Abstract
Objective
This study was designed to characterize and compare the maternal and newborn epidemiological characteristics through analysis of environmental factors, socio-demographic characteristics, and clinical characteristics between the different clinical subtypes of preterm birth (PTB): Idiopathic (PTB-I), premature rupture of the membranes (PTB-PPROM) and medically indicated (PTB-M). The two subtypes PTB-I and PTB-PPROM grouped are called spontaneous preterm births (PTB-S).
Methods
A retrospective, observational study was conducted in 1.291 preterm non-malformed singleton live-born children to nulliparous and multiparous mother’s in Tucumán-Argentina between 2005 and 2010. Over 50 maternal variables and ten newborn variables were compared between the different clinical subtypes. The comparisons were done to identify heterogeneity between subtypes of preterm birth: (PTB-S) vs. (PTB-M), and within spontaneous subtype: (PTB-I) vs. (PTB-PPROM). In the same way, two conditional logistic multivariate regressions were used to compare the odds ratio (OR) between PTB-S and PTB-M, as well as PTB-I and PTB-PPROM. We matched for maternal age when comparing maternal variables and gestational age when comparing infant variables.
Results
The PTB-I subtype was characterized by younger mothers of lower socioeconomic status, PTB-PPROM was characterized by environmental factors resulting from inflammatory processes, and PTB-M was characterized by increased maternal or fetal risk pregnancies.
Conclusions
The main risk factor for PTB-I and PTB-M was having had a prior preterm delivery, however previous spontaneous abortion was not a risk factor, suggesting a reproductive selection mechanism.
Keywords: preterm birth, clinical subtypes, epidemiological features, pregnancy
1. Introduction
Preterm birth (PTB) is considered a complex and heterogeneous trait resulting from the interplay of several genetic and environmental factors[1,2].
The greatest single risk factor for delivering a preterm neonate is a history of a prior PTB[3]. The role of other proposed predictors such as maternal and/or genetic factors, environment, or of a synergy or interaction between these remains unknown[4].
Preterm birth can be categorized by its clinical presentation: idiopathic (PTB-I), premature rupture of membranes (PTB-PPROM) and medically induced (PTB-M). The clinical subtypes PTB-I and PTB-PPROM are together designated spontaneous preterm birth (PTB-S)[5,6]. Given the heterogeneity within clinical subtypes of preterm birth, it is helpful to examine the subtypes separately in terms of etiology and management[7,8,9]. In addition, identifying specific characteristics of each clinical subtype is essential to employing effective prevention methods of preterm birth[10].
Many studies have investigated the differences between subtypes of PTB, using term deliveries as a control. The intention of this work was not to estimate population relative risk, but to compare demographic and genetics components that provide clues to the heterogeneity and etiology between clinical subtypes. Differences among these preterm birth subtypes have not been fully explored in Latin America and few epidemiological studies of clinical subtypes have been completed[11,12]. The purpose of the current study was to characterize and compare the maternal epidemiological characteristics through analysis of environmental factors, socio-demographic characteristics, and clinical characteristics between the different clinical subtypes.
2. Methods
2.1. Study Population
This retrospective, observational study was conducted in women delivering preterm singleton live-born children without malformations at Nuestra Señora de la Merced Maternity Hospital in Tucumán-Argentina between July 2005 and December 2010. This study is part of an international collaborative project aimed at elucidating etiological genetic factors involved in PTB through candidate gene evaluation and DNA sequencing[13].
More than 80% of all newborns in the Tucumán province are delivered at Nuestra Señora de la Merced. The premature delivery rate in the general population was 10.6% during the period of the study. The ethnic/race population was mostly South American Amerindian with Latin European admixture (98.7%)[14,15].
