Abstract
Objective
Risk-reducing salpingo-oophorectomy (RRSO) is recommended for women with BRCA mutation due to increased risk of pelvic serous carcinoma. Serous tubal intraepithelial carcinoma (STIC) is a pathologic finding of unknown clinical significance. This study evaluates the clinical outcome of patients with isolated STIC.
Materials/Methods
We retrospectively reviewed the medical records of consecutive patients with a germline BRCA1/2 mutation or a high-risk personal or family history of ovarian cancer who underwent RRSO between January 2006 and June 2011. All patients had peritoneal washings collected. All surgical specimens were assessed using the sectioning and extensively examining the fimbria protocol, with immunohistochemistry when indicated. p53 signature lesions and secretory cell outgrowths were excluded.
Results
Of 593 patients who underwent RRSO, isolated STIC was diagnosed in 12 patients (2%). Five patients (42%)were BRCA1 positive, 5 patients (42%)were BRCA2 positive, and 2 patients (17%) had high-risk family history. Preoperatively, all patients with STIC had normal CA-125 levels and/or pelvic imaging results. Seven patients underwent hysterectomy and omentectomy, 6 patients (46%) had pelvic node dissections, and 5 patients (39%) had para-aortic node dissections. With the exception of positive peritoneal washings in 1 patient, no invasive or metastatic disease was identified. No patient received adjuvant chemotherapy. At median follow-up of 28 months (range, 16–44 months), no recurrences have been identified.
Conclusions
Among the cases of isolated STIC after RRSO reported in the literature, the yield of surgical staging is low, and short-term clinical outcomes are favorable. Peritoneal washings are the most common site of disease spread. Individualized management is warranted until additional data become available.
Keywords: Serous tubal intraepithelial carcinoma, Prophylactic salpingo-oophorectomy, Ovarian carcinoma
As early as 1969, investigators postulated amultifocal origin of ovarian carcinoma, suggesting that the genesis of these tumors may occur outside the ovary and even potentially arise from the fallopian tube.1–5 However, it was not until recently that the fallopian tube specifically came to be seen as a leading potential site of origin for pelvic serous carcinomas.6,7 Some of the data supporting this include the identification of pathologic abnormalities in the fallopian tubes after risk-reducing salpingo-oophorectomy (RRSO) for germline BRCA mutation carriers, and the similarity of p53 mutations in concurrent serous tubal intraepithelial carcinoma (STIC) and invasive serous carcinoma.
Women with germline BRCA mutations are recommended to undergo RRSO to decrease their risk of pelvic serous carcinoma. It has been shown that occult fallopian tube carcinomas are more common in RRSO specimens than are occult ovarian carcinomas.8 Immunohistochemical techniques (particularly p53 and Mib1) have allowed for closer examination of the fallopian tube from which distinct patterns of abnormalities have been identified. One such abnormality is STIC. Diagnosis of STIC is made with a combination of morphologic and immunohistochemical evaluation. Morphologic features include increased nuclear to cytoplasmic ratio, enlarged nuclei with prominent nucleoli, lack of ciliated cells, loss of polarity, and a complete lack of stromal invasion. Immunohistochemical stains supporting a diagnosis of STIC are p53 (overexpressed or null phenotype) and Ki-67. Serous tubal intraepithelial carcinomas have been defined by these histologic findings and can be distinguished from p53 signature and serous tubal intraepithelial (STIL) lesions, which carry some but not all of the unique histologic features of STIC.9,10 In addition, fallopian tube abnormalities, including STIC, have been reported with greater frequency when the sectioning and extensively examining the fimbria (SEE-FIM) protocol, a more detailed sectioning of the tubal fimbriated end, is completed.8,11–14 SEE-FIM, developed at Brigham and Women’s Hospital, entails fixation of the entire tube and longitududinal sectioning of the distal 2 cm of the fibriated end of the fallopian tube into 4 pieces followed by serial sectioning every 2 to 3 mm of the entire length of the fallopian tube. SEE-FIM ensures a comprehensive evaluation of the fallopian tube; and in studies where it has been used, the detection rate for STIC is higher.15
To date, the literature on STIC has focused on the incidence of the finding, not clinical follow-up. The prognostic significance of isolated STIC after RRSO and the next steps in management remain undefined. The open lumen of the fallopian tube into the peritoneal cavity raises a concern that cells could exfoliate from the fallopian tube, implant on a peritoneal surface, and develop into a pelvic serous carcinoma. A direct mechanism through which STIC leads to invasive serous carcinoma has yet to be defined. Pertinent questions regarding need for surgical staging, frequency with which invasive lesions are identified from staging procedures, and whether there is benefit to adjuvant chemotherapy have not been answered. The purpose of the current study was to identify the rate of isolated STIC among patients with RRSO after the adoption of the SEE-FIM protocol at Memorial Sloan-Kettering Cancer Center in 2006, assess the clinical outcome of these cases, and review the literature reported to date. The data will help guide management of STIC lesions identified at RRSO.
