Abstract
Brain-derived neurotrophic factor (BDNF) regulates neuronal survival and growth and promotes synaptic plasticity. Recently, researchers have begun to explore the relationship between peripheral BDNF levels and autism spectrum disorder (ASD), but the findings are inconsistent. We undertook the first systematic review and meta-analysis of studies examining peripheral BDNF levels in ASD compared with healthy controls. The PubMed, Embase, and Cochrane Library databases were searched for studies published before February 2016. Fourteen studies involving 2,707 participants and 1,131 incident cases were included. The meta-analysis provided evidence of higher peripheral BDNF levels in ASD compared with controls [standardized mean difference (SMD) = 0.63, 95% confidence interval (95% CI) = 0.18–1.08; P = 0.006]. Subgroup analyses revealed higher BDNF levels in ASD compared with controls for both serum [SMD = 0.58, 95% CI = 0.11–1.04; P = 0.02] and plasma [SMD = 1.27, 95% CI = 0.92–1.61; P < 0.001]. Studies of childhood yielded similar cumulative effect size [SMD = 0.78, 95% CI = 0.31–1.26; P = 0.001], while this was not true for the studies of adulthood [SMD = 0.04, 95% CI = −1.72–1.80; P = 0.97]. This meta-analysis suggests that peripheral BDNF levels are a potential biomarker of ASD.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by abnormalities in social interaction, impairment in language and communication, restrictive or repetitive interests, and stereotyped behaviours and movements1. ASD includes autistic disorder, Asperger syndrome, and pervasive developmental disorder not otherwise specified1. Over the last decade, the prevalence of ASD has been reported as 11.3 per 1,000 with a male-to-female ratio of 3–4:12. There is growing evidence that ASD may be influenced by genetic, neurological, environmental and immunological factors3,4. However, the underlying mechanism of ASD has not yet been identified. Behavioural abnormalities are not evident until approximately 12–18 months of age5,6. Furthermore, ASD individuals vary enormously in clinical manifestation, severity, developmental trajectory, and treatment response. This complexity has propelled an intensive search to identify biomarkers to aid clinicians in achieving earlier diagnoses and in predicting treatment response7.
Brain-derived neurotrophic factor (BDNF) is a small protein found throughout the central nervous system (CNS) and peripheral blood. BDNF plays an important regulatory function in cell proliferation, migration, and survival during neurodevelopment and can also modulate axonal and dendritic outgrowth, synapse formation, neurotransmitter release and other neuroplastic processes8,9. Converging lines of evidence implicate the role of BDNF in the pathophysiology of ASD. It is reported that the concentration of BDNF in serum and the CNS are closely correlated in rats10. However, evidence of such a correlation in humans is still lacking. Therefore, whether altered BDNF values in the periphery reflect altered BDNF levels in the human CNS requires further investigation. Scientists assume that peripheral BDNF levels mirror and indirectly reflect BDNF levels in the brain11. This indirect measure of BDNF levels is much easier to acquire than direct assessments of BDNF levels in the CNS. For this reason, the concentration of BDNF in peripheral blood may be a useful potential biological marker for ASD.
The concept of the “periphery as a window to the brain” has led to an ever-increasing number of clinical studies assessing peripheral BDNF levels in ASD. However, reports of peripheral BDNF levels in ASD are inconsistent. Some studies have reported that serum BDNF is significantly reduced in ASD compared with healthy controls12,13, while other studies have reported higher serum BDNF levels in children with ASD compared with controls14,15,16. Similarly, two studies that have examined BDNF levels in neonatal specimens from individuals later diagnosed with ASD have yielded inconsistent results17,18.
Thus, we undertook a systematic review of studies assessing peripheral BDNF levels in ASD and controls, followed by a series of meta-analyses to provide an overall estimate of the effect size and between-study heterogeneity of the association between peripheral BDNF levels and ASD.