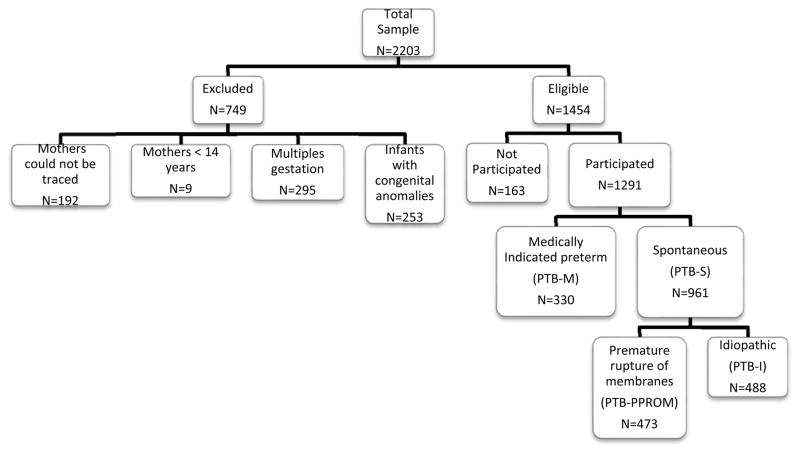
Premature infants, infants delivered at less than 37 weeks of gestational age, were identified using the daily delivery room records (births were registered within 24 hours or in the immediate postpartum period). During the study period, 2.203 preterm live-born children were identified. Of these children, 192 were excluded because they could not be traced (8.7%). An additional 253 (11.5%) infants were excluded due to congenital anomaly, 295 (13.4%) due to multiple gestations; and 9 (0.4%) due to maternal ageless than 14 years. Of the remaining 1.454 eligible cases, a total of 1.291 (88.8%) families agreed to participate in the study (Figure 1). All of the enrolled subjects signed an informed consent form that, together with the protocol, had been approved by the Centro de Educación Médica e Investigaciones Clínicas (CEMIC) Ethics Committee (IRB 00001745 – IORG 0001315) and the University of Iowa IRB (protocol 200411759).
Figure 1.
Data set selection from Nuestra Señora de la Merced Maternity Hospital (Tucumán-Argentina)
2.2. Data source and collection procedures
Women who agreed to participate were personally interviewed by a qualified team member from a large South-American hospital-based network (ECLAMC)[16]. The ECLAMC is a clinical- epidemiological research and surveillance program for birth defects in South America, since 1967, that involves a voluntary collaboration between a wide network of hospitals and health professionals.
The information collected was recorded on standardized antenatal, obstetrics, and neonatal clinical research forms. The forms included the standardized clinical perinatal record[17], ECLAMC clinical records[16], and a structured questionnaire designed to collect information on socio-demographics, clinical history, maternal reproductive history, obstetrical complications, and neonatal outcomes. All coded records were reviewed by the paediatricians and obstetricians involved in the study. The local monitor reviewed the clinical research forms or contacted the patient if data was missing or discordant.
2.3. Outcome definition and ascertainment
2.3.1. Preterm delivery
Preterm delivery was defined as a live-birth at less than 37 weeks of gestation[18]. The gestational age was estimated from the date of the last menstrual period (LMP). If the date of the LMP was uncertain, an ultrasound examination was performed before 22 weeks of estimated gestation to establish gestational age[19]. If an ultrasound estimate or LMP was not available, the case was excluded. In the event of a difference greater than seven days between the ultrasound result and the LMP, the former was used as the estimate for gestational age.
2.3.2. Clinical subtypes
Three clinical subtypes were identified in accordance with recently developed research guidelines for conducting genetic epidemiologic studies of PTB[7,20–21]:
preterm labor leading to PTB, called idiopathic PTB (PTB-I);
preterm premature rupture of membranes (PTB-PPROM), and
medically indicated iatrogenic PTB (PTB-M).
Data on the onset of labor were regularly registered in the clinical perinatal record by the obstetrician. Based on this categorization, preterm deliveries at the hospital were classified by researchers as:
PTB-I: preterm labor leading to PTB (idiopathic), when diagnosed as cervical change (dilation and effacement) due to regular uterine activity. A rupture of membranes was only included in this category when the rupture occurred during active labor.
PTB-PPROM: preterm premature rupture of the membranes diagnosed by pelvic examination, or obvious leakage of fluid from the cervix into the posterior fornix. If it was unclear whether PPROM had occurred, either a sample of fluid was examined by microscopy looking for “arborization” or an ultrasound was performed searching for oligohydramnios. Membrane rupture had to occur before the onset of labor regardless of the means of delivery.
PTB-M: a medical condition existed that required delivery before 37 weeks gestation (i.e. preeclampsia).
The first two subtypes (PTB-I and PTB-PPROM) are frequently grouped in one category called spontaneous preterm births (PTB-S).
Preterm births were classified into subtypes by two independent researchers. If there was a disagreement, the case was reviewed by the principal investigator.
2.4. Definition of Variables
Sociodemographic characteristics of the parents and clinical history of the mother
The following characteristics of the mother were analyzed: maternal and paternal age; maternal and paternal level of instruction (defined as low: incomplete primary school (less seven years of school education), medium: complete primary, but incomplete secondary school (7–12 years of school education) and high: complete secondary school at university (more than 12 years of school education)); marital status (single or married); cohabitation with current male partner; change of sexual partner between current and previous pregnancy; and number of previous pregnancies.
