Abstract
The cholinesterase inhibitor, rivastigmine, ameliorates cognitive dysfunction and is approved for the treatment of Alzheimer's disease (AD). Rivastigmine is a dual inhibitor of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE); however, the impact of BuChE inhibition on cognitive dysfunction remains to be determined. We compared the effects of a selective BuChE inhibitor, N1-phenethylnorcymserine (PEC), rivastigmine and donepezil (an AChE-selective inhibitor) on cognitive dysfunction induced by amyloid-β peptide (Aβ1–40) in mice. Five-week-old imprinting control region (ICR) mice were injected intracerebroventricularly (i.c.v.) with either Aβ1–40 or the control peptide Aβ40–1 on Day 0, and their recognition memory was analyzed by a novel object recognition test. Treatment with donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) 20 min prior to, or immediately after the acquisition session (Day 4) ameliorated the Aβ1–40 induced memory impairment, indicating a beneficial effect on memory acquisition and consolidation. In contrast, none of the investigated drugs proved effective when administrated before the retention session (Day 5). Repeated daily administration of donepezil, rivastigmine or PEC, on Days 0–3 inclusively, ameliorated the cognitive dysfunction in Aβ1–40 challenged mice. Consistent with the reversal of memory impairments, donepezil, rivastigmine or PEC treatment significantly reduced Aβ1–40 induced tyrosine nitration of hippocampal proteins, a marker of oxidative damage. These results indicate that BuChE inhibition, as well as AChE inhibition, is a viable therapeutic strategy for cognitive dysfunction in AD.
Keywords: Acetylcholinesterase, Butyrylcholinesterase, Amyloid-β
1. Introduction
Alzheimer's disease (AD) is the leading cause of dementia in the elderly population and is characterized by progressive cognitive decline and neuropsychiatric disturbances. Accumulation of extracellular amyloid-beta (Aβ) and the formation of Aβ plaques and intracellular neurofibrillary tangles in the brain are pathological hallmarks of AD [1]. Aberrant proteolytic processing of amyloid precursor protein (APP) [2–4] results in the formation of Aβ oligomers and diffusible ligands that may damage synapses, impair memory, and ultimately induce neuronal dysfunction and neurotoxicity [5–8]. Since the major form of Aβ contains 40 amino acids (Aβ1–40) [2–4], we and others have generated partial models of AD in rodents by intracerebroventricular (i.c.v.) injection of Aβ1–40 to induce clinical signs reminiscent of AD, including learning and memory deficits and impairment of the cholinergic system [9,10].
The deficit of presynaptic markers is indication of a neuronal degeneration which is the real cause of the profound depletion of acetylcholine (ACh) within the hippocampus, basal forebrain and cortical areas [1–4]. Since changes in cholinergic regulation are a contributing factor to the cognitive dysfunction observed in AD patients, the selective acetylcholinesterase (AChE) inhibitor, donepezil, is widely utilized for symptomatic treatment of patients [11]. The cholinesterase inhibitor rivastigmine also ameliorates cognitive dysfunction and is similarly used in the treatment of AD. Unlike donepezil, rivastigmine is a dual inhibitor of AChE and butyrylcholinesterase (BuChE) [12–14]. However, the functional relevance of BuChE inhibition in cognitive dysfunction remains largely unknown.
AChE is principally associated with neurons and axons, while BuChE is primarily expressed and secreted by glial cells within the brain [15]. Yet studies by Darvesh et al. [16,17] indicate that specific neurons use BuChE rather than AChE to cleave presynaptic ACh. Indeed, some 10–15% of cholinergic neurons in the human hippocampus and amygdala express BuChE in their cell bodies and proximal dendrites, in lieu of AChE [16,17]. In the healthy human brain, AChE and BuChE are found in the ratio of 4:1. However, in the brains of AD patients AChE activity can decline by up to 45% during disease progression, reflecting the disappearance of neurons and axons to which it is associated, while BuChE activity can be elevated by up to 2-fold [17], thereby altering this ratio considerably.
Highly selective, reversible, central nervous system (CNS)-penetrable BuChE inhibitors, e.g., N1-phenethyl-norcymserine (PEC) and its analogues, have recently been developed and are permitting elucidation of the physiological role of brain BuChE. Selective BuChE inhibition has been shown to elevate cortical extracellular ACh levels in rats in a manner similar to that achieved by selective AChE inhibition using donepezil or by dual AChE/BuChE inhibition using rivastigmine [18]. In addition to elevating brain ACh, PEC has been shown to augment long-term potentiation (LTP; a molecular correlate of learning), improve cognitive performance in aged healthy rats, and lower brain levels of Aβ1–40 and Aβ1–42 in transgenic mice overexpressing human Aβ [19]. However, the effect of selective BuChE inhibition on Aβ-induced cognitive dysfunction remains unclear.
Aβ is widely recognized to mediate oxidative stress in the brain, particularly in AD [20,21]. An increase in protein nitration, a marker of widespread oxidative damage, is correlated with the severity of cognitive dysfunction in humans as well as animals [22–27]. We have previously demonstrated that continuous i.c.v. infusion of Aβ1–40 stimulates a time-dependent expression of inducible nitric oxide synthase (iNOS) and an overproduction of nitric oxide (NO) within the rat hippocampus [28]. In addition, tyrosine nitration contributes to Aβ-induced, and oxidative damage-mediated, cognitive dysfunction in rats and mice [29–32]. Recently, we have also demonstrated that tyrosine nitration of hippocampal proteins, specifically neurofilament light chain, correlates with the severity of cognitive impairments in mice [29,31].