Results
Literature search
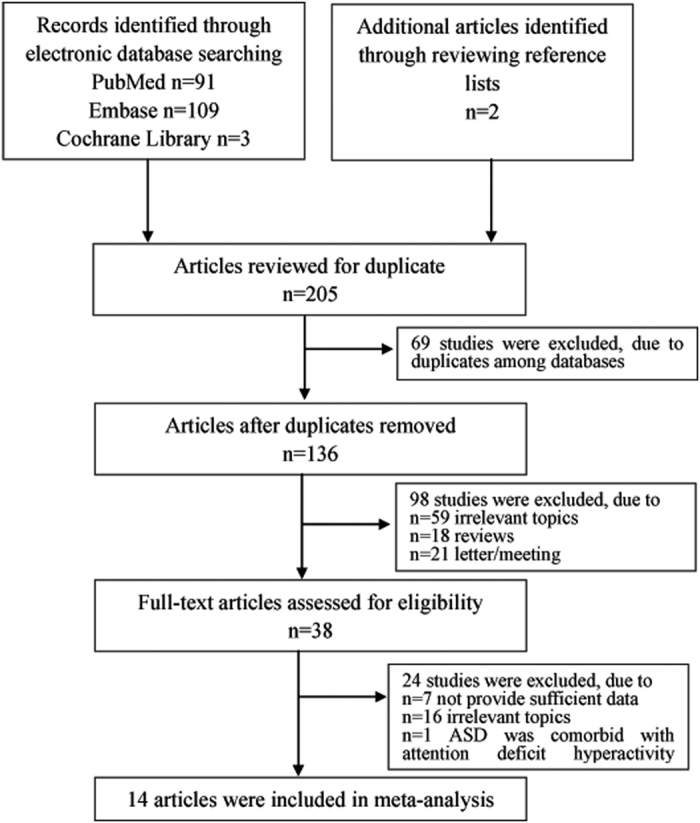
The initial search yielded a total of 205 citations: 91 from PubMed, 109 from Embase, 3 from Cochrane Library and 2 from reviewing references. After excluding 69 duplicate studies, 59 with irrelevant topics, 18 reviews and 21 letters/meetings, 38 studies of peripheral BDNF levels in ASD were identified and subjected to detailed evaluation. Subsequently, 16 studies were excluded due to irrelevant outcomes. Seven reports that lacked sufficient data (raw data mean and standard deviation (SD) or median and interquartile range (IQR)) were also excluded. One report was excluded because the ASD was comorbid with attention deficit hyperactivity disorder. Finally, 14 studies, including 2,707 participants and 1,131 incident cases, fulfilled all of the inclusion criteria and were included in the meta-analysis. A detailed flow chart of the search and selection process is presented in Fig. 1.
Figure 1. Flow chart of the study selection process to identify studies eligible for the systematic review.
Study characteristics
The characteristics of the fourteen selected studies are presented in Table 1. All of the studies were published between 2001 and 2016. 14 studies including 2,707 participants and 1,131 incident cases were included in this meta-analysis. Three studies were conducted in the United States18,19,20, three in China16,21,22, three in Japan13,23,24, one in Denmark17, one in Ireland14, one in Saudi Arabia25, one in India26, and one in Italy15. The sample sizes varied widely, ranging from 1813,24 to 35917 ASD individuals and from 1624 to 74117 controls. Similarly, the mean age of the ASD and control individuals varied broadly and ranged from 017,18,19,20 to 22.2 ± 2.213 years old. The systematic review identified two different biomaterials used for BDNF assays: serum13,15,16,17,18,19,20,21,22,23,24,25,26 and plasma14. Moreover, 11 studies assessed the BDNF levels using enzyme-linked immunosorbent assay (ELISA) as the analytical procedure13,14,15,16,20,21,22,23,24,25,26, while 3 adopted Luminex17,19,20 and 1 used recycling immunoaffinity chromatography (RIAC)18.
Table 1. Characteristics of the fourteen studies included in the meta-analysis.
| Author Year | Country | Sample size ASD controls | Age Mean ± SD (range) ASD controls | Sex (F/M) ASD controls | Analytical technology | Biomaterial | BDNF Mean ± SD ASD controls | Unit of measure | Adjusted founders |
|---|---|---|---|---|---|---|---|---|---|
| Abdallah17 | Denmark | 359741 | 00 | 68/291146/595 | Luminex | Serum | 6.77 ± 3.466.94 ± 3.51 | ng/ml | Age, gender |
| Correia14 | Ireland | 14650 | 7.17.5 | nr | ELISA | Plasma | 40.44 ± 13.8723.26 ± 12.34 | ng/ml | nr |
| Croen19 | America | 84159 | 00 | nr | Luminex | Serum | 0.0541 ± 0.0400.0537 ± 0.048 | ng/ml | Age, gender |
| Halepoto25 | Saudi Arabia | 6025 | 6 ± 1.727.04 ± 1.74 | nr | ELISA | Serum | 0.392 ± 0.2430.290 ± 0.162 | ng/ml | Age, gender |
| Hashimoto13 | Japan | 1818 | 21.2 ± 2.122.2 ± 2.2 | 0/180/18 | ELISA | Serum | 25.6 ± 2.1561.6 ± 10.9 | ng/ml | Age |