MATERIALS AND METHODS
After obtaining approval from the Memorial Sloan-Kettering Cancer Center Institutional Review Board, clinical and pathologic databases were queried to identify all patients with a diagnosis of STIC between January 2006 and June 2011. All patients were planned for RRSO with or without hysterectomy, at the discretion of the surgeon and the patient. The procedures were performed with traditional laparoscopy, robotic-assisted laparoscopy and, rarely, via laparotomy. Patients with a concurrent diagnosis of pelvic serous carcinoma (endometrial, ovarian, fallopian tube, or primary peritoneal) were excluded. The medical, operative, and pathology records of the identified patients were reviewed. Data were collected including demographics, genetic testing results, preoperative assessment, pathology results, postoperative testing, follow-up recommendations, and recurrence details.
At the time of the pathologic review, all specimens were assessed using the SEE-FIM protocol. Serous tubal intraepithelial carcinoma was defined using a combination of morphologic evaluations to distinguish it from p53 signatures, STIL, and invasive carcinoma. Immunohistochemistry was performed only when nuclear atypia was present, and a diagnosis of STIC was considered based on review of sections stained by hematoxylin and eosin. Morphologic considerations included the following: nuclear/cytoplasmic ratio, nuclear pleomorphism, epithelial stratification with loss of polarity, irregular epithelial thickness, and exfoliation of cells into the tubal lumen. Immunohistochemical stains included p53 and Mib-1. Elevated Mib-1 (>15% nuclear cell staining) and abnormal p53 staining (null phenotype or >60% nuclear cell staining) were used as supportive evidence of the diagnosis. All histologic evaluations were performed by pathologists with advanced training in gynecologic pathology and reviewed at the gynecologic pathology division conference to determine a consensus diagnosis.
RESULTS
During the study period, 593 patients underwent RRSO for known BRCA mutation or high-risk personal or family history of ovarian carcinoma. Patients who underwent bilateral salpingo-oophorectomy exclusively for hormonal treatment of breast cancer or who had an abnormal imaging or CA-125 test result preoperatively were excluded. Of the 593 patients, 189 patients (31.9%) carried germline BRCA1 mutations, 186 patients (31.4%) carried germline BRCA2 mutations, 18 patients (3%) carried BRCA mutations of unknown significance, 104 patients (17.5%) were BRCA negative, and 94 patients (15.9%) had not undergone genetic testing. There were 2 patients (0.3%) with germline BRCA mutations documented in clinic notes, but there were no mutation details available. The SEE-FIM protocol for RRSO was fully implemented by January 2006, and the first subsequent diagnosis of STIC was made in July 2006. Twelve patients with isolated STIC were identified (Table 1). The overall frequency of STIC in patients who underwent RRSO was 2%. When categorized according to BRCA status, 5 (2.65%) of 189 women with germline BRCA1 mutations were found to have a STIC lesion, 5 (2.69%) of 186 BRCA2 carriers and 0 (0%) of 104 patients negative for BRCA germline mutations. In the untested population (94 women), 2 women (2.13%) had a diagnosis of a STIC lesion.
TABLE 1.
Patients’ characteristics
| Patients with STIC | 12 |
|---|---|
| Age, mean (SD), yrs | 54 (39–76) |
| Germline BRCA mutation, n (%) | |
| BRCA1 | 5 (42) |
| BRCA2 | 5 (42) |
| BRCA unknown | 2 (17) |
| Preoperative assessment, n (%) | |
| CA125 | 9 (75) |
| Pelvic imaging | 10 (83) |
| STIC location, n (%) | |
| unilateral | 10 (83) |
| bilateral | 2 (17) |
| single focus | 9 (75) |
| multifocal | 3 (25) |
| Peritoneal washings positive, n (%) | |
| Yes | 1 (8) |
| No | 11 (92) |
| Follow-up, median (range), mos | 28 (16–44) |
The 12 patients with isolated STIC at the time of RRSO had a mean age of 54 years (range, 39–77 years). Five women were carriers of a BRCA1 mutation; 4 women had a BRCA2 mutation; 1 woman had a BRCA2 rearrangement; and for 2 patients, the BRCA status was unknown. The 2 women of unknown BRCA status both had high-risk personal and family histories for ovarian carcinoma. Both had a personal history of bilateral breast cancer and a family history of breast and ovarian cancer. Ten (83%) of the 12 women with STIC identified at RRSO had preoperative CA-125 testing. All values were normal for the reference laboratory, ranging from5 to 29U/mL; 6 patients (50%) had a CA-125 level of less than 10 U/mL. Both of the women who did not have preoperative CA-125 testing had CA-125 assayed after the time of RRSO; in both cases, the results were less than 10 U/mL. Ten (83%) of the patients with STIC identified at RRSO had preoperative ultrasound, and all results were normal. All patients with STIC at the time of RRSO had either a normal CA-125 level or imaging preoperatively.