In the present study we compared the effects of rivastigmine and PEC with those of donepezil on cognitive dysfunction in Aβ-injected mice using a novel object recognition test, and investigated any correlation with oxidative damage via tyrosine nitration of hippocampal proteins.
2. Materials and methods
2.1. Animals
Male, 5-week-old imprinting control region (ICR) mice were used throughout the study (Nihon SLC Co., Shizuoka, Japan). They were housed in a controlled environment (23 ± 1 °C, 50 ± 5% humidity), maintained on a 9:00 a.m. to 9:00 p.m. light cycle and allowed access to food and water ad libitum. All experiments were performed in accordance with the Guidelines for Animal Experiments of Nagoya University Graduate School of Medicine. All animal procedures and care conformed to the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006).
2.2. Treatment and experimental design
Aβ1–40 (obtained from Bachem, Bubendorf, Switzerland) was dissolved in saline to a stock concentration of 1.0 mg/ml and stored at −20 °C before use. The reverse peptide, Aβ40–1 (Bachem) was utilized as a control and prepared in the same way. The dissolved stock solutions of Aβ1–40 and Aβ40–1 peptides were incubated at 37 °C for 4 days, to allow aggregation prior to administration [31]. Incubated Aβ1-40, or Aβ40–1 was administered by i.c.v. injection as described previously [29,31,33,34]. Briefly, a microsyringe with a 28-gauge stainless-steel needle 3.0 mm long was used for all experiments. Mice were anesthetized lightly with ether, and the needle was inserted unilaterally 1 mm to the right of the midline point, at an equal distance between the eyes and the ears and perpendicular to the plane of the skull. Thereafter, an i.c.v. injection of 5 μl peptide (5 μg) or vehicle was delivered gradually over 3 min. Mice recovered rapidly and within 1 min post-injection exhibited normal behavior. The administration site was confirmed in preliminary experiments and neither the insertion of the needle, nor the volume of injection, significantly influenced survival, behavioral response or cognitive function.
Donepezil (Aricept™; Eisai, Tokyo, Japan) and rivastigmine (Exelon®, Novartis Pharma AG, Basel, Switzerland) were dissolved in saline. PEC (kindly supplied by Dr. Nigel H. Greig, synthesized to >99.9% chiral and chemical purity [35] to provide a characterized compound with an IC50 value for BuChE: 5 nM and AChE: <30 μM) was dissolved in saline containing 0.05% ethanol and 0.15% Tween 80. Donepezil (0.3, 1.0 mg/kg), rivastigmine (0.03, 0.3, 1.0 mg/kg) and PEC (1.0, 3.0 mg/kg) were administered via the intraperitoneal (i.p.) route at various time points before and during the different phases of the novel object recognition test (described in Section 2.4).
2.3. Locomotor activity
Locomotor activity was measured using an infrared detector (Neuroscience Company, Tokyo, Japan) in a plastic box (32 × 22 × 15 cm), as described previously [36]. Mice were injected with donepezil (0.3, 1.0 mg/kg), rivastigmine (0.03, 0.3, 1.0 mg/kg) or PEC (1.0, 3.0 mg/kg) and the locomotor activity was measured for 60 min.
2.4. Novel object recognition test
Novel object recognition analysis was performed on Days 3–5 after the i.c.v. injection of Aβ1–40 (Day 0) [29,37]. This method has been previously used as a measure of cognitive dysfunction in mouse models of natural aging and AD [38]. A plastic chamber (35 × 35 × 35 cm) was used in low light conditions during the light phase of the light/dark cycle. The procedure consisted of three different phases: a habituation phase, an acquisition phase and a retention phase. On Day 3 (habituation phase), mice were individually subjected to a single familiarization session of 10 min, during which time they were introduced into the empty arena to become familiar with the apparatus. On Day 4 (acquisition phase), the animals were subjected to a single 10 min session, during which two floor-fixed objects (A and B) were placed in a symmetric position from the center of the arena, 15 cm apart from one another and 8 cm from the nearest wall. Mice were allowed to explore the objects in the open field. On Day 5 (retention phase), mice were allowed to explore the open field in the presence of two objects: the familiar object A and a novel object C, each of a different shape and color but of similar size (A and C). A recognition index, calculated for each mouse, was expressed as the ratio (TC × 100)/(TA + TC), where TA and TC are the times spent during the retention phase on object A and object C.
The time spent exploring individual object (nose pointing toward the object at a distance ≤1 cm) was recorded by a blinded investigator. Drug and vehicle treatments were administrated once i.p., either 20 min before the acquisition phase, immediately after the acquisition phase, or 20 min before the retention phase. Alternatively, the drugs were administered once a day for 4 days (Day 0–3 inclusively) after i.c.v. injection of Aβ1–40 and then the mice were subjected to novel object recognition analysis on Days 3–5 as before.
2.5. Sample preparation for Western blot analysis
Removed hippocampus were placed on an ice-cold glass plate and immediately frozen and stored at −80 °C. Hippocampal protein extracts were obtained by homogenization in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer containing 20 mM Tris–HCl (pH 7.6), 150 mM sodium chloride, 2 mM EDTA–2Na, 50 mM sodium fluoride, 1 mM sodium vanadate, 1% (v/v) Nonidet P-40, 1% (w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS). The RIPA buffer was supplemented with complete protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany). Homogenates were centrifuged at 13,000 × g for 20 min to obtain the desired supernatant of the extracts.