| Kasarpalkar26 | India | 4829 | 7.47.4 | nr | ELISA | Serum | 250.56 ± 92.7225.16 ± 79.54 | ng/ml | Age |
| Katoh-Semba23 | Japan | 56218 | <60<60 | nr | ELISA | Serum | 0.359 ± 0.2770.331 ± 0.187 | ng/ml | Age |
| Miyazaki24 | Japan | 1816 | 7.6 ± 6.123.3 ± 0.9 | 1/1711/5 | ELISA | Serum | 25.22 ± 2.4517.5 ± 2.00 | ng/ml | nr |
| Nelson18 | America | 6954 | 00 | 8/6127/27 | RIAC | Serum | 0.0374 ± 0.01990.0133 ± 0.005 | ng/ml | nr |
| Nelson20 | America | 2720 | 00 | nr | Luminex and ELISA | Serum | 3.404 ± 1.1313.299 ± 0.844 | ng/ml | Mean gestational age, birth weight |
| Ricci15 | Italy | 2929 | 2–212–21 | 2/272/27 | ELISA | Serum | 0.728 ± 0.4550.351 ± 0.347 | ng/ml | Age, gender |
| Wang16 | China | 7575 | 4.0 ± 1.254.0 ± 1.25 | 13/6213/62 | ELISA | Serum | 17.59 ± 5.5511.21 ± 2.79 | ng/ml | Age, gender |
| Zhang21 | China | 6060 | 3.78 ± 1.223.78 ± 1.22 | 12/4812/48 | ELISA | Serum | 17.6 ± 5.711.5 ± 3.1 | ng/ml | Age, gender |
| Meng22 | China | 8282 | 4.02 ± 1.274.02 ± 1.27 | 17/6517/65 | ELISA | Serum | 17.75 ± 5.4311.49 ± 2.85 | ng/ml | Age, gender |
ASD: autism spectrum disorder; nr: not reported; F/M: female/male; RIAC: recycling immunoaffinity chromatography; ELISA: enzyme-linked immunosorbent assay.
Meta-analysis of peripheral BDNF levels in ASD
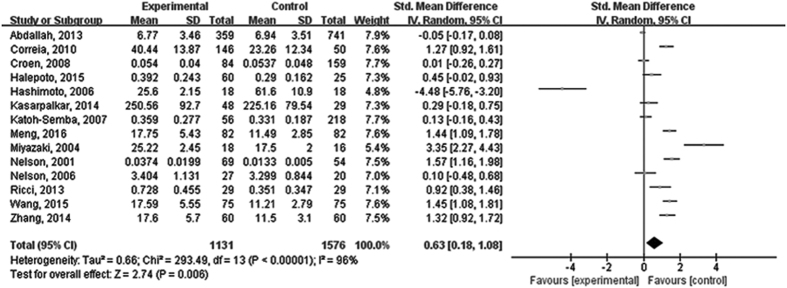
Based on estimates pooled from 14 studies, a significantly higher level of BDNF was found in ASD compared with controls [standardized mean difference (SMD) = 0.63, 95% CI = 0.18–1.08; P = 0.006)]. However, there was significant statistical heterogeneity across studies (I2 = 96%, P < 0.001) (Fig. 2).
Figure 2. Forest plot of random-effect between-group meta-analysis of peripheral BDNF levels in persons with ASD and healthy controls.
ASD: autism spectrum disorder.
Quality evaluation
The results of the quality assessment of the included studies are shown in Table 2. Fourteen studies were of high quality, with an average score of 7.4.
Table 2. Quality assessment of the included studies based on the Newcastle–Ottawa Scale.
| Publication year | Study design | Selection | Comparability | Exposure/Outcome | Total scores |
|---|---|---|---|---|---|
| Abdallah17 | Cross-section | ★★★★ | ★★ | ★★ | 8 |
| Correia14 | Cross-section | ★★★ | ★ | ★★ | 6 |
| Croen19 | Case-control | ★★★★ | ★★ | ★★ | 8 |
| Halepoto25 | Cross-section | ★★★★ | ★★ | ★★ | 8 |
| Hashimoto13 | Cross-section | ★★★ | ★★ | ★★ | 7 |
| Kasarpalkar26 | Cross-section | ★★★★ | ★★ | ★★ | 8 |
| Katoh-Semba23 | Cross-section | ★★★ | ★★ | ★★ | 7 |
| Miyazaki24 | Cross-section | ★★★★ | ★ | ★★ | 7 |
| Nelson18 | Case-control | ★★★★ | ★ | ★★ | 7 |
| Nelson20 | Case-control | ★★★★ | ★★ | ★★ | 8 |
| Ricci15 | Cross-section | ★★★ | ★★ | ★★ | 7 |
| Wang16 | Cross-section | ★★★★ | ★★ | ★★ | 8 |
| Zhang21 | Cross-section | ★★★★ | ★★ | ★★ | 8 |
| Meng22 | Cross-section | ★★★ | ★★ | ★★ | 7 |
Publication bias
The funnel plot was symmetric (Fig. 3). Moreover, Begg’s and Egger’s tests did not reveal significant evidence of publication bias among the included studies (Begg’s test, P = 1.000; Egger’s test, P = 0.156).