Risk-reducing salpingo-oophorectomy was performed laparoscopically in 10 (83%) of the 12 patients, and robotically in 2 patients (17%). Serous tubal intraepithelial carcinoma was identified bilaterally in 2 patients (17%) and unilaterally in 10 patients (83%). Additionally, the STICs were characterized as multifocal in 3 women (25%) and unifocal in 9 women (75%). One woman had a concurrent serous adenofibroma, and 1 woman had evidence of endosalpingiosis. No endometriosis was seen. Washings were performed in all 12 patients and were negative in 11 (92%) cases. Ten patients (83%) underwent dilation and curettage, and all specimens were benign. No other procedures were performed at the time of RRSO. Four patients had additional postoperative imaging, and none showed evidence of metastatic disease. The 2 women who did not have preoperative imaging are among those who had postoperative imaging.
With the exception of 1 patient whose condition was diagnosed early during the study period, all patients were recommended to undergo a staging procedure. Three women declined surgical staging, and 1 woman elected to have her staging procedure with a local provider. The remaining 7 women underwent staging at Memorial Sloan-Kettering Cancer Center. Three procedures were performed with standard laparoscopy, and 4 procedures were performed with robotic assistance. All 7 women underwent hysterectomy, omentectomy, and peritoneal washings. Of the 7 staging procedures, pelvic and para-aortic nodal dissection was performed in 5 cases and pelvic node dissection alone was performed in 1 case (14%). A mean of 17 (range, 4–34) pelvic nodes and 10 (range, 7–19) paraaortic nodes were removed. Peritoneal biopsies were taken in 6 patients (86%) and diaphragm biopsies in 3 patients (43%). All surgical staging results were benign, with the exception of 1 patient with peritoneal washing suspicious for carcinoma; this finding was consistent with the cytology read from the washings performed at the time of RRSO.
No recommendation for adjuvant chemotherapy was made to any of the patients. Follow-up recommendations were based on discussion with individual patients and included combinations of CA-125 testing, imaging, and office visits. Eleven patients have available follow-up data. After a median follow-up of 28 months (range, 16–44 months), there have been no recurrences noted. One patient had a diagnosis of pancreatic carcinoma 32 months after RRSO and died of her disease 2 months later.
Given the variation of STIC lesions identified, 2 patients warranted additional clarification of clinical outcome due to the extent of their histologic findings (Table 2). One of these, patient 32, is a 44-year-old woman with a personal and family history of breast cancer who had a diagnosis of a BRCA1 185delAG mutation 3 months before her RRSO. Before surgery, she had a CA-125 level of 29 U/mL and had not had any imaging. She was found to have bilateral STIC with multiple foci in each tube. Her staging procedure included hysterectomy, omentectomy, and bilateral pelvic lymph node dissection, as well as biopsies of the pelvic and diaphragm peritoneum. All specimens were benign. She is now 19 months from diagnosis, and her CA-125 level has declined to 6 U/mL. She remains without evidence of disease and is under observation with semiannual physician visits. The patient with positive peritoneal washings (patient 30) is a 77-year-old woman with a personal history of breast cancer who had a BRCA2 6174delT mutation identified before RRSO. Pathologic findings revealed STIC in multiple foci on a single fallopian tube and positive washings. Her CA-125 level was 15 U/mL. She returned to the operating room for staging, at which time she had washings, hysterectomy, omentectomy, pelvic peritoneal biopsies, and resection of an umbilical nodule. All pathologic specimens were benign, but the washings were suspicious, not diagnostic, for adenocarcinoma. After counseling regarding her options, the patient elected to proceed with observation consisting of office visits and CA-125 assays every 3 months, with extension of the visit interval anticipated. At her most recent evaluation, 16 months after RRSO, her CA-125 level was 20 U/mL, her CT scan demonstrated severe diverticulosis, and her PET scan was negative for metastatic disease.