2.6. Western blot analysis
Following protein content analysis by Lowry method, equal amounts (20 μg) of protein sample were resolved by 10% SDS–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, MA, USA) for 1 h with 15 V. Membranes were incubated in 3% (w/v) skimmed milk in phosphate-buffered saline containing 0.05% (v/v) Tween 20 for 2 h at room temperature, and probed with anti-nitrotyrosine mouse monoclonal 1A6 antibody (1:1000 diluted in 3% (w/v) skimmed milk, Millipore, catalog number 05-233) for overnight at 4 °C and anti-β-actin goat polyclonal antibody (1:1000 diluted in 3% (w/v) skimmed milk) for 1 h at room temperature (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Immunoreactive complexes on the membrane were detected using Enhanced Chemiluminescence Plus Western Blotting Detection Reagents (GE Healthcare Japan, Tokyo, Japan) and analyzed by Atto Light-Capture system (Atto, Tokyo, Japan). Exposure time was 3 min.
2.7. Statistical analysis
All results are expressed as mean ± standard error (SE) values. Statistical significance was determined with a one-way analysis of variance (ANOVA) followed by the Bonferroni's multiple comparisons test, with p < 0.05 as the threshold to define a significant level of difference.
3. Results
3.1. Effects of donepezil, rivastigmine and PEC on baseline locomotor activity
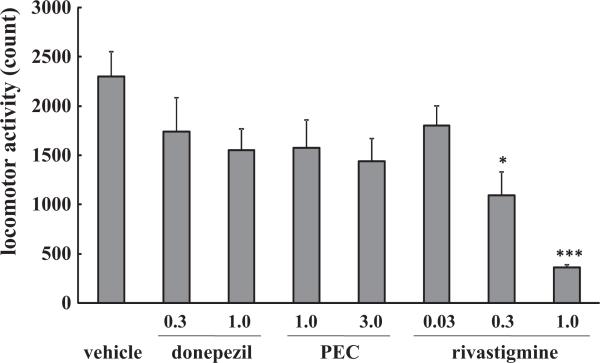
Donepezil (0.3, 1.0 mg/kg), PEC (1.0, 3.0 mg/kg) and rivastigmine (0.03 mg/kg) had no significant effect on baseline locomotor activity. At higher doses (0.3 and 1.0 mg/kg) rivastigmine significantly decreased locomotor activity in a dose-dependent manner (p < 0.05, p < 0.001 vs. control mice, respectively, Fig. 1).
Fig. 1.
Effects of donepezil, rivastigmine and PEC on baseline locomotor activity in mice. Mice were injected i.p. with donepezil (0.3, 1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) and the baseline locomotor activity was measured over 60 min, in the absence of Aβ peptide injection. Control mice were injected (i.p.) with saline. Values indicate the mean ± SE (n = 5). F(7,32) = 5.736, p < 0.001; *p < 0.05 and ***p < 0.001 vs. control.
3.2. Effects of a single administration of donepezil, rivastigmine and PEC on cognitive dysfunction induced by Aβ1–40 in mice
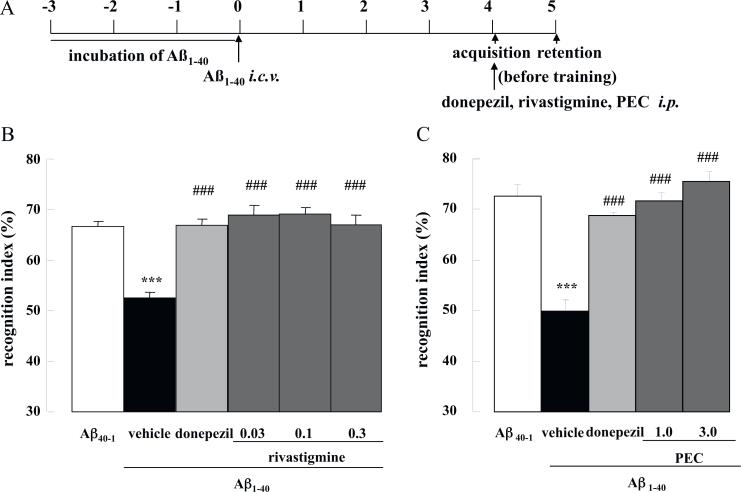
Mice were challenged with Aβ1–40 by i.c.v. injection and their cognitive ability was examined using a novel object recognition paradigm. First, the effect of cholinesterase inhibitors (ChEIs) on memory acquisition was investigated. Donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) was administered i.p. 20 min before the acquisition phase on Day 4 (Fig. 2A). The time exploring the two objects during the acquisition session (Day 4) was unaffected by Aβ1–40, Aβ40–1, donepezil or PEC treatment (Table 1). Rivastigmine (0.03 and 0.1 mg/kg) similarly had no effect, but the drug at a dose of 0.3 mg/kg significantly reduced interaction time with the two objects compared with saline-treated Aβ1–40 controls (p < 0.001, Table 1). During the retention phase of the task (Day 5), mice challenged with Aβ1–40 proved unable to discriminate between the unfamiliar and familiar objects, and displayed a significantly decreased recognition index compared with those challenged with the control reverse peptide, Aβ40–1 (p < 0.001, Fig. 2B and C). Treatment with donepezil, rivastigmine or PEC 20 min before acquisition fully ameliorated the Aβ1–40-induced impairment of memory recognition (p < 0.001 for all drugs at all doses vs. saline-treated Aβ1–40 controls, Fig. 2B and C).
Fig. 2.
Effects of donepezil, rivastigmine and PEC on memory acquisition in Aβ1–40-injected mice. Mice were injected with Aβ1–40 on Day 0, and subjected to the novel object recognition task on Days 4–5 (A). Donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) were administered i.p. 20 min before the acquisition session on Day 4 (B and C). Values indicate the mean ± SE (n = 8 for B, n = 8 for C). F(5,42) = 18.4, p < 0.001 (B); F(4,35) = 28.32, p < 0.001 (C); ***p < 0.001 vs. Aβ40–1control; p < 0.001; ### vs. vehicle-treated Aβ1–40 control.