Figure 3. Funnel plot of random-effect between-group meta-analysis of peripheral BDNF levels in persons with ASD and healthy controls.
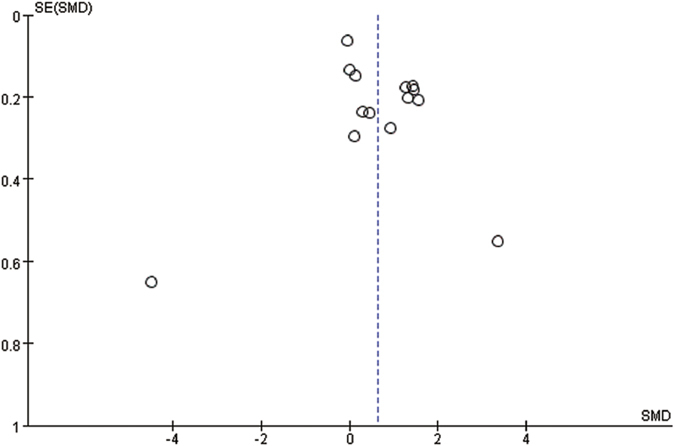
ASD: autism spectrum disorder.
Subgroup analysis
Of the fourteen studies included in this meta-analysis, thirteen described BDNF levels assessed in serum. One study reported result assessed in plasma. Higher BDNF levels were found in ASD compared with controls in both subgroups of studies. The pooled SMD was 0.58 (95% CI = 0.11–1.04, P = 0.02) for BDNF levels assessed in serum and 1.27 (95% CI = 0.92–1.61, P < 0.001) for BDNF levels assessed in plasma (Table 3).
Table 3. Summary results of peripheral BDNF levels in persons with ASD and healthy controls.
| Variables | No. of comparisions | No. of subjects |
Meta-analysis |
Heterogeneity |
Test for subgroup differences |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD | Controls | SMD | 95% CI | P-value | I2 | P-value | I2 | P-value | |||
| Biomaterial | |||||||||||
| Serum | 13 | 985 | 1526 | 0.58 | 0.11 | 1.04 | 0.02 | 96 | <0.001 | 81.6 | 0.02 |
| Plasma | 1 | 146 | 50 | 1.27 | 0.92 | 1.61 | <0.001 | Not applicable | Not applicable | ||
| Condition | |||||||||||
| ASD | 5 | 538 | 974 | 0.64 | 0.1 | 1.19 | 0.02 | 92 | <0.001 | 0 | 0.82 |
| autism | 9 | 593 | 602 | 0.55 | −0.03 | 1.13 | 0.06 | 94 | <0.001 | ||
| Subject age | |||||||||||
| Childhood | 10 | 1010 | 1295 | 0.78 | 0.31 | 1.26 | 0.001 | 96 | <0.001 | 0 | 0.42 |
| Adulthood | 4 | 121 | 281 | 0.04 | −1.72 | 1.8 | 0.97 | 97 | <0.001 | ||
| Analytical technology | |||||||||||
| ELISA | 11 | 619 | 622 | 0.66 | 0.12 | 1.2 | 0.02 | 94 | <0.001 | 96.6 | <0.001 |
| Luminex | 3 | 470 | 920 | −0.03 | −0.14 | 0.08 | 0.56 | 0 | 0.84 | ||
| RIAC | 1 | 69 | 54 | 1.57 | 1.16 | 1.98 | <0.001 | Not applicable | Not applicable | ||
| Subject age & analytical technology | |||||||||||
| Childhood | |||||||||||
| ELISA | 7 | 498 | 341 | 0.93 | 0.54 | 1.33 | <0.001 | 85 | <0.001 | 97.2 | <0.001 |
| Luminex | 3 | 470 | 920 | −0.03 | −0.14 | 0.08 | 0.56 | 0 | 0.84 | ||
| RIAC | 1 | 69 | 54 | 1.57 | 1.16 | 1.98 | <0.001 | Not applicable | Not applicable | ||
| Adulthood | |||||||||||
| ELISA | 4 | 121 | 281 | 0.04 | −1.72 | 1.8 | 0.97 | 97 | <0.001 | Not applicable | Not applicable |
ASD: autism spectrum disorder; No.: number; CI: confidence interval; RIAC: recycling immunoaffinity chromatography; ELISA: enzyme-linked immunosorbent assay.