TABLE 2.
Reported cases of patients with a diagnosis of STIC at RRSO
| Patient Number |
Institution | Age | BRCA Status |
Procedures | Nodal Sampling |
Washings | Adjuvant Treatment (No. cycles) |
Follow-Up |
|---|---|---|---|---|---|---|---|---|
| 1 | A8 | 64 | BRCA1 | TAH, BSO, omentectomy | None | Negative | Unknown | Unknown |
| 2 | B12 | 61 | BRCA1 | BSO | None | Negative | None | 38 m ned |
| 3 | B | 48 | BRCA1 | BSO | None | Negative | None | 7 m ned |
| 4 | B | 49 | BRCA1 | TAH, USO | None | None | None | 87 m ned |
| 5 | C14 | 44 | BRCA1 | TAH, BSO | None | Negative | Carboplatin/ paclitaxel |
Median PFS, 36 months |
| 6 | C | 44 | BRCA2 | TAH, BSO, “staging” | Unknown | Positive | Carboplatin/ paclitaxel |
|
| 7 | C | 66 | BRCA2 | TAH, BSO, staging | Unknown | Negative | Carboplatin/ Paclitaxel |
|
| 8 | D16–18 | 52 | BRCA1 | BSO | Unknown | Negative | None | Ned at surgery |
| 9 | D | 46 | BRCA1 | BSO | Unknown | Negative | None | ned at surgery |
| 10 | D | 53 | BRCA1 | BSO | None | Negative | None | 99 m ned |
| 11 | D | 45 | BRCA1 | BSO | None | Negative | None | 80 m ned |
| 12 | D | 47 | BRCA1 | LAVH, BSO | None | Positive | Carboplatin/ paclitaxel (6) |
150 m ned |
| 13 | D | 46 | BRCA2 | BSO | None | Negative | Carboplatin/ Paclitaxel (3) |
58 m ned |
| 14 | D | 65 | BRCA2 | LAVH, BSO | None | Negative | Carboplatin/ paclitaxel (3) |
138 m ned |
| 15 | E19,20 | 48 | BRCA1 | BSO | None | Negative | None | Ned at surgery |
| 16 | E | 40 | BRCA1 | BSO | None | Negative | None | Ned at surgery |
| 17 | E | 67 | BRCA1 | BSO | None | Negative | None | Ned at surgery |
| 18 | E | 43 | BRCA1 | TLH, BSO, omentectomy | None | Positive | None | 40 m ned |
| 19 | E | 60 | BRCA2 | BSO | None | Suspicious | None | 24 m ned |
| 20 | E | 60 | BRCA2 | BSO | None | Negative | None | 12 m ned |
| 21 | E | 60 | unknown high risk family history |
BSO | None | Negative | None | 48 m ned |
| 22 | E | 44 | unknown high risk family history |
BSO | None | Negative | None | Ned at surgery |
| 23 | E | 55 | unknown high risk family history |
TLH, BSO, omx | None | Positive | None | 28 m ned |
| 24 | F20 | 44 | BRCA1 | BSO | None | None | None | Primary peritoneal carcinoma, 72 m |
| 25 | G21 | 57 | BRCA2 | Unknown | Unknown | Negative | 26 m ned | |
| 26 | G | 50 | BRCA2 | Unknown | Unknown | Negative | 8 m ned | |
| 27 | G | 56 | BRCA2, variant of unknown significance |
Unknown | Unknown | Negative | 2 m ned | |
| 28 | H18,22,23 | 51 | BRCA1 | BSO, omental biopsies | None | Negative | None | 88 m ned |
| 29 | H | 52 | BRCA1 | BSO, omental biopsies | None | Positive | None | 101 m ned |
| 30 | H | 50 | BRCA1 | BSO, omental biopsies | None | Negative | None | 64 m ned |
| 31 | H | 43 | BRCA2 | BSO, omental biopsies | None | Negative | None | 47 m ned |
| 32 | I18 | 73 | BRCA1 | no staging | None | Negative | None | 133 m ned |
| 33 | I | 54 | BRCA1 | no staging | None | Negative | None | 40 m ned |
| 34 | I | 49 | BRCA1 | no staging | None | Negative | None | Primary peritoneal carcinoma, 43 m |
| 35 | I | 61 | BRCA1 | staging with nodes | Yes | Negative | None | 99 m ned |
| 36 | I | 58 | BRCA1 | staging with nodes | Yes | Negative | None | 78 m ned |
| 37 | I | 76 | BRCA2 | staging without nodes | None | Negative | None | 75 m ned |
| 38 | J | 44 | BRCA1 | TAH, omentectomy, PLND, biopsies | Yes | Negative | None | 19 m ned |
| 39 | J | 39 | BRCA1 | TAH, omentectomy, PPALND | Yes | Negative | None | 20 m ned |
| 40 | J | 65 | BRCA1 | TAH, omentectomy, PPALND, biopsies | Yes | Negative | None | 29 m ned |
| 41 | J | 44 | BRCA1 | TAH, omentectomy, PPALND, biopsies | Yes | Negative | None | 30 m ned |