Lifestyle features
The following lifestyle characteristics were analyzed (all were yes/no responses): smoking before, during, or at the beginning of pregnancy; smoking in the family; alcohol consumption during pregnancy; alcohol consumption during the weekend; coffee intake during pregnancy; maternal employment status during pregnancy; psychological illness; physical recreational activities before and during of pregnancy; illicit drugs use; hypocaloric diet during pregnancy; and severe life events classified as described in Khashan et al.[22].
Pregnancy care and complications
The following variables were analyzed (all were yes/no responses unless otherwise indicated): low number of prenatal visits (<5), difficulty in conceiving, fertility treatment required to conceive, cesarean delivery, body weight gain (<6, 7–12 and >13 Kg), low pregnancy maternal body mass index (BMI <18.5 kg/m2), anemia during pregnancy, medication for anemia in the previous year, use of corticosteroids, asthma, medication during pregnancy, vitamins before pregnancy, surgery before and/or during pregnancy, vaginal bleeding, urinary tract infection, periodontal infections, thyroid disorders, tooth cavity, complications after prenatal diagnosis, diabetes, diagnosis of disease during pregnancy, sexually transmitted diseases (STDs), fibroma, hypertension-related disorders, preeclampsia, infection during pregnancy (indicated by vaginal discharge), positive chagas test, positive toxoplasmosis test, blood transfusion during pregnancy, and immunizations during pregnancy.
Reproductive History
All previous pregnancies were classified as either pregnancy loss (spontaneous abortion or still-birth) or live birth (preterm or term newborn). The reproductive history of the adverse outcome was based on the patient’s self-report and, in 70% of the cases, was confirmed by the mother’s clinical records.
Perinatal Outcomes
We retrieved neonatal outcome data, including gestational age (<28, 29–31, 32–33, 34–37 weeks)[23], birthweight (<1500, 1501–2500, >2501 grams), small for gestational age (SGA) (defined as birth weight less than the 10th percentile for gestational age), gender, and neonatal morbidities (sepsis, patent ductus arteriosus, intracranial hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, APGAR≤3 (1 minute and 5 minutes), mechanical ventilation, and death during hospitalization).
2.5. Statistical analysis
Analysis of variance (ANOVA) was used to compare means for maternal age, gestational age and birth weight between the clinical subtypes. Univariate comparisons were done to identify heterogeneity between subtypes of preterm birth: 1) spontaneous (PTB-S=PTB-I+PTB-PPROM) vs. medically indicated (PTB-M); and 2) within spontaneous subtype PTB-I vs. PTB-PPROM. For nominal variables we used a chi-square test with 2 degrees of freedom, and one degree of freedom for each comparison. In the same way, two conditional logistic multivariate regressions were used to compare the odds ratio (OR) between PTB-S and PTB-M, as well as PTB-I and PTB-PPROM. We matched by maternal age (in years) when comparing maternal variables, and matched by gestational age (in weeks) when comparing infant variables. The dependent variable in the conditional logistic model was preterm subtype (0=PTB-S, 1=PTB-M for the first comparison and 0=PTB-I, 1=PTB-PPROM for the second comparison). The independent variables included in the model were the 28 variables statistically significant at p<0.10 in univariate analysis (see Table 1). Given the number of variables analyzed, the strategy of choosing a significance level (< 0.10) was for balancing the amount of variables included in the regression model without losing relevant variables. By the same motive, the Bonferroni correction was not used. Robust standard errors were estimated to control outlier values. Variables that had more than 5% missing data were excluded to avoid problems with sample selection.
Table I.