We conducted a meta-analysis of the five studies of ASD and the nine studies of autism. The pooled SMD was 0.64 (95% CI = 0.1–1.19, P = 0.02) for the studies of ASD and 0.55 (95% CI = −0.03–1.13, P = 0.06) for the studies of autism (Table 3).
We conducted a meta-analysis of the ten studies of childhood and the four studies of adulthood. The pooled SMD was 0.78 (95% CI = 0.31–1.26, P = 0.001) for the studies of childhood and 0.04 (95% CI = −1.72–1.80, P = 0.97) for the studies of adulthood (Table 3).
We also conducted a meta-analysis of the studies based on the analytic technology used: Luminex, ELISA or RIAC. The effect size of the difference in BDNF levels measured in ASD and controls when these different analytical technologies were applied was 0.66 (95% CI = 0.12–1.2, P = 0.02) for ELISA, −0.03 (95% CI = −0.14–0.08, P = 0.56) for Luminex, and 1.57 (95% CI = 1.16–1.98, P < 0.001) for RIAC (Table 3).
Meta-analysis of the studies based on both subject age and the analytical technology used showed that the pooled SMD was 0.93 (95% CI = 0.54–1.33, P < 0.001) for the studies of childhood and 0.04 (95% CI = −1.72–1.8, P = 0.97) for the studies of adulthood when applying ELISA. The pooled SMD was −0.03 (95% CI = −0.14–0.08, P = 0.56) for the studies of childhood and used Luminex as the analytical method. The pooled SMD was 1.57 (95% CI = 1.16–1.98, P < 0.001) for the study of childhood and employed RIAC as the analytical method (Table 3).
Meta-regression analysis
We performed meta-regression analyses in an exploratory attempt to identify the sources of heterogeneity between the studies and the effect of moderators. Using univariable meta-regression models, we found a positive relationship between gender and BDNF levels (slope = 0.06, 95% CI = 0.01–0.11; P = 0.024). There was no relationship between the mean age, study design or confounders adjustment and BDNF levels (Table 4).
Table 4. Meta-regression of peripheral BDNF levels in persons with ASD and healthy controls.
| Moderator | No. of comparisions | No. of subjects |
Meta-regression |
Proportion of variance explained | ||||
|---|---|---|---|---|---|---|---|---|
| ASD | Controls | Slope | 95% CI | P-value | R2 analog | |||
| Age (mean, years) | 12 | 1046 | 1329 | −0.1028 | −0.285 | 0.079 | 0.237 | 4.66 |
| Gender (% male) | 8 | 710 | 1075 | 0.0628 | 0.0117 | 0.114 | 0.024 | 58.18 |
| Study design | 14 | 1131 | 1576 | 0.248 | −1.9765 | 2.4726 | 0.812 | 0 |
| Confounders adjustment | 14 | 1131 | 1576 | −1.8329 | −3.992 | 0.326 | 0.089 | 19.18 |
ASD: autism spectrum disorder; No.: number; CI: confidence interval.
Sensitivity analysis
The influence of each study on the overall estimate was assessed by removing studies one by one and comparing the pooled estimate from the remaining thirteen studies to the pooled estimate from all fourteen studies. The results revealed higher peripheral BDNF levels in ASD compared with controls in all 14 analyses, indicating that the removal of any one study would not alter the overall results.
Discussion
Over the fourteen studies, 2,707 participants and 1,131 incident cases were included in this meta-analysis. A random-effect model was used to compute the pooled estimates because there was significant between-study heterogeneity. The pooled SMD indicated that the peripheral BDNF level was higher in ASD compared to the controls. Sensitivity analysis showed that the pooled results were robust. The symmetrical funnel plot and the results of Begg’s and Egger’s tests also suggested the lack of significant publication bias.
BDNF, which is the most abundant neurotrophin in the CNS, can cross the blood-brain barrier. Therefore, its levels in serum and plasma are highly correlated with the levels in cerebrospinal fluid (r = 0.8)10,27. Studies have shown that BDNF protein levels in serum and the brain are similar in developmental rats, with a positive correlation between serum and cortical BDNF levels10. In addition, Klein et al.28 reported a significant positive correlation between whole blood and hippocampal BDNF levels in rats. Furthermore, they demonstrated that blood and plasma BDNF levels also reflected brain-tissue BDNF levels in rats and pigs. However, BDNF levels in blood are undetectable in other species, such as mice. Additionally, evidence of such a correlation in humans is still lacking. Therefore, whether altered BDNF values in the periphery reflect altered BDNF levels in the human CNS requires further investigation.