Table 1.
Effects of donepezil, rivastigmine and PEC on the combined exploration time of two objects during the acquisition session in the novel object recognition test.
| Drug | Dose (mg/kg) | Time (s) | |
|---|---|---|---|
| Treatment group A | |||
| Aβ40-1 | Saline | - | 39.6 ± 1.4 |
| Aβ1-40 | Saline | - | 42.2 ± 1.4 |
| Aβ1-40 | Donepezil | 1.0 | 42.0 ± 1.3 |
| Aβ1-40 | Rivastigmine | 0.03 | 41.0 ± 0.9 |
| Aβ1-40 | Rivastigmine | 0.1 | 39.2 ± 1.4 |
| Aβ1-40 | Rivastigmine | 0.3 | 19.0 ± 2.7*** |
| Treatment group B | |||
| Aβ40-1 | Saline | - | 56.0 ± 4.1 |
| Aβ1-40 | Saline | - | 57.0 ± 6.1 |
| Aβ1-40 | Donepezil | 1.0 | 50.3 ± 4.2 |
| Aβ1-40 | PEC | 1.0 | 55.5 ± 3.8 |
| Aβ1-40 | PEC | 3.0 | 65.9 ± 3.6 |
Mice were injected with Aβ1-40 on Day 0, and were subjected to the novel object recognition test on Day 3-5. Donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) and saline (A) or donepezil (1.0 mg/kg), PEC (1.0, 3.0 mg/kg) or saline (B) were administered i.p. 20 min before the acquisition session on Day 4. Values indicate the mean ± SE (n = 8 for A, n = 5 for B). F(5,42) = 31.67, p < 0.001 (A); F(4,20) = 1.98, p = 0.137 (B)
p<0.001 vs. saline-treated Aβ1-40.
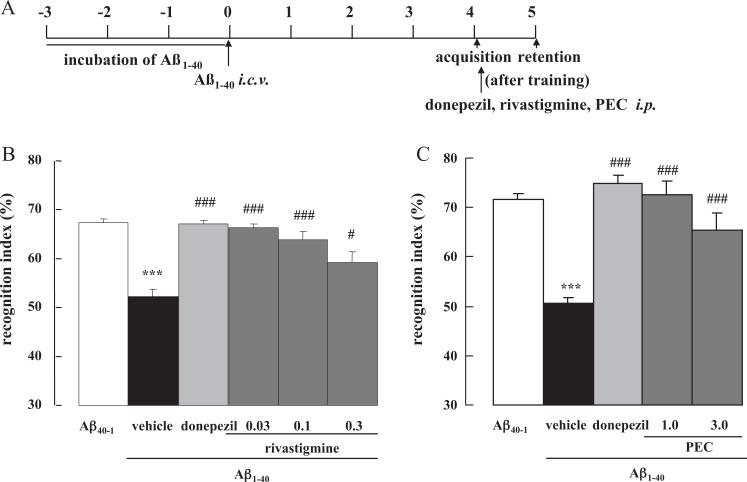
The effect of ChEIs on memory consolidation was then investigated. When donepezil, rivastigmine or PEC was administered immediately after the acquisition session on Day 4 (Fig. 3A), they significantly ameliorated the Aβ1–40 induced cognitive dysfunction compared with the saline-injected Aβ1–40 control mice (p < 0.001 for all drugs at all doses vs. saline-treated Aβ1–40 controls, except for rivastigmine 0.3 mg/kg where p < 0.05, Fig. 3B and C).
Fig. 3.
Effect of donepezil, rivastigmine and PEC on memory consolidation in Aβ1–40-injected mice. Mice were injected with Aβ1–40 on Day 0 and subjected to the novel object recognition task on Day 4–5 (A). Donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) were administered i.p. immediately after the acquisition session on Day 4 (B and C). Values indicate the mean ± SE (n = 8 for B, n = 6 for C). F(5,42) = 17.99, p < 0.001 (B); F(4,26) = 25.25, p < 0.001 (C); ***p < 0.001 vs. Aβ40–1 control; #p < 0.05 and ###p < 0.001 vs. vehicle-treated Aβ1–40 control.
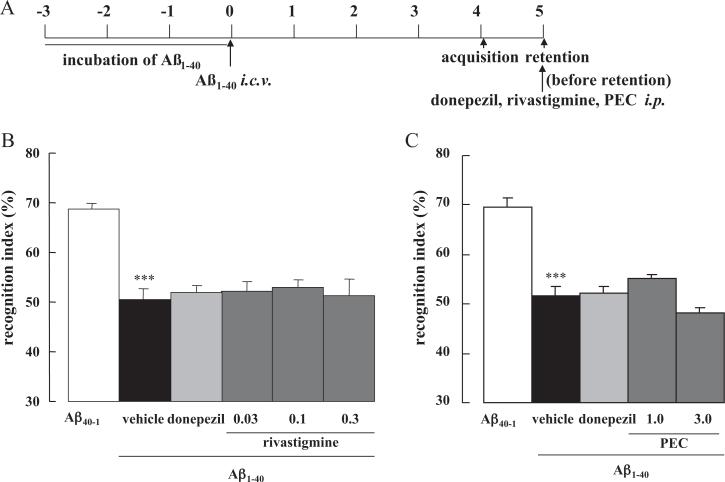
Finally the effect of ChEIs on memory retrieval was investigated. When donepezil, rivastigmine or PEC was administered 20 min before the retention session on Day 5 (Fig. 4A), none of the drugs improved the cognitive dysfunction induced by Aβ1–40 (Fig. 4B and C), in contrast to the previous dosing regimens
Fig. 4.