Phenotype characteristics by clinical subtypes
| PTB-I n=473 |
PTB-PPROM n=488 |
PTB-M n=330 |
F | P(a) | |
|---|---|---|---|---|---|
| Mother’s age (years) X±SD | 22.9±5.5 | 24.5±6.2 | 26.9± 6.7* | 40.1 | <0.001 |
| Father’s age (years) X±SD | 26.2± 7.2 | 27.5 ±7.4 | 29.4± 7.4 | 17.9 | <0.001 |
|
| |||||
| Sociodemographic characteristics of the parents | |||||
|
| |||||
| PTB-I (%) | PTB-PPROM (%) | PTB-M (%) | X2 | P(b) | |
|
| |||||
| Maternal age | |||||
| <20 years | 32.77%* | 25.82% | 15.45% | 43.9 | <0.001 |
| 20–34 years | 64.48% | 66.8% | 74.24% | ||
| >34 years | 2.75% | 7.38% | 10.3% * | ||
| Mother’s level of education | |||||
| Low | 49.89% | 47.75% | 47.27% | 8.6 | 0.072** |
| Medium | 43.77% | 44.05% | 40.61% | ||
| High | 6.34% | 8.20% | 12.12% * | ||
| Father’s level of education | |||||
| Low | 57.29% | 56.97% | 54.77% | 7.4 | 0.116 |
| Medium | 39.11% | 38.73% | 38.48% | ||
| High | 3.62% | 4.34% | 7.69% | ||
| Number of previous pregnancy | |||||
| 1 | 23.04% | 23.77% | 17.27% | 15.9 | 0.003** |
| 2 | 13.11% | 10.45% | 12.73% | ||
| ≥ 3 | 21.7% | 28.28% | 34.55% * | ||
| Married | 15.22% | 15.37% | 28.18% * | 26.4 | <0.001** |
| Cohabitation with the partner | 77.59% | 76.43% | 82.12% | 1.3 | 0.511 |
| Changing paternity | 64.69% * | 55.33% | 50.91% | 9.3 | 0.009** |
|
| |||||
| Lifestyle features | |||||
|
| |||||
| Smoking | |||||
| Before pregnancy | 42.71% | 47.34% | 41.82% | 3.4 | 0.185 |
| During pregnancy | 21.78% | 24.39% | 20.61% | 1.8 | 0.402 |
| In the family | 41.86% | 44.88% | 44.24% | 1.0 | 0.597 |
| At the beginning of pregnancy | 23.26% | 25.00% | 21.52% | 1.3 | 0.502 |
| Alcohol consumption | |||||
| During pregnancy | 13.74% | 12.50% | 13.64% | 0.5 | 0.775 |
| During pregnancy at the weekend | 29.18% | 27.05% | 29.09% | 0.6 | 0.726 |
| Coffee intake during pregnancy | 51.37% | 52.66% | 52.12% | 0.2 | 0.905 |
| Illicit drugs use | 1.90% | 1.02% | 1.21% | 1.4 | 0.486 |
| Low calorie diet during pregnancy | 8.67% | 11.07% | 12.12% | 2.8 | 0.245 |
| Physical activities | |||||
| before pregnancy | 17.97% | 18.44% | 22.42% * | 2.9 | 0.224 |
| during pregnancy | 4.44% | 4.51% | 2.42% | 2.7 | 0.259 |
| Any employment work outside the home | 21.78% | 24.39% | 31.52% * | 10.1 | 0.006** |
| Psychological illness | 1.27% | 1.84% | 1.52% | 0.5 | 0.759 |
| Stress | 32.56% | 29.51% | 36.36% | 4.2 | 0.120 |
|
| |||||
| Pregnancy care and complications | |||||
|
| |||||
| Prenatal visits (<5) | 52.52% * | 48.10% | 35.05% | 27.5 | <0.001** |
| Use of corticosteroid | 41.23% | 59.02% * | 55.15% | 32.7 | <0.001** |
| Difficulty in conceiving | 8.88% | 10.45% | 13.94% * | 5.1 | 0.079** |
| Treatment to conceive | 1.9% | 2.05% | 3.03% | 2.0 | 0.362 |
| Cesarean deliveries | 11.85% | 26.95% | 98.5% * | 17.9 | <0.001** |
| Previous spontaneous abortions | 23.04% | 26.43% | 28.48% | 3.2 | 0.197 |
| Previous preterm birth | 22.41% | 18.85% | 25.15% | 4.8 | 0.091** |
| Anemia during pregnancy | 39.75% | 49.59% * | 43.33% | 7.0 | 0.030** |
| Anemia medication (previous year) | 6.13% | 9.22% | 6.06% | 4.7 | 0.094** |
| Asthma | 2.54% | 2.05% | 4.55% | 4.6 | 0.100 |
| Vaginal bleeding | 40.59% | 48.57% * | 35.76% | 13.9 | <0.001** |
| Tooth cavity | 78.22% | 78.07% | 76.36% | 0.6 | 0.726 |