Interestingly, we found that peripheral BDNF levels were higher in the ASD samples from childhood but not from adulthood. Evidence from animal models of ASD suggests that BDNF levels increase in the foetal brain29,30. In the ASD model of sodium valproate (VPA) exposure in utero, VPA administration has been shown to increase BDNF protein levels in the feotal mouse brain 5- to 6-fold in vitro and in vivo29. Higher BDNF expression in the foetal brain was also demonstrated in the BTBR T+tf/J ASD model30. BDNF hyperactivity during early life may play an aetiological role in ASD. Early BDNF hyperactivity could result in the overgrowth of brain tissue, which is found in many ASD children31. Increased BDNF levels in children with ASD may reflect a regional compensatory mechanism in response to late brain maturation32. Therefore, accurately detecting BDNF levels is important to analyse the brain development in children with ASD.
Different analytical technologies for the assessment of BDNF were used across the studies. Four studies of adulthood and seven studies of childhood used ELISA to analyse BDNF levels. BDNF levels were found to be significantly increased in the childhood studies but not in the adulthood studies, further demonstrating the difference in peripheral BDNF levels between the age groups. Furthermore, subgroup analyses revealed that BDNF levels were significantly higher in children with ASD compared with controls when the levels were measured through ELISA and RIAC, but not when they were measured using Luminex. Therefore, the ELISA and RIAC assays may have been more sensitive than the Luminex assay in detecting BDNF levels in the included studies.
Significant heterogeneity was found in this analysis. To clarity the sources of heterogeneity and make a more comprehensive analysis, we performed the subgroup analyses and meta-regression. We found that three different factors contributed to the heterogeneity. First, the positive SMD is significantly higher in plasma-based samples than that in serum-based samples (Table 3). This difference may due to the difference of clotting factors between plasma and serum. Second, that different analytic technologies used in this study may also contribute to heterogeneity (Table 3). Finally, the gender factor especially for the percentage of male was also one of the reasons for the heterogeneity (Table 4).
Our study had some advantages. First, this is the first comprehensive meta-analysis conducted to assess the association between peripheral BDNF levels and ASD. Second, the sensitivity analysis indicated that the removal of individual studies did not alter the final results, which increased the robustness of the conclusions of this analysis. Third, no significant publication bias was detected, suggesting that the results are reliable.
However, our meta-analysis also had some limitations. Firstly, detailed information regarding medication use was not provided in some studies. Medication could have influenced peripheral BDNF levels in ASD. Thus, future research should take into consideration the possible effect of medication on peripheral BDNF levels. Secondly, the potential correlation between the severity of ASD and BDNF levels was not assessed because few studies have analysed the relationship between the clinical severity of ASD and BDNF levels. The association between the severity of ASD and BDNF should be evaluated in future studies. Thirdly, the meta-analysis of BDNF levels in ASD generated a pooled result that largely originated from cross-sectional studies. Therefore, we cannot draw any conclusions about causality. We do not know if an increase in BDNF levels is a cause or consequence of ASD development. Fourthly, there was significant heterogeneity in this analysis, which may have affected the precision of the overall results.
In conclusion, this meta-analysis indicated that peripheral BDNF levels are higher in ASD compared with controls, suggesting that peripheral BDNF levels may serve as a potential biomarker for the diagnosis of ASD. Future studies need to clarify the influence of medication, the causal nature of the relationship and the association between the severity of clinical ASD symptoms and BDNF levels.
Methods
Literature search
Two authors searched the PubMed, Embase and Cochrane Library databases for relevant articles published before February 2016 using both Medical Subject Heading (MeSH) terms and the free text terms “ASD,” “autism,” “autism spectrum disorder,” “autistic disorder,” “Asperger syndrome,” “pervasive developmental disorder,” and “BDNF,” “brain-derived neurotrophic factor,” and “peripheral,” “levels,” “serum,” “plasma,” “urine,” “saliva,” “blood,” “platelets,” “cerebrospinal fluid,” “red blood cells.” In addition, the references of the included articles and previous meta-analyses were manually searched to identify additional studies.
We restricted the search to human studies published in English. The titles and abstracts of the retrieved studies were reviewed to exclude studies that were clearly irrelevant. Then, two authors independently read the full text of the remaining studies to assess their eligibility according to the inclusion criteria. Disagreements about the inclusion/exclusion of a study were resolved by a third author, who independently examined the studies, and consensus was reached.
