37 °C for 1 h, rinsed with warm PBS, and lysed with 2% Triton-X. Release of LDH was assessed in the resulting solution with an LDH assay as per the manufacturer’s protocol (Roche Molecular Diagnostics, Pleasanton, CA).
4.3.3 Mechanical tests
A tensile test was performed for native rat aorta (before decellularization), rat aorta ECM, and heparinized rat aorta ECM using either “click” chemistry or carbodiimide chemistry adhering to ASTM guidelines, and their elastic moduli calculated as described previously.[5]
4.4 Evaluation of heparinized polymer-ECM composites in vivo
4.4.1 Surgery
A rat abdominal aorta interposition model was used to evaluate ECM-based vascular grafts. Briefly, Male Sprague Dawley rats (200–250g) were anesthetized by isoflurane inhalation (1–5%) using VetEquip inhalation anesthesia system. The abdomen of the anesthetized rat was entered through a midline incision. The abdominal aorta was exposed and clamped in both proximal and distal regions using micro-vessel clips and then is excised between the clamps (Figure 4. A). A segment (1cm in length) of ECM-based graft (ECM control, polymer-ECM composite, heparinized composite via “click” chemistry, and heparinized composite via carbodiimide crosslinking) was connected to the abdominal aorta using an end-to-end anastomosis technique with interrupted stitches at each end. Branches of aortic grafts were tied with silk sutures to prevent bleeding (Figure 4. B). Following reperfusion of blood flow through the graft, the incision was closed in layers. No systemic anticoagulation therapy was used either during or post operation. Ultrasound imaging with (Figure 4. C) and without (Figure 4. D) Doppler was performed to monitor graft patency weekly, using an M7/M7T Diagnostic Ultrasound System (Mindray Bio-Medical Electronics, Shenzhen, China) with an L14-6S probe.
4.4.2. Histological and Immunofluorescence Staining
Four weeks after the implantation surgeries, the animals were sacrificed and the grafts excised including both anastomoses and fixed in 4% formaldehyde solution. Each graft (1cm long) was divided into five regions from the proximal to the distal end, including the two anastomoses sites, with 2 mm per segment. Each segment was embedded in paraffin and sectioned with a microtome with 5 μm thickness. The sections were stained with H&E, Masson’s trichrome and von Kossa staining after deparaffinization and rehydration following standard protocols. Bright field microscopy was then used to image the sections with 4x and 10x objective (Nikon TE2000U, Melville, NY). Immunofluorescence staining for eNOS (endothelial cell marker), α-SMA (smooth muscle cell marker) and CD68 (macrophage marker) was performed. Primary antibody (eNOS, α-SMA or CD68, 1:200 dilution, Santa Cruz) was added to the sections and incubated in a humid chamber overnight at 4°C. After washing with PBS, the sections were then incubated with fluorescence conjugated secondary antibody (1: 500 dilution, Life Technologies) for 2 hours at room temperature. All immunofluorescence staining slides were imaged with fluorescence microscopy. (Nikon TE2000U, Melville, NY).
4.4.3. Evaluation
The histological and immunofluorescent images were analyzed for the degree of intimal hyperplasia, endothelial coverage and inflammation with ImageJ (National Institute of Health, Bethesda, MD). To quantify the degree of intimal hyperplasia, the ratio of the area of intimal hyperplasia (α-SMA positive region) to the circumference of the graft (μm2 μm−1 graft circumference) was calculated as previously described.[51] Endothelial coverage was defined as the circumferential length of the endothelial cell layer (eNOS positive) on the inner surface, and expressed as the percentage of total graft circumference. Inflammation was analyzed by calculating the area of macrophages (CD68 positive) per area of tissue surrounding the vascular graft.
4.5 Statistical Analysis
All statistical data are expressed as means ± standard deviation. Data were analyzed using one way ANOVA with a Tukey-Kramer post-test using SigmaStat (San Jose, CA). For all comparisons, p<0.05 was considered statistically significant.
Supplementary Material
Supplemental Figure 1. Representative stress-strain curve for heparinized polymer-ECM composites prepared with either “click” or carbodiimide chemistry. A higher value of elastic modulus was observed for samples prepared via the carbodiimide chemistry.