Effect of donepezil, rivastigmine and PEC on memory retrieval in Aβ1–40-injected mice. Mice were injected with Aβ1–40 on Day 0 and subjected to the novel object recognition task on Day 4–5 (A). Donepezil (1.0 mg/kg), rivastigmine (0.03, 0.1, 0.3 mg/kg) or PEC (1.0, 3.0 mg/kg) were administered i.p. 20 min before the retention session on Day 5 (B and C). Values indicate the mean ± SE (n = 6 for B, n = 4 for C). F(5,30) = 11.76, p < 0.001 (B); F(4,11) = 24.56, p < 0.001 (C); ***p < 0.001 vs. Aβ40–1 control.
3.3. Amelioration of an Aβ1–40-induced cognitive dysfunction by repeated daily administration of donepezil, rivastigmine or PEC
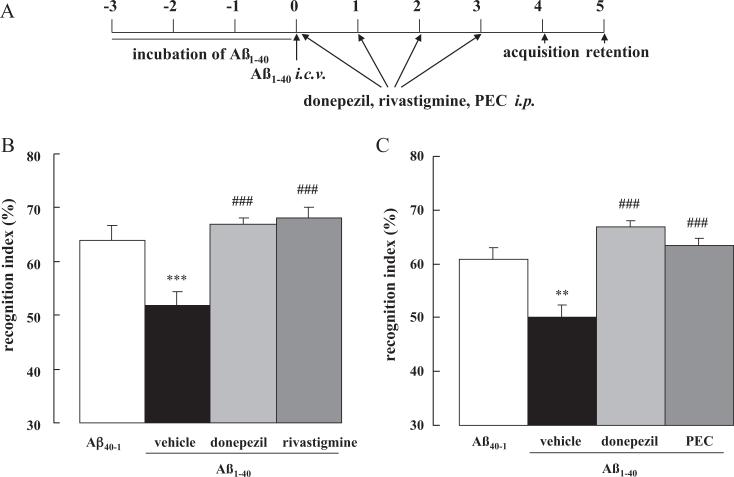
Subsequently, we evaluated the effects of repeated daily treatment of donepezil (1.0 mg/kg), rivastigmine (0.03 mg/kg) or PEC (1.0 mg/kg) on memory impairment induced by Aβ1–40. No intergroup differences were evident with regard to the overall object exploration time during the acquisition session (data not shown). During the retention phase (Day 5), mice challenged with Aβ1–40 and administered saline proved unable to discriminate between unfamiliar and familiar objects, and displayed a significantly decreased recognition index in comparison with the reverse-peptide controls (p < 0.001 and p < 0.01 for treatment groups A and B, respectively, Fig. 5A–C). Repeated treatment with donepezil, rivastigmine and PEC significantly enhanced new object discrimination in Aβ1–40-injected mice (p < 0.001 for all drugs vs. saline-treated Aβ1–40 controls, Fig. 5B and C), fully ameliorating the impairment.
Fig. 5.
Effect of repeated treatment with donepezil, rivastigmine and PEC on cognitive dysfunction in Aβ1–40-injected mice. Donepezil (1.0 mg/kg), rivastigmine (0.3 mg/kg) and PEC (1.0 mg/kg) were repeatedly administered i.p. once a day for 4 days, starting immediately after the i.c.v. injection of Aβ1–40 on Day 0. The mice were subjected to the novel object recognition task on Day 4–5. Values indicate the mean ± S.E. (n = 8 for B, n = 10 for C). F(3,29) = 42.28, p < 0.001 (B); F(3,40) = 9.42, p < 0.001 (C); **p < 0.01 and ***p < 0.001 vs. Aβ40–1 control; ###p < 0.001 vs. vehicle-treated Aβ1–40 control.
3.4. Tyrosine nitration of proteins induced by Aβ1–40 in the hippocampus of mice
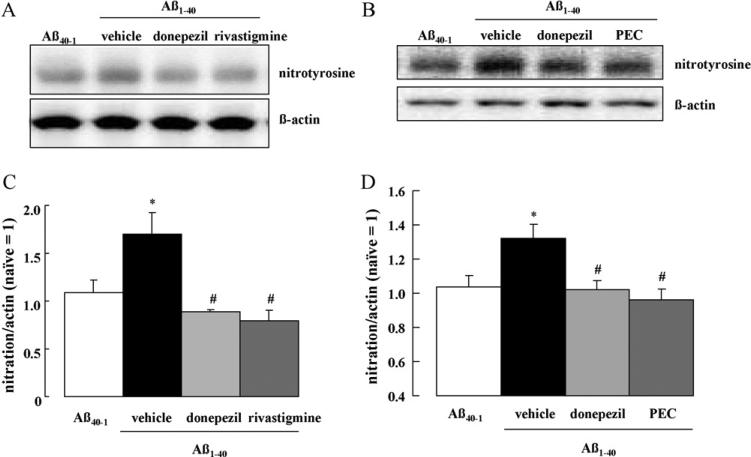
We have previously demonstrated that memory impairment induced by Aβ1–40 in mice is associated with an increase in oxidative stress and tyrosine nitration of hippocampal proteins [31]. Accordingly, we evaluated the effects of repeated daily treatments of donepezil, rivastigmine and PEC on tyrosine nitration of hippocampal proteins. Injection of Aβ1–40 i.c.v. induced extensive tyrosine nitration compared with Aβ40–1, which was detected as a single band of approximately 70 kDa (p < 0.05 vs. Aβ40–1 control, Fig. 6). Repeated 4-day treatments with donepezil, rivastigmine and PEC fully reduced the elevated tyrosine nitration generated by Aβ1–40 (p < 0.05 for all drugs vs. saline-treated Aβ1–40 controls, Fig. 6). By contrast, acute treatment with donepezil, rivastigmine or PEC failed to change the levels of tyrosine nitration of hippocampal proteins (data not shown).