| Complications after prenatal diagnosis | 1.48% | 1.23% | 3.33% | 5.3 | 0.072** |
| Periodontal infections | 56.66% | 49.18% | 54.55% | 5.2 | 0.072** |
| Urinary infection | 41.23% | 41.8% | 41.21% | 0.1 | 0.942 |
| Infection during pregnancy | 44.82% * | 39.14% | 36.36% | 6.1 | 0.049** |
| Diabetes | 0.21% | 0.61% | 0.61% | 1.0 | 0.592 |
| Diagnosis of some disease during pregnancy | 8.88% | 13.52% | 16.06% | 10.2 | 0.007** |
| STDs | 0.85% | 0.61% | 0.3% | 0.9 | 0.630 |
| Fibroma | 1.27% | 1.43% | 2.12% | 1.0 | 0.615 |
| Hypertensive disorders | 1.69% | 2.87% | 6.67% * | 15.2 | 0.001** |
| Preeclampsia | 2.20% | 2.61% | 7.08% * | 17.4 | 0.001** |
| Thyroid disorders | 2.54% | 3.48% | 1.21% | 4.1 | 0.127 |
| Chagas positive test | 0.63% | 2.25% * | 0.61% | 7.0 | 0.030** |
| Toxoplasmosis positive test | 28.96% | 28.07% | 29.09% | 0.9 | 0.629 |
| Medication during pregnancy | 79.28% | 79.30% | 80.91% | 0.7 | 0.706 |
| Vitamins before pregnancy | 3.81% | 6.15% | 3.33% | 4.6 | 0.099** |
| Surgery | |||||
| Before pregnancy | 5.29% | 3.89% | 4.85% | 1.1 | 0.574 |
| During pregnancy | 1.06% | 1.84% | 0.91% | 1.7 | 0.424 |
| Blood transfusion during pregnancy | 1.69% | 2.46% | 3.33% | 2.3 | 0.317 |
| Immunizations during pregnancy | 87.74% | 85.25% | 86.67% | 1.3 | 0.515 |
| Body weight gain | |||||
| ≤6 kg | 31.95% | 32.56% | 24.25% | 22.1 | <0.001** |
| 7–12 Kg | 49.43% | 46.05% | 43.19% | ||
| ≥13 kg | 18.62% | 21.4% | 32.56% * | ||
| BMI (<18.5 Kg/m2) | 14.67% | 13.52% | 10.03% | 3.3 | 0.188 |
|
| |||||
| Perinatal outcomes | |||||
|
| |||||
|
PTB-I n=473 |
PTB-PPROM n=488 |
PTB-M n=330 |
F | P (a) | |
|
| |||||
| Gestational age (weeks) X±SD | 32.91 ± 3.18 | 32.94± 2.93 | 33.15 ± 2.68 | 0.7 | 0.490 |
| Birth-weight (grs.)X±SD | 1879.56 ± 584.56 | 1853.77 ± 575.73 | 1803.89 ± 577.97 | 1.7 | 0.189 |
|
| |||||
| PTB-I (%) | PTB-PPROM (%) | PTB-M (%) | X2 | P(b) | |
|
| |||||
| SGA (<p10) | 25.37% | 31.15% | 45.45% * | 36.5 | <0.001** |
| Gestational age (weeks) | |||||
| <28 | 12.71% | 10.29% | 8.23% | 9.3 | 0.188 |
| 29–31 | 14.41% | 18.31% | 15.55% | ||
| 32–33 | 15.68% | 16.05% | 20.43% | ||
| 34–37 | 57.2% | 55.35% | 55.79% | ||
| Birth-weight | |||||
| <1500 | 27.48% | 28.89% | 33.03% | 5.4 | 0.094** |
| 1501–2500 | 58.35% | 60.25% | 54.24% | ||
| >2501 | 14.16% | 10.86% | 12.73% | ||
| Gender (male) | 54.33% | 52.25% | 48.18% | 2.9 | 0.226 |
| Retinopathy | 10.39% | 12.43% | 10.98% | 0.7 | 0.688 |
| Patent ductus arteriosus | 13.61% * | 6.63% | 11.95% | 9.9 | 0.007** |
| Mechanical ventilation requirement | 27.17%* | 21.35% | 33.12% * | 13.5 | 0.001** |
| Necrotizing Enterocolitis | 10.15% | 7.89% | 12.62% | 4.8 | 0.097** |
| Intercranial hemorrhage | 23.13% | 21.91% | 17.88% | 3.1 | 0.091** |
| Sepsis | 19.62% | 21.72% | 21.43% | 0.5 | 0.777 |
| Reanimation | 47.4% | 45.07% | 51.84% | 3.0 | 0.223 |
| Apgar (≤3) | |||||
| 1 minute | 92.81% | 93.03% | 93.03% | 0.02 | 0.989 |
| 5 minute | 96.83% | 98.57% | 98.18% | 3.67 | 0.159 |
| Mortality during hospitalization | 10.31% | 7.30% | 9.21% | 2.5 | 0.287 |
Note: PTB-I, preterm labor leading to PTB (idiopathic) ; PTB-PPROM, premature rupture of membranes ; PTB-M, medically indicated preterm ; N, number of cases for each clinical subtype; STDs, sexually transmitted diseases; BMI, body mass index; SGA, Small for gestational age.