Supplemental Figure 2. Von Kossa staining for calcification in the vascular wall of (A) the ECM control graft, (D) heparinized composite (carbodiimide) graft, and at the anastomoses of (B) polymer-ECM composite graft, (C) heparinized composite (click) graft. Dark black color indicates calcification in Von Kossa staining, while pink color indicates cell nuclei (nuclear fast red). Scale bar = 100 μm.
Acknowledgments
We recognize the Northwestern University Microsurgery Core that performed the surgeries. This work is supported by the Dixon Translational Research Grants Innovation Award from Northwestern Memorial Foundation, the Robert R. McCormick Foundation, National Institute of Health (5R01EB017129-02 and 5K08DK101757), American Heart Association (AHA) Midwest Affiliate Postdoctoral Fellowship (14POST20160091), and the Chicago Biomedical Consortium (CBC) Postdoctoral Award (PDR-008).
References
- 1.Albers M, Battistella VM, Romiti M, Rodrigues AAE, Pereira CAB. Journal of vascular surgery. 2003;37:1263. doi: 10.1016/s0741-5214(02)75332-9. [DOI] [PubMed] [Google Scholar]
- 2.Quint C, Arief M, Muto A, Dardik A, Niklason LE. Journal of vascular surgery. 2012;55:790. doi: 10.1016/j.jvs.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmeister H, Plasenzotti R, Walter I, Plass C, Bastian F, Rieder E, Sipos W, Kaider A, Losert U, Weigel G. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;87B:95. doi: 10.1002/jbm.b.31074. [DOI] [PubMed] [Google Scholar]
- 4.Dahl SL, Koh J, Prabhakar V, Niklason LE. Cell transplantation. 2003;12:659. [PubMed] [Google Scholar]; Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT. Science translational medicine. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 5.Jiang B, Akgun B, Lam RC, Ameer GA, Wertheim JA. Acta biomaterialia. 2015;18:50. doi: 10.1016/j.actbio.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murugesan S, Xie J, Linhardt RJ. Current topics in medicinal chemistry. 2008;8:80. doi: 10.2174/156802608783378891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conklin B, Richter E, Kreutziger K, Zhong DS, Chen C. Medical engineering & physics. 2002;24:173. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Badylak SF, Freytes DO, Gilbert TW. Acta Biomaterialia. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA. Advanced Materials. 2006;18:1493. [Google Scholar]
- 10.van Lith R, Gregory EK, Yang J, Kibbe MR, Ameer GA. Biomaterials. 2014;35:8113. doi: 10.1016/j.biomaterials.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang B, Perrin L, Kats D, Meade T, Ameer G. Biomaterials. 2015;69:110. doi: 10.1016/j.biomaterials.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G. Biomaterials. 2013;34:30. doi: 10.1016/j.biomaterials.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piterina AV, Cloonan AJ, Meaney CL, Davis LM, Callanan A, Walsh MT, McGloughlin TM. International Journal of Molecular Sciences. 2009;10:4375. doi: 10.3390/ijms10104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catto V, Farè S, Freddi G, Tanzi MC. ISRN Vascular Medicine. 2014;2014:27. [Google Scholar]
- 15.Ravi S, Chaikof EL. Regenerative medicine. 2010;5:107. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davie EW. Journal of Biological Chemistry. 2003;278:50819. doi: 10.1074/jbc.X300009200. [DOI] [PubMed] [Google Scholar]; Hirsh J, Raschke R. CHEST Journal. 2004;126:188S. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L. Proceedings of the National Academy of Sciences. 2009;106:10086. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Motlagh D, Webb AR, Ameer GA. Tissue engineering. 2005;11:1876. doi: 10.1089/ten.2005.11.1876. [DOI] [PubMed] [Google Scholar]
- 19.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, Wagner DD. Proceedings of the National Academy of Sciences. 2006;103:16900. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt CE, Baier JM. Biomaterials. 2000;21:2215. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 21.Park S-N, Park J-C, Kim HO, Song MJ, Suh H. Biomaterials. 2002;23:1205. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 22.Pilipchuk SP, Vaicik MK, Larson JC, Gazyakan E, Cheng MH, Brey EM. Journal of Biomedical Materials Research Part A. 2013;101:2883. doi: 10.1002/jbm.a.34602. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y, Tsai C-C, Liang H-C, Sung H-W. Biomaterials. 2002;23:2447. doi: 10.1016/s0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- 24.Badylak SF, Gilbert TW. Seminars in Immunology. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borschel GH, Huang Y-C, Calve S, Arruda EM, Lynch JB, Dow DE, Kuzon WM, Dennis RG, Brown DL. Tissue engineering. 2005;11:778. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 26.Lemson MS, Tordoir JHM, Daemen MJAP, Kitslaar PJEHM. European Journal of Vascular and Endovascular Surgery. 2000;19:336. doi: 10.1053/ejvs.1999.1040. [DOI] [PubMed] [Google Scholar]