Fig. 6.
Effect of repeated treatment with donepezil, rivastigmine and PEC on tyrosine nitration of hippocampal protein in Aβ1–40-injected mice. Donepezil (1.0 mg/kg), rivastigmine (0.3 mg/kg) and PEC (1.0 mg/kg) were repeatedly administered once a day for 4 days, starting immediately following the i.c.v. injection of Aβ1–40 on Day 0. On Day 4, tyrosine nitration of hippocampal proteins was analyzed by Western blotting. Values indicate the mean ± SE (n = 8 for C, n = 15 for D). F(3,28) = 4.77, p < 0.01 (C); F(3,56) = 3.968, p < 0.05 (D); *p < 0.05 vs. Aβ40–1 control; #p < 0.05 vs. vehicle-treated Aβ1–40 control.
4. Discussion
In the current study, the independent roles of BuChE and AChE in the cholinergic amelioration of Alzheimer-associated cognitive impairment were evaluated, using a novel object recognition paradigm. This behavioral test is considered to involve visual learning and recognition processing, which are particularly affected during AD [39,40]. Aggregated Aβ1–40 oligomers have been previously shown to impair LTP and synapse formation and induce neuronal dysfunction using in vitro and in vivo model systems [5–8]. Following i.c.v. injection of aggregated Aβ1–40 into the brains of mice in our model of AD, a clear and consistent deficit in recognition index was apparent when measured 4 days later, in accordance with prior studies [9,10,41]. We demonstrate for the first time that a selective BuChE inhibitor, PEC, completely reversed the cognitive impairment induced by Aβ1–40, in a way comparable to that achieved by either selective AChE inhibition (donepezil) or by dual inhibition of AChE and BuChE (rivastigmine). A single treatment with donepezil, rivastigmine or PEC reversed the impairment of recognition memory when mice were treated 20 min before, or immediately after, the acquisition session. However, when these same drugs were administrated 20 min before the retention phase, they had no impact on memory impairment in Aβ1–40-challenged mice. Wise et al. [42] examined the effect of donepezil on spatial memory by radial-arm maze task and demonstrated that donepezil (0.3 and 1.0 mg/kg) improved spatial memory when administered 20 min before the acquisition session. Alternatively, when given immediately after the acquisition phase or 30 min prior to the retention test, donepezil failed to affect spatial memory [43]. These results together with our present findings suggest that both AChE and BuChE inhibitors, separately (i.e., donepezil and PEC) or in combination (i.e., rivastigmine), have beneficial effects on memory impairment induced by Aβ1–40, likely through an action on memory acquisition and/or consolidation, but not via the process of memory retrieval. Moreover, it has been reported that PEC at the dose of 5 mg/kg induced an increase in cortical extracellular ACh levels with a moderate BuChE inhibition [18]. Greig et al. [19] have also demonstrated that the extracellular ACh level in rat parietal cortex slightly but significantly increase after PEC (1.25 mg/kg) treatment. Since the doses of PEC (1.0, 3.0 mg/kg) used in this experiment have been previously shown to have no effect on AChE activity [18,19], ameliorative effect of PEC on Aβ-induced cognitive impairment may be associated with transient increase in extracellular level of ACh through BChE inhibition. Interestingly, it has been noted by Wise et al. [42] that the combination of two effective drugs with separate mechanisms at subthreshold doses can provide synergistic action. Since rivastigmine inhibits both of AChE and BuChE, the effective dose of rivastigmine (0.03 mg/kg) that ameliorated memory impairment in our study proved lower than that of donepezil (1.0 mg/kg) and PEC (1.0 mg/kg). It remain unclear if the effective dose of rivastigmine increase ACh level in the brain although approximately 2–3 fold increases in cortical extracellular ACh level has been detected followed the administration of the drug at the dose of 0.6 mg/kg [18]. Furthermore, whether or not such a benefit of dual cholinesterase inhibitor vs. a selective one would translate into any clinically relevant difference remains unknown. These points should be addressed in future studies.
Similarly, repeated daily treatment with donepezil, rivastigmine or PEC for 4 days, fully reversed the memory impairment induced by Aβ1–40. The mechanism(s) underpinning this action remain to be elucidated but are likely different from that associated with the cognitive improvement evident after acute treatment. The repeated drug treatments ended 24 h before the acquisition phase, at a time when the cholinergic actions of all the assessed drugs are predicted to be minimal. In contrast, the single acute administration of the same drugs closer to the time of the acquisition phase, produced elevated brain ACh levels during acquisition [18]. A potential mechanism underlying the behavioral improvements provided by repeated administration of AChE and BuChE inhibitors may derive from protection against the Aβ1–40 challenge that induces behavioral deficit.
During the early stages of AD pathology, peroxynitrite-mediated damage is associated with progressive cognitive impairment [22,43–45]. It has been reported that injection of preaggregated Aβ1-42 peptide into the nucleus basalis magnocellularis of rats results in an increase in the level of iNOS immunoreactivity through cyclooxygenase-2 induction [46]. Recent proteomic studies have identified several protein targets of oxidative modification in rat brain injected with Aβ1-42 into the nucleus basalis magnocellularis [47]. Aβ upregulates iNOS, mediating peroxynitrite protein damage via hyperphosphorylation of extracellular signal-regulated kinase (ERK), while the selective inhibition of ERK or iNOS abolishes Aβ–induced neurotoxicity [48,49] and memory impairment [50].