Study selection
Studies were eligible for analysis if they met all of the following criteria: (1) they were about the association between peripheral BDNF levels and ASD in vivo; and (2) they provided the raw data mean and SD or median and IQR.
The following types of studies were excluded: (1) reviews, case reports, case-only studies, animal studies, and simple commentaries; (2) overlapping publications; (3) publications lacking measures of peripheral BDNF levels, including pharmacological, genetic, brain imaging, and post-mortem studies; (4) studies in which ASD was comorbid with other conditions; and (5) studies that showed BDNF levels in dot plot and histogram format but did not provide numerical results.
Data extraction
Two authors extracted data from the included articles, with particular regard to the following: first author’s name, publication year, country of region, number of cases and controls, age of subjects (mean ± SD), percentage of females and males, analytical technology employed, biomaterial evaluated, BDNF level (mean ± SD), unit of measure and adjusted confounders. BDNF levels were measured from serum and plasma, and different units of measurement were used across studies (ng/ml or pg/ml). Therefore, we reported all BDNF levels in ng/ml (1 ng/ml = 1000 pg/ml). If the data were presented using the median (IQR) format, then the formula “IQR/1.35” was used to calculate SD. If participants overlapped between studies, the study with the largest sample size was included in the meta-analysis.
Quality evaluation
Two authors independently assessed the quality of each included study using the Newcastle-Ottawa Quality Assessment Scale (NOS) to determine the quality of selection, comparability, exposure, and outcome of study participants, with a maximum score of 9 points. We divided the study quality into three categories: (1) high quality (scored 7–9); (2) moderate quality (scored 4–6); and (3) low quality (scored 0–3). Disagreements were resolved through mutual discussion.
Statistical analysis
The SMD was used to assess the association between peripheral BDNF levels and ASD. We pooled the SMD across studies using the Mantel-Haenszel formula (fixed-effect model) or the DerSimonian-Laird formula (random-effect model). A fixed-effect model was chosen when heterogeneity was low; otherwise, a random-effect model was adopted. Across-study heterogeneity was evaluated using the I2 and Q statistics; these statistics provide a quantitative measure of inconsistency across studies, with suggested thresholds for low (25–50%), moderate (50–75%) and high (>75%) heterogeneity. The Q statistic was considered significant if P < 0.1, and I2 > 50% indicated high heterogeneity. The results of the analyses are shown in forest plots.
Potential publication bias was assessed via visual inspection of the funnel plot. Begg’s and Egger’s tests were used to estimate the severity of publication bias, with P < 0.05 considered statistically significant.
We analysed subgroups of studies to examine the source of potential heterogeneity based on the biomaterial used (serum or plasma), condition (ASD or autism) and subject age (childhood < 18 years old or adulthood ≥18 years old). The analytical technologies employed included Luminex, ELISA and RIAC.
Unrestricted maximum likelihood random effects meta-regressions of effect size were performed with mean age, gender (% male), study design and confounder adjustment as moderators to determine whether these covariates influenced the effect size.
We carried out the sensitivity analysis by removing studies one by one and comparing the SMD of the remaining studies to the SMD for all studies. Statistical analysis was performed using Stata 12.0 (Stata Corp, College Station, Texas, USA) and Cochrane Collaboration Review Manager 5.1.2 (Cochrane Collaboration, Oxford, UK) software.
Additional Information
How to cite this article: Zheng, Z. et al. Peripheral brain-derived neurotrophic factor in autism spectrum disorder: a systematic review and meta-analysis. Sci. Rep. 6, 31241; doi: 10.1038/srep31241 (2016).
Acknowledgments
This work was supported by the National Science Foundation of China (No. 81330016 to Dezhi Mu and No. 81270724 to Yi Qu), a grant from the Major State Basic Research Development Program (2013CB967404), grants from the Ministry of Education of China (313037, IRT0935), a grant from the State Commission of Science Technology of China (2012BAI04B04), grants from the Science and Technology Bureau of Sichuan Province (2014SZ0149, 2016TD0002), and a grant from the clinical discipline program (neonatology) of the Ministry of Health of China (1311200003303).