| 42 | J | 45 | BRCA1 | TAH, omentectomy, PPALND, biopsies | Yes | Negative | None | 26 m ned |
| 43 | J | 67 | BRCA2 | BSO | None | Negative | None | 33 m ned |
| 44 | J | 50 | BRCA2 | Offered, declined | None | Negative | None | 34 m ned |
| 45 | J | 46 | BRCA2 | Offered, declined | None | Negative | None | 20 m ned |
| 46 | J | 68 | BRCA2 | Performed at outside hospital | None | Negative | None | 41 m ned |
| 47 | J | 77 | BRCA2 | TAH, omentectomy, biopsies | None | Positive | None | 16 m ned |
| 48 | J | 60 | unknown high risk family history |
offered, declined | None | Negative | None | 23 m ned |
| 49 | J | 47 | unknown high risk family history |
TAH, omentectomy, PPALND, biopsies |
Yes | Negative | None | 44 m ned |
DISCUSSION
The data presented here support overall favorable short-term clinical outcomes for patients with STIC: no recurrences and no evidence of distant disease at the time of subsequent surgical staging. Until now, most information regarding the clinical course of patients with STIC has been embedded in articles regarding the frequency of occult malignancy at the time of RRSO, with limited clinical outcomes data. Nine main series, in some cases presented in more than one publication to allow for updates, provide clinical information about the 37 previously published cases of STIC (Table 2).8,12,14,16–23 There are additional publications on frequency of STIC, which do not provide follow-up information and are not included in this discussion.
In the current report, the incidence of STIC lesions identified at the time of RRSO was found to be 2%; this is consistent with prior reports in the literature, in which the incidence ranges from 0.6% to 7%. The differences seen among these reports could be easily attributed to statistical variation of small sample sizes and a rare event. However, more concrete components of study design and patient population are likely contributory. For example, BRCA mutation was an inclusion criteria for most but not all of the prior reports; for some, high-risk personal and family history was sufficient for inclusion.11,16,17,19,20,24 As demonstrated by Manchanda et al, the risk of an occult lesion is lower among patients without documented BRCA mutation. In this series, no patients known to be BRCA negative were found to have a STIC lesion. Similarly, none of the previously published 37 cases of isolated STIC were patients known to be BRCA negative. In one case, a 56-year-old woman with a personal history of breast cancer at the age of 28 had a BRCA2 mutation of unknown significance (ALA2306Pro). Age is a risk factor for the development of malignancy in patients with BRCA mutations; hence, the guiding recommendation is that patients with BRCA1 and BRCA2 mutations undergo RRSO between the ages of 35 and 40 after completion of childbearing. Patients who undergo risk-reducing surgery at a later age have a higher incidence of STIC on final pathology.16 Powell et al recently compared the age at diagnosis of STIC to the age at diagnosis of an occult carcinoma and found that patients with STIC were in fact older than those with an invasive carcinoma. In each of the reported studies, the mean or median age was between 46 and 51 years, with patients completing RRSO anywhere from age 30 to 76 years. In addition, the extent to which the fallopian tube is examined can affect the frequency of finding occult histologic changes. Our current report reflects the rate of STIC in which all RRSO specimens were examined with the SEE-FIM protocol, in contrast to the other publications included in Table 3.15,25
TABLE 3.