More specifically, our previous studies indicate an involvement of tyrosine nitration of hippocampal proteins, especially neurofilament light chain, by Aβ peptides [29–31], which was associated with memory impairment in Aβ-challenged mice [31]. We subsequently investigated whether treatment with ChEIs could alter this observed nitration of hippocampal proteins. Repeated daily treatment with donepezil, rivastigmine and PEC prevented the increase in tyrosine nitration of proteins in the hippocampus while ameliorating Aβ1–40-induced memory impairment. In contrast, single administration of the same agents failed to affect the tyrosine nitration of hippocampal proteins in similarly challenged mice, implicating the involvement of cholinergically-mediated neuro-protective actions with repeated administration. ACh has known anti-inflammatory actions; therefore it is possible that increased expression of AChE and BuChE reduces ACh levels, triggering a process of inflammation, characteristic of AD [51,52]. The restoration of such balance by AChE and/or BuChE inhibition may have the potential to mitigate pro-inflammatory signaling cascades. Hwang et al. [53] suggested that donepezil may attenuate microglial production of NO and tumor necrosis factor-α (TNF-α) and suppress iNOS, interleukin-1-β, and TNF-α gene expression. Together with results in the current study, these findings suggest that inflammatory responses, including tyrosine nitration of proteins, may be reduced by chronic therapeutic treatment with AChE and/or BuChE inhibitors.
5. Conclusions
In the current study, we demonstrated that both selective BuChE inhibition mediated by PEC, and selective AChE inhibition mediated by donepezil, provide beneficial effects on the cognitive dysfunction apparent in Aβ1–40-challenged mice. Furthermore, the dual AChE/BuChE inhibitor rivastigmine also demonstrated beneficial effects on memory acquisition and consolidation. This may involve direct classical cholinergic augmentation, together with a cholinergically-mediated protective action, potentially via the reduction of inflammation.
These data support further investigation of dual AChE/BuChE inhibition in preclinical and clinical studies to maximize therapeutic efficacy, and the evaluation of selective BuChE inhibition as a new therapeutic strategy for cognitive dysfunction in AD.
Acknowledgements
This study was supported in part by the following funding sources: (i) grants-in-aid for Scientific Research (No. 22390046, 23659135) from the Japan Society for the Promotion of Science; (ii) a grant from the global COE program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; (iii) a grant from the Academic Frontier Project for Private Universities, matching fund subsidy from MEXT, 2007–2011; (iv) a grant from the Regional Joint Research Program supported by grants to Private Universities to Cover Current Expenses from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); (v) a grant from Novartis Pharma KK and ONO Pharmaceutical Co., Ltd. (vi) The Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- APP
amyloid protein precursor
- ANOVA
analysis of variance
- Aβ
amyloid beta
- AD
Alzheimer's disease
- BuCh
butyrylcholine
- BuChE
butyrylcholinesterase
- ChEIs
cholinesterase inhibitors
- ERK
extracellular signal-regulated kinase
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- PEC
N1-phenethyl-norcymserine
References
- 1.Small DH, McLean CA. Alzheimer's disease and the amyloid beta protein: what is the role of amyloid? J Neurochem. 1999;73:443–9. doi: 10.1046/j.1471-4159.1999.0730443.x. [DOI] [PubMed] [Google Scholar]
- 2.Estus S, Golde TE, Kunishita T, Blades D, Lowery D, Eisen M, et al. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992;255:726–8. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- 3.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255:728–30. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- 4.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–5. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 5.Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989;245:417–20. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- 6.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991;207:367–8. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- 7.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–4. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 8.Howlett DR, Jennings KH, Lee DC, Clark MS, Brown F, Wetzel R, et al. Aggregation state and neurotoxic properties of Alzheimer beta-amyloid peptide. Neurodegeneration. 1995;4:23–32. doi: 10.1006/neur.1995.0003. [DOI] [PubMed] [Google Scholar]
- 9.McDonald MP, Dahl EE, Overmier JB, Mantyh P, Cleary J. Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behav Neural Biol. 1994;62:60–7. doi: 10.1016/s0163-1047(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Nitta A, Saito T, Hu J, Nabeshima T. Changes in ciliary neurotrophic factor content in the rat brain after continuous intracerebroventricular infusion of beta-amyloid(1–40) protein. Neurosci Lett. 1995;201:155–8. doi: 10.1016/0304-3940(95)12161-7. [DOI] [PubMed] [Google Scholar]
- 11.Small DH, Mok SS, Bornstein JC. Alzheimer's disease and Abeta toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–8. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 12.Bullock R, Lane R. Executive dyscontrol in dementia, with emphasis on sub-cortical pathology and the role of butyrylcholinesterase. Curr Alzheimer Res. 2007;4:277–93. doi: 10.2174/156720507781077313. [DOI] [PubMed] [Google Scholar]
- 13.Naik RS, Hartmann J, Kiewert C, Duysen EG, Lockridge O, Klein J. Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase-deficient mice. J Pharm Pharm Sci. 2009;12:79–85. doi: 10.18433/j3mk59. [DOI] [PubMed] [Google Scholar]
- 14.Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Curr Med Res Opin. 2006;22:49–59. doi: 10.1185/030079906x80279. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam M, Guillozet A, Shaw P, Quinn B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol Dis. 2002;9:88–93. doi: 10.1006/nbdi.2001.0462. [DOI] [PubMed] [Google Scholar]
- 16.Darvesh S, Grantham DL, Hopkins DA. Distribution of butyrylcholinesterase in the human amygdala and hippocampal formation. J Comp Neurol. 1998;393:374–90. [PubMed] [Google Scholar]