P value for analysis of variance F test,
P value for Pearson chi-square test (2 degrees of freedom)
High frequency,
Independent variables (P values <0.10) included in the multivariate analysis
To compare the reproductive history between clinical subtypes, we created five maternal risk groups: 1) primigravidity, 2) multigravidity without previous adverse outcomes, 3) multigravidity with at least one previous preterm birth and no previous spontaneous abortions, 4) multigravidity with at least one previous spontaneous abortion and no previous preterm births, and 5) multigravidity with at least one previous preterm birth and one previous spontaneous abortion. The dependent variable was preterm subtype and the independent variables were the maternal risk groups, using multigravidity without previous adverses outcomes as a reference group. The analysis was adjusted for the number of previous pregnancies.
3. Results
During the study period, 1.291 preterm live-born children were identified that qualified for inclusion. Of the 1.291 cases, that participated in the study, 504 preterm singleton were from primigravidity and 787 from multigravidity mother’s. PTB-PPROM represents 37.9% of the sample (N=488), PTB-I, 36.6% (N=473), and PTB-M 25.5% (N=330).
Significant differences were observed in maternal and paternal age means between subtypes (F=40.1, p<0.001; F=17.9, p<0.001, respectively) with lowest age in PTB-I and highest in PTB-M.
3.1. Maternal Characteristics
3.1.1. PTB-S vs PTB-M
Mothers with PTB-M were characterized by the following variables: increased maternal age, greater degree of schooling, greater number of previous pregnancies, married, having a job outside of the house, difficulty conceiving, higher number of cesarean deliveries, increased frequency of arterial hypertension, increased frequency of preeclampsia and greater weight gain during pregnancy (Table I). When matched by maternal age in multivariate analysis, PTB-M mothers were often married, had increased frequency of arterial hypertension, increased frequency of preeclampsia, showed greater pregnancy weight gain (>13 Kg) and greater number of prenatal visits than PTB-S (Table II). The rest of the variables incorporated in the model (p < 0.10, see table I) did not reach statistical significance.
Table II.
Significant maternal and neonatal outcomes by clinical subtypes
| Maternal Characteristics (matched by maternal age) | ||||
|---|---|---|---|---|
| PTB-M vs PTB-S (Reference: Subtype PTB-S) | OR | 95% CI | P | |
| Married | 1.47 | 1.00 | 2.21 | 0.057* |
| Hypertensive disorders | 2.17 | 1.01 | 5.16 | 0.048* |
| Preeclampsia | 2.50 | 2.00 | 4.30 | 0.003* |
| Body weight gain (>13Kg) | 1.50 | 1.00 | 2.25 | 0.047* |
| Less than 5 antenatal care visits (<5) | 0.62 | 0.44 | 0.87 | 0.006* |
|
| ||||
| PTB-PPROM vs PTB-I (Reference: Subtype PTB-I) | OR | 95% CI | P | |
|
| ||||
| Use of corticosteroid | 1.63 | 1.08 | 2.65 | 0.019* |
| Vaginal bleeding | 1.79 | 1.21 | 2.67 | 0.004* |
| Infection during pregnancy | 0.59 | 0.40 | 0.85 | 0.008* |
|
| ||||
| Neonatal outcomes (matched by gestational age) | ||||
|
| ||||
| PTB-M vs PTB-S (Reference: Subtype PTB-S) | OR | 95% CI | P | |
|
| ||||
| Very low birth-weight (<1500 gr) | 2.95 | 1.82 | 4.78 | <0.001** |
| Mechanical ventilation requirement | 2.09 | 1.49 | 2.92 | <0.001** |
| SGA (<p10) | 2.11 | 1.63 | 2.73 | <0.001** |
| Intracranial hemorrhage | 0.55 | 0.36 | 0.80 | 0.004* |
|
| ||||
| PTB-PPROM vs PTB-I (Reference: Subtype PTB-I) | OR | 95% CI | P | |
|
| ||||
| Patent ductus arteriosus | 2.26 | 1.20 | 4.35 | 0.011* |
| SGA (p<10) | 0.75 | 0.56 | 0.99 | 0.047* |
Note: PTB-S, spontaneous preterm birth; PTB-I, preterm labor leading to PTB (idiopathic); PTB-PPROM, premature rupture of membranes; PTB-M, medically indicated iatrogenic PTB.