Clinical reports on STIC at RRSO
| Author | No. RRSO Cases |
No. STIC Cases |
Age Range (Years) |
Inclusion Criteria | Pathology Protocol |
|---|---|---|---|---|---|
| Lamb*16 | 113 | 4 (3.5%) | 47–65 | BRCA mutation carrier or high-risk personal/family history |
2–3 mm sectioning for 23/30 patients |
| Carcangiu12 | 50 | 3 (6%) | 48–61 | BRCA mutation carrier | 2–12 slides reviewed per tube or ovary, for 20 patients the tubes and ovaries were submitted in their entirety |
| Powell23 | 111 | 4 (3.6%) | 43–52 | BRCA mutation carrier | Rigorous pathology protocol |
| Callahan14 | 122 | 3 (2.5%) | 44–66 | BRCA mutation carrier | 2–3 mm sectioning for patients before 2/2005, SEE-FIM 2/2005 and after |
| Finch8 | 159 | 1 (0.6%) | 64 | BRCA mutation carrier | 2 mm sectioning for 91/159 patients |
| Manchanda19,20 | 308 | 9 (3%) | 40–67 | BRCA mutation carrier or high risk personal/family history |
2–3 mm sectioning for all patients |
| Wethington (current study) |
593 | 12 (2%) | 39–77 | BRCA mutation carrier or high-risk personal/family history |
SEE-FIM |
| Total | 1456 | 36 (2%) | 39–77 |
Cataloging the clinical course of these patients to increase understanding and inform future clinical recommendations is critical. Combining this series of 12 patients and the 37 previously published cases, there is clinical information regarding 49 patients in the published literature. Two patients reported in the literature have developed a primary peritoneal carcinoma (PPC). The first case has not been formally published in a series on RRSO but was described in an editorial on the topic. She had a germline BRCA1 mutation and developed PPC 6 years after RRSO. At the time of RRSO, she had no peritoneal washing assessment and the pathologic examination of the fallopian tubes was not done with SEE-FIM. After her diagnosis of PPC, the RRSO specimen was retrospectively examined with a more detailed sectioning and revealed a single focus of STIC.20 The second patient is the only person who has developed PPC after diagnosis of an isolated STIC at the time of RRSO. She was 49 at the time of RRSO. She had a BRCA1 mutation and developed PPC43 months after the RRSO after an elevation in CA-125 level was noted. She was treated with primary debulking surgery and chemotherapy and is currently alive without evidence of disease 16 months later. The numbers here are too small to draw conclusions; however, this rate of PPC, 2% (1 of the 48 women reported in a case series), is in line with published literature reporting 1% to 4% risk of developing primary peritoneal cancer after RRSO.8,26
A variety of clinical approaches to the care of patients after RRSO have been described. As is the case for previous reports, this series is limited by a small cohort and individualized treatment plans. Although a median follow-up of 28 months might be considered short, the literature on PPC after RRSO suggests that a high-risk time period is in the first 2 years.21,27–29 A challenge lies in developing recommendations for appropriate next steps once the diagnosis of STIC has been made, in particular, the role of surgical staging, chemotherapy, and surveillance.
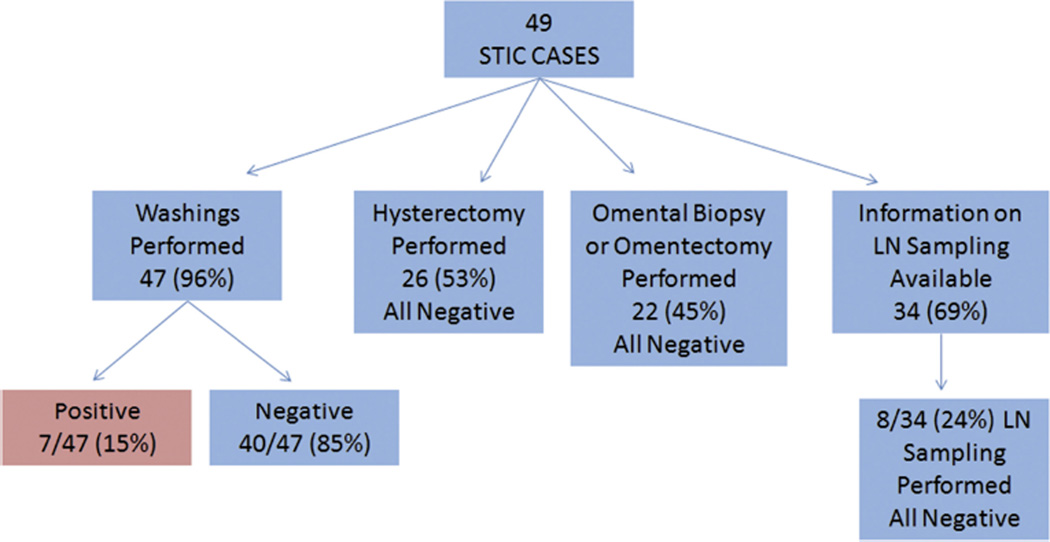
Of the 49 patients reported in the literature (including the current series), 47 patients (96%) had washings obtained, and 26 patients (53%) underwent hysterectomy. Twenty-two patients (45%) underwent omental biopsy or omentectomy, with no invasive disease identified in the omental specimen. For the 34 women whose information regarding lymph node sampling was available, 8 women (24%) had lymph nodes removed and none were positive. Complete information on the extent of surgical staging is available for 27 patients (Fig. 1). Some publications indicate patients underwent “staging,” but the authors do not provide the details regarding what was included as part of the staging procedure, specifically regarding peritoneal biopsies, omentectomy, and lymph node dissection. Washings are the one component of the evaluation, along with complete removal of both tubes and ovaries, which was consistently performed. The importance of washings is supported by the aggregate data; 15% of the patients were