- 17.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–8. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 18.Cerbai F, Giovannini MG, Melani C, Enz A, Pepeu G. N1phenethyl-norcymserine a selective butyrylcholinesterase inhibitor, increases acetylcholine release in rat cerebral cortex: a comparison with donepezil and rivastigmine. Eur J Pharmacol. 2007;572:142–50. doi: 10.1016/j.ejphar.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 19.Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA. 2005;102:17213–8. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 21.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010;19:341–53. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 22.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–7. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–9. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim HC, Yamada K, Nitta A, Olariu A, Tran MH, Mizuno M, et al. Immunocyto-chemical evidence that amyloid beta (1-42) impairs endogenous antioxidant systems in vivo. Neuroscience. 2003;119:399–419. doi: 10.1016/s0306-4522(02)00993-4. [DOI] [PubMed] [Google Scholar]
- 26.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 27.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–93. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Tran MH, Yamada K, Olariu A, Mizuno M, Ren XH, Nabeshima T. Amyloid beta-peptide induces nitric oxide production in rat hippocampus: association with cholinergic dysfunction and amelioration by inducible nitric oxide synthase inhibitors. FASEB J. 2001;15:1407–9. doi: 10.1096/fj.00-0719fje. [DOI] [PubMed] [Google Scholar]
- 29.Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25–35). Behav Brain Res. 2007;180:139–45. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Alkam T, Nitta A, Mizoguchi H, Saito K, Seshima M, Itoh A, et al. Restraining tumor necrosis factor-alpha by thalidomide prevents the amyloid beta-induced impairment of recognition memory in mice. Behav Brain Res. 2008;189:100–6. doi: 10.1016/j.bbr.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Alkam T, Nitta A, Mizoguchi H, Itoh A, Murai R, Nagai T, et al. The extensive nitration of neurofilament light chain in the hippocampus is associated with the cognitive impairment induced by amyloid beta in mice. J Pharmacol Exp Ther. 2008;327:137–47. doi: 10.1124/jpet.108.141309. [DOI] [PubMed] [Google Scholar]
- 32.Tran MH, Yamada K, Nakajima A, Mizuno M, He J, Kamei H, et al. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta-peptide-induced cholinergic dysfunction. Mol Psychiatry. 2003;8:407–12. doi: 10.1038/sj.mp.4001240. [DOI] [PubMed] [Google Scholar]
- 33.Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–93. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 34.Alkam T, Nitta A, Furukawa-Hibi Y, Niwa M, Mizoguchi H, Yamada K, et al. Oral supplementation with Leu-Ile, a hydrophobic dipeptide, prevents the impairment of memory induced by amyloid beta in mice via restraining the hyperphosphorylation of extracellular signal-regulated kinase. Behav Brain Res. 2010;210:184–90. doi: 10.1016/j.bbr.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Yu Q, Holloway HW, Utsuki T, Brossi A, Greig NH. Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer's disease. J Med Chem. 1999;42:1855–61. doi: 10.1021/jm980459s. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–25. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–25. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitta A, Itoh A, Hasegawa T, Nabeshima T. Beta-Amyloid protein-induced Alzheimer's disease animal model. Neurosci Lett. 1994;170:63–6. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 39.Haley GE, Berteau-Pavy F, Berteau-Pavy D, Raber J. Novel image-novel location object recognition task sensitive to age-related cognitive decline in nondemented elderly. Age (Dordr) 2011 doi: 10.1007/s11357-010-9204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werheid K, Clare L. Are faces special in Alzheimer's disease? Cognitive conceptualisation, neural correlates, and diagnostic relevance of impaired memory for faces and names. Cortex. 2007;43:898–906. doi: 10.1016/s0010-9452(08)70689-0. [DOI] [PubMed] [Google Scholar]
- 41.Games D, Khan KM, Soriano FG, Keim PS, Davis DL, Bryant K, et al. Lack of Alzheimer pathology after beta-amyloid protein injections in rat brain. Neurobiol Aging. 1992;13:569–76. doi: 10.1016/0197-4580(92)90057-5. [DOI] [PubMed] [Google Scholar]
- 42.Wise LE, Iredale PA, Stokes RJ, Lichtman AH. Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology. 2007;32:1805–12. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- 43.Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953:135–43. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- 44.Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, et al. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184:1425–33. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster B, Hansen L, Adame A, Crews L, Torrance M, Thal L, et al. Astroglial activation of extracellular-regulated kinase in early stages of Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:142–51. doi: 10.1097/01.jnen.0000199599.63204.6f. [DOI] [PubMed] [Google Scholar]
- 46.Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, et al. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–74. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- 47.Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, et al. Proteomic identification of proteins oxidized by Abeta(1-42) in synaptosomes: implications for Alzheimer's disease. Brain Res. 2005;1044:206–15. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 48.Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, et al. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–9. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapoport M, Ferreira A. PD98059 prevents neurite degeneration induced by fibrillar beta-amyloid in mature hippocampal neurons. J Neurochem. 2000;74:125–33. doi: 10.1046/j.1471-4159.2000.0740125.x. [DOI] [PubMed] [Google Scholar]
- 50.Diaz A, Mendieta L, Zenteno E, Guevara J, Limon ID. The role of NOS in the impairment of spatial memory and damaged neurons in rats injected with amyloid beta 25–35 into the temporal cortex. Pharmacol Biochem Behav. 2011;98:67–75. doi: 10.1016/j.pbb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Rao AA, Sridhar GR, Das UN. Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer's disease. Med Hypotheses. 2007;69:1272–6. doi: 10.1016/j.mehy.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–79. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang J, Hwang H, Lee HW, Suk K. Microglia signaling as a target of donepezil. Neuropharmacology. 2010;58:1122–9. doi: 10.1016/j.neuropharm.2010.02.003. [DOI] [PubMed] [Google Scholar]