Footnotes
Author Contributions Z.Z. and L.Z. designed the study and wrote the manuscript. T.Z. performed the literature searches and collected the data. J.H. and Y.Q. conducted the statistical analysis. D.M. revised the manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association (2013). [Google Scholar]
- Wingate M. et al. Prevalence of autism spectrum disorders-autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 61, 1–19 (2012). [PubMed] [Google Scholar]
- Matelski L. &Van de Water J. Risk factors in autism: Thinking outside the brain. J Autoimmun. 67, 1–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M. & Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 350; doi: 10.1126/science.aab3897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S., Cardy J. O. & Zwaigenbaum L. Differentiating autism spectrum disorder from other developmental delays in the first two years of life. Dev Disabil Res Rev. 17, 130–140 (2011). [DOI] [PubMed] [Google Scholar]
- Wan M. W. et al. Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. J Child Psychol Psychiatry. 54, 763–771 (2013). [DOI] [PubMed] [Google Scholar]
- Walsh P., Elsabbagh M., Bolton P. & Singh I. Insearch of biomarkers for autism: scientific, social and ethical challenges. Nat RevNeurosci. 12, 603–612 (2011). [DOI] [PubMed] [Google Scholar]
- Binder D. K. & Scharfman H. E. Brain-derived neurotrophic factor. Growth Factors. 22, 123–131 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T. & Michel T. M. The role of neurotrophic factors in autism. Mol Psychiatry. 16, 478–490 (2011). [DOI] [PubMed] [Google Scholar]
- Karege F., Schwald M. & Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 328, 261–264 (2002). [DOI] [PubMed] [Google Scholar]
- Fernandes B. S. et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. 20, 1108–1119 (2015). [DOI] [PubMed] [Google Scholar]
- Taurines R. et al. Altered peripheral BDNF mRNA expression and BDNF protein concentrations in blood of children and adolescents with autism spectrum disorder. J Neural Transm (Vienna). 121, 1117–1128 (2014). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 30, 1529–1531 (2006). [DOI] [PubMed] [Google Scholar]
- Correia C. T. et al. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 9, 841–848 (2010). [DOI] [PubMed] [Google Scholar]
- Ricci S. et al. Altered cytokine and BDNF levels in autism spectrum disorder. Neurotox Res. 24, 491–501 (2013). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. Increased serum levels of brain-derived neurotrophic factor in autism spectrum disorder. Neuroreport. 26, 638–641 (2015). [DOI] [PubMed] [Google Scholar]
- Abdallah M. W. et al. Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr Scand. 128, 61–69 (2013). [DOI] [PubMed] [Google Scholar]
- Nelson K. B. et al. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 49, 597–606 (2001). [PubMed] [Google Scholar]
- Croen L. A. et al. Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Res. 1, 130–137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G. et al. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int J Dev Neurosci. 24, 73–80 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang Q. B., Jiang L. F., Kong L. Y. & Lu Y. J. Serum Brain-derived neurotrophic factor levels in Chinese children with autism spectrum disorders: a pilot study. Int J Dev Neurosci. 37, 65–68 (2014). [DOI] [PubMed] [Google Scholar]
- Meng W. D. et al. Elevated serum brain-derived neurotrophic factor (BDNF) but not BDNF gene Val66Met polymorphism is associated with autism spectrum disorders. Mol Neurobiol. doi: 10.1007/s12035-016-9721-9 (2016). [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R. et al. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int J Dev Neurosci. 25, 367–372 (2007). [DOI] [PubMed] [Google Scholar]
- Miyazaki K. et al. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 26, 292–295 (2004). [DOI] [PubMed] [Google Scholar]
- Halepoto D. M., Bashir S., Zeina R. & Al-Ayadhi L. Y. Correlation between Hedgehog (Hh) protein family and brain-derived neurotrophic factor (BDNF) in autism spectrum disorder (ASD). J Coll Physicians Surg Pak. 25, 882–885 (2015). [PubMed] [Google Scholar]
- Kasarpalkar N. J., Kothari S. T. & Dave U. P. Brain-derived neurotrophic factor in children with autism spectrum disorder. Ann Neurosci. 21, 129–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F. et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 109, 143–148 (2002). [DOI] [PubMed] [Google Scholar]
- Klein A. B. et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 14, 347–353 (2011). [DOI] [PubMed] [Google Scholar]
- Almeida L. E., Roby C. D. & Krueger B. K. Increased BDNF expression in fetal brain in the valproic acid model of autism. Mol Cell Neurosci. 59, 57–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. R. et al. Altered expression levels of neurodevelopmental proteins in fetal brains of BTBR T+tf/J mice with autism-Like behavioral characteristics. J Toxicol Environ Health A. 78, 516–523 (2015). [DOI] [PubMed] [Google Scholar]
- Lainhart J. E. & Lange N. Increased neuron number and head sizein autism. JAMA. 306, 2031–2032 (2011). [DOI] [PubMed] [Google Scholar]
- Shim S. H. et al. Increased levels of plasma brain-derived neurotrophic factor (BDNF) in children with attention deficit-hyperactivity disorder (ADHD). Prog Neuropsychopharmacol Biol Psychiatry. 32, 1824–1828 (2008). [DOI] [PubMed] [Google Scholar]