found to have positive washings at the time of RRSO. Thus, we support peritoneal washings as a component of all RRSO procedures. Some series include hysterectomy as part of risk-reducing surgery to ensure that all tubal tissue is excised. As data increasingly demonstrate, the distal tube, and not the interstitial component, is the primary site of both STIC and occult invasive carcinomas. Thus, hysterectomy may not be required at the time of RRSO for pelvic serous carcinoma prevention. However, it may be prudent to consider hysterectomy at the time of RRSO for other issues pertinent to the gynecologic history. Owing to the small number of cases in the literature and the significant institutional variation, there is limited room for broad conclusions to be made regarding the necessity for, and the most appropriate extent of, surgical staging after the diagnosis of STIC at the time of RRSO. Individualized patient decisions should include the extent of peritoneal cavity assessment at the time of RRSO, results of washings, and whether or not the outcome of a staging procedure would change recommendations for adjuvant therapy.
FIGURE 1.
Cumulative staging information for STIC cases identified with RRSO.
Treatment recommendations for patients with STIC, positive washings, and no other evidence of invasive malignancy at time of RRSO are important. As stated, 7 reported cases (15%) had positive washings, with the remainder of the surgical pathology without evidence of metastatic disease. Among these patients, 2 patients received chemotherapy and 4 patients did not. Of the 2 patients who received chemotherapy, only 1 patient had a surgical staging procedure. The parameters of the staging procedure are not clearly defined. Follow-up on the 7 patients with positive washings and no other evidence of disease ranges from 16 to 150 months, and there have been no recurrences. Of note, there were 4 patients with STIC and negative washings who received adjuvant chemotherapy. At a median follow-up of 58 months, no documented recurrences have been reported (Table 2). Chemotherapy was given by 1 institution to all patients in their cohort regardless of washings. The presence of positive washings implies circulating malignant cells in the peritoneal cavity, prompting some institutions to offer adjuvant chemotherapy. However, in the absence of invasive disease, particularly if there has been a negative staging procedure, observation remains a reasonable option and obviates the potential for chemotherapy-induced adverse effects.
Finally, our study examined the use of disease surveillance schedules. The data from the publication of Powell et al and this series seem to be the only 2 reports with details as to the follow-up recommendations made to patients.14,18,23,25 The options for follow-up include annual visit with review of systems and examination, tumor marker assays (CA-125 and/or HE4), and imaging (ultrasound, computed tomographic scan, and magnetic resonance imaging). We agree with Powell et al that although no screening system has been found that successfully identifies patients with primary peritoneal cancer before diffuse spread of disease, patients with STIC identified at the time of RRSO fall into a risk category that necessitates continued surveillance, which should be determined at the discretion of the patient and oncologist but could include a combination of review of systems, physical examination, and CA-125 testing.
In the coming decade, the number of women who have undergone RRSO with SEE-FIM will likely increase and additional cases of STIC with clinical follow-up will be reported. In parallel, molecular characterization of STIC, and investigation into its relevance to the pathogenesis of pelvic serous carcinomas will contribute additional pertinent and potentially prognostic information. In the meantime, the body of data currently available is presented here and emphasizes the association of isolated STIC at the time of RRSO with germline BRCA mutation, the importance of obtaining peritoneal washings (as they are positive in 15% of the cases), and the overall favorable short-term outcomes for patients with isolated STIC at the time of RRSO.