Note:
P value < 0.05,
P value < 0.01
3.1.2. PTB-I vs PTB-PPROM
Within spontaneous type, PTB-I mothers were characterized by lower maternal age, increased frequency of changing paternity, lower prenatal visits, and higher frequency of infectious illnesses. Meanwhile, mothers with PTB-PPROM showed increased use of corticosteroids and increased frequency of anemia, vaginal bleeding (Table I). When matched by maternal age in multivariate analysis, use of corticosteroids during pregnancy and increased frequency of vaginal bleeding were observed in PTB-PPROM and higher frequency of infectious diseases was observed in PTB-I (Table II). The rest of the variables incorporated in the model (p < 0.10, see table I) did not reach statistical significance.
3.2. Neonatal outcomes
3.2.1. PTB-S vs PTB-M
No significant differences were observed for means of gestational age (F=0.7, p=0.490) or mean of birthweight (F=1.7, p=0.189) between subtypes.
Medically induced preterm infants had increased frequency of the following variables: small for gestational age (<p10), mechanical ventilation requirement. Multivariate analysis matched by gestational age (in weeks) showed higher risk for very low birth-weight, mechanical ventilation requirement and small for gestational age (<p10)(SGA) in PTB-M with relation to PTB-S, meanwhile PTB-S had higher risk of intracranial hemorrhage (Table II). The rest of the variables incorporated in the model (p < 0.10, see table I) did not reach statistical significance.
3.2.2. PTB-I vs PTB-PPROM
Greater frequency of patent ductus arteriosus, mechanical ventilation requirement was observed in PTB-I compared to PTB-PPROM (Table I). When matched by gestational age only greater frequency of patent ductus arteriosus was still observed. PTB-PPROM had a higher risk for small for gestational age (p<10)(SGA) (Table II). The rest of the variables incorporated in the model (p < 0.10, see table I) did not reach statistical significance.
3.3. Reproductive history outcomes
No significant differences were found when we compared reproductive history between PTB-S and PTB-M.
PTB-I showed a higher frequency of primigravid mothers and mothers with previous PTB than PTB-PPROM. No significant differences between clinical subtypes were observed when comparing mothers who has a previous spontaneous abortion and mothers with both adverse outcomes (previous PTB and previous spontaneous abortion) (Table III).
Table III.
Reproductive history for risk groups
| PTB-S vs PTB-M | |||||
|---|---|---|---|---|---|
| Risk group | Subtypes | OR | 95% CI | P | |
| Multigravidity without previous outcome adverse | PTB-S | 1.00 | - | - | - |
| Primigravidity | PTB-M | 1.07 | 0.76 | 1.51 | 0.692 |
| Previous preterm birth alone | PTB-M | 0.84 | 0.55 | 1.28 | 0.413 |
| Previous miscarriage alone | PTB-M | 0.88 | 0.59 | 1.32 | 0.550 |
| Previous preterm + miscarriage | PTB-M | 0.78 | 0.47 | 1.27 | 0.313 |
|
| |||||
| PTB-I vs PTB-PPROM | |||||
|
| |||||
| Risk group | Subtypes | OR | 95% CI | P | |
|
| |||||
| Multigravidity without previous outcome adverse | PTB-PPROM | 1.00 | - | - | - |
| Primigravidity | PTB-I | 1.43 | 1.02 | 2.02 | 0.039* |
| Previous preterm birth alone | PTB-I | 1.80 | 1.15 | 2.80 | 0.010* |
| Previous miscarriage alone | PTB-I | 1.10 | 0.73 | 1.66 | 0.647 |
| Previous preterm + miscarriage | PTB-I | 1.07 | 0.63 | 1.81 | 0.793 |
Note: PTB-S, spontaneous preterm birth; PTB-I, preterm labor leading to PTB (idiopathic); PTB-PPROM, premature rupture of membranes ; PTB-M, medically indicated iatrogenic PTB.
P value < 0.05
4. Discussion
In the currently study, we found significant differences in epidemiological characteristic of mothers of premature newborns among the different clinical subtypes.
4.1. Clinical subtypes distribution
PTB-PPROM represented 37.9% of the sample, PTB-I 36.6%, and PTB-M 25.5%. This distribution was different from that described in the literature. The rate of preterm births according to clinical subtypes was previously reported[23], as about 30–35% PTB-M, 40–45% PTB-I, and 25–30% PTB-PPROM. Disagreements in the distribution between clinical subtypes may be due to a different ethnic mixtures across populations and regions[14,24]. Previous studies reported that genetic admixture in Argentina showed South Amerindian ancestry admixed with European [14, 15]. Furthermore, this sample excluded preterm twin pregnancies and preterm infants with malformations, which could modify the distribution of subtypes. Interactions of environmental factors, such as infection (intra-amniotic infection, vaginal bacterial), smoking, and nutrition, with specific genes could also result in different phenotypic expression[25].
4.2. Maternal epidemiological characteristics
We investigated several phenotypic features and epidemiological characteristics that could characterize the different clinical subtypes. More than 50 maternal variables and 10 newborn variables were compared between the three clinical subtypes, but only a few showed significant differences.
This observation indicates that different subtypes share some epidemiological characteristics. This finding is consistent with other studies that provide some evidence suggesting heterogeneity of risk factors for clinical subtypes of PTB[20,21]. Some studies have shown the risk of PTB-S was directly related to low socioeconomic status, low education, inadequate weight gain during pregnancy, single marital status, extremes of maternal age (<20 and >35), maternal smoking and maternal alcohol abuse[26,27].
In our study, PTB-I was characterized by younger maternal age, primigravidity, high frequency of changing paternity, single marital status, higher frequency of infectious diseases during pregnancy, and less prenatal visits compared to the other two subtypes. This suggests a profile of mothers with low socioeconomic and educational status compared with PTB-PPROM and PTB-M. Similar socio-demographic characteristics for PTB-I have been reported by other authors[10,28–29]. When the data were adjusted for maternal age, the main outcomes did not change.
PTB-PPROM was characterized by a higher frequency of vaginal bleeding and anemia during pregnancy, two risk factors already reported in the literature[23,30]. When the comparisons were matched for maternal age, significant differences were observed with high frequencies of use of corticosteroids during pregnancy and increased frequency of vaginal bleeding. The increased use of corticosteroids was not surprising as corticosteroids are a common prophylaxis for neonatal respiratory distress when early labor is identified[31,32].
PTB-M was characterized by increased maternal age, married marital status, higher number of prenatal visits, and increased frequency of complications or disease during pregnancy. The increased frequency of complications or disease resulted in more clinical follow up in the PTB-M group compared with PTB-PPROM and PTB-I. Finally, when the comparisons were matched by maternal age, the following significant differences were observed: more likely to be married, increased frequency of arterial hypertension, increased frequency of preeclampsia and increased weight gain (>13 Kg). Hypertensive disorders and preeclampsia were the major pregnancy complications experienced by PTB-M, consistent with previous studies[33,34]. There is a disagreement about gestational weight gain (GWG) being associated with decreased or increased risk of PTB. In our study, we found an association between high GWG (>13Kg) and preeclampsia with PTB-M. Increased maternal weight gain during pregnancy could explain the increasing risk of certain maternal complications, such as preeclampsia[35].
These results were expected according to the clinical management of the condition of each subtype. We were unable to find statistical differences between the clinical subtypes with regards to environmental exposure or lifestyle risk factors, such as alcohol use, coffee consumption or smoking.
Numerous studies have shown that alcohol use[36], and smoking during pregnancy increases the risk of preterm birth, low birth-weight and developmental problems[37–38]. These risk factors were analyzed in previous studies using term live-birth control groups. Our study did not replicate these findings, but that could be because we only compared preterm clinical subtypes.
4.3. Reproductive history
PTB-I showed a greater frequency of primigravidity and high frequency of previous preterm birth in relation to PTB-PPROM, but not in relation to PTB-M. This is an important finding in our study that could indicate a real difference between the two spontaneous clinical subtypes (PTB-I and PTB-PPROM), but not between PTB-I and PTB-M as suggested by other authors[28,39]. Few studies have analyzed differences in recurrence between PTB-I and PTB-PPROM, as these subtypes are frequently grouped in one category called spontaneous preterm births (PTB-S). The low recurrence for PTB-PPROM in relation to the other two clinical subtypes could indicate PTB-PPROM would be affected by more environmental factors such as inflammatory processes[25].
The increased risk in primigravid women has previously been reported[40], but increased risk in both clinical subtypes (PTB-I and PTB-M) is a new finding.
The differences between clinical subtypes in the recurrence were only found in