- 27.Ballyk PD, Walsh C, Butany J, Ojha M. Journal of Biomechanics. 1997;31:229. doi: 10.1016/s0197-3975(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 28.Haruguchi H, Teraoka S. J Artif Organs. 2003;6:227. doi: 10.1007/s10047-003-0232-x. [DOI] [PubMed] [Google Scholar]
- 29.Newby AC, Zaltsman AB. The Journal of Pathology. 2000;190:300. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Castellot J, Favreau L, Karnovsky M, Rosenberg R. Journal of Biological Chemistry. 1982;257:11256. [PubMed] [Google Scholar]
- 31.Majack RA, Clowes AW. Journal of cellular physiology. 1984;118:253. doi: 10.1002/jcp.1041180306. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Nature medicine. 2001;7:738. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 33.Szmitko PE, Wang C-H, Weisel RD, de Almeida JR, Anderson TJ, Verma S. Circulation. 2003;108:1917. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 34.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A. Nature medicine. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Circulation. 2003;108:2710. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 36.Wong WT, Huang NF, Botham CM, Sayed N, Cooke JP. Circulation research. 2012;111:1363. doi: 10.1161/CIRCRESAHA.111.247213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melchiorri AJ, Hibino N, Fisher JP. Tissue Engineering Part B: Reviews. 2013;19:292. doi: 10.1089/ten.teb.2012.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iredale JP. Journal of Clinical Investigation. 2007;117:539. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W. Cell. 2009;139:891. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S, Lakatta EG. Yonsei medical journal. 2012;53:258. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldonado M, Nam J. BioMed research international. 2013;2013 [Google Scholar]
- 42.Shao J-S, Cheng S-L, Sadhu J, Towler DA. Hypertension. 2010;55:579. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey MT, Pillarisetti S, Xiao H, Vyavahare NR. Journal of Biomedical Materials Research Part A. 2003;66:93. doi: 10.1002/jbm.a.10543. [DOI] [PubMed] [Google Scholar]; Steinhoff G, Stock U, Karim N, Mertsching H, Timke A, Meliss RR, Pethig K, Haverich A, Bader A. Circulation. 2000;102:Iii. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- 44.Assmann A, Akhyari P, Delfs C, Flögel U, Jacoby C, Kamiya H, Lichtenberg A. Journal of Surgical Research. 2012;176:367. doi: 10.1016/j.jss.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Johnson RC, Leopold JA, Loscalzo J. Circulation research. 2006;99:1044. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 46.Fleisch H, Schibler D, Maerki J, Frossard I. Nature. 1965;207:1300. doi: 10.1038/2071300b0. [DOI] [PubMed] [Google Scholar]
- 47.Schurgers LJ, Cranenburg EC, Vermeer C. Thromb Haemost. 2008;100:593. [PubMed] [Google Scholar]
- 48.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Journal of the American Society of Nephrology. 2005;16:2920. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 49.Ellman GL, Courtney KD, Andres V, Featherstone RM. Biochemical pharmacology. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 50.Farndale RW, Sayers CA, Barrett AJ. Connective tissue research. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 51.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, Walpoth BH. Circulation. 2008;118:2563. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative stress-strain curve for heparinized polymer-ECM composites prepared with either “click” or carbodiimide chemistry. A higher value of elastic modulus was observed for samples prepared via the carbodiimide chemistry.
Supplemental Figure 2. Von Kossa staining for calcification in the vascular wall of (A) the ECM control graft, (D) heparinized composite (carbodiimide) graft, and at the anastomoses of (B) polymer-ECM composite graft, (C) heparinized composite (click) graft. Dark black color indicates calcification in Von Kossa staining, while pink color indicates cell nuclei (nuclear fast red). Scale bar = 100 μm.