Acknowledgments
The Project Hope for Ovarian Cancer Research and Education funded a portion of this research.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Bannatyne P, Russell P. Early adenocarcinoma of the fallopian tubes. A case for multifocal tumorigenesis. Diagn Gynecol Obstet. 1981;3:49–60. [PubMed] [Google Scholar]
- 2.Pauerstein CJ. Pathophysiology of the fallopian tube. Clin Obstet Gynecol. 1974;17:89–119. doi: 10.1097/00003081-197406000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Russell P. The pathological assessment of ovarian neoplasms. III: The malignant “epithelial” tumours. Pathology. 1979;11:493–532. doi: 10.3109/00313027909059027. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff JD, Julian CG. Multiple malignancy in the upper genital canal. Am J Obstet Gynecol. 1969;103:810–822. doi: 10.1016/0002-9378(69)90579-1. [DOI] [PubMed] [Google Scholar]
- 5.Yeung HH, Bannatyne P, Russell P. Adenocarcinoma of the fallopian tubes: a clinicopathological study of eight cases. Pathology. 1983;15:279–286. doi: 10.3109/00313028309083506. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Vang R, Junge J, et al. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–1614. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 9.Agoff SN, Mendelin JE, Grieco VS, et al. Unexpected gynecologic neoplasms in patients with proven or suspected BRCA-1 or-2 mutations: implications for gross examination, cytology, and clinical follow-up. Am J Surg Pathol. 2002;26:171–178. doi: 10.1097/00000478-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Vang R, Visvanathan K, Gross A, et al. Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;31:243–253. doi: 10.1097/PGP.0b013e31823b8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agoff SN, Garcia RL, Goff B, et al. Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol. 2004;28:1112–1114. doi: 10.1097/01.pas.0000131554.05732.cd. [DOI] [PubMed] [Google Scholar]
- 12.Carcangiu ML, Peissel B, Pasini B, et al. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol. 2006;30:1222–1230. doi: 10.1097/01.pas.0000202161.80739.ac. [DOI] [PubMed] [Google Scholar]
- 13.Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 15.Rabban JT, Krasik E, Chen LM, et al. Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am J Surg Pathol. 2009;33:1878–1885. doi: 10.1097/PAS.0b013e3181bc6059. [DOI] [PubMed] [Google Scholar]
- 16.Lamb JD, Garcia RL, Goff BA, et al. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol. 2006;194:1702–1709. doi: 10.1016/j.ajog.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Leeper K, Garcia R, Swisher E, et al. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol. 2002;87:52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 18.Powell CB, Swisher EM, Cass I, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol. 2013;129:364–371. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manchanda R, Abdelraheim A, Johnson M, et al. Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. BJOG. 2011;118:814–824. doi: 10.1111/j.1471-0528.2011.02920.x. [DOI] [PubMed] [Google Scholar]
- 20.Manchanda R, Drapkin R, Jacobs I, et al. The role of peritoneal cytology at risk-reducing salpingo-oophorectomy (RRSO) in women at increased risk of familial ovarian/tubal cancer. Gynecol Oncol. 2012;124:185–191. doi: 10.1016/j.ygyno.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Reitsma W, de Bock GH, Oosterwijk JC, et al. Support of the ‘fallopian tube hypothesis’ in a prospective series of risk-reducing salpingo-oophorectomy specimens. Eur J Cancer. 2013;49:132–141. doi: 10.1016/j.ejca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Powell CB. Occult ovarian cancer at the time of risk-reducing salpingo-oophorectomy. Gynecol Oncol. 2006;100:1–2. doi: 10.1016/j.ygyno.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Powell CB, Chen LM, McLennan J, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer. 2011;21:846–851. doi: 10.1097/IGC.0b013e31821bc7e3. [DOI] [PubMed] [Google Scholar]
- 24.Paley PJ, Swisher EM, Garcia RL, et al. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176–180. doi: 10.1006/gyno.2000.6071. [DOI] [PubMed] [Google Scholar]
- 25.Rabban JT, Barnes M, Chen LM, et al. Ovarian pathology in risk-reducing salpingo-oophorectomies from women with BRCA mutations, emphasizing the differential diagnosis of occult primary and metastatic carcinoma. Am J Surg Pathol. 2009;33:1125–1136. doi: 10.1097/PAS.0b013e31819e986a. [DOI] [PubMed] [Google Scholar]
- 26.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacha OM, Gregoire J, Grondin K, et al. Effectiveness of risk-reducing salpingo-oophorectomy in preventing ovarian cancer in a high-risk French Canadian population. Int J Gynecol Cancer. 2012;22:974–978. doi: 10.1097/IGC.0b013e318257b936. [DOI] [PubMed] [Google Scholar]
- 28.Finkelman BS, Rubinstein WS, Friedman S, et al. Breast and ovarian cancer risk and risk reduction in Jewish BRCA1/2 mutation carriers. J Clin Oncol. 2012;30:1321–1328. doi: 10.1200/JCO.2011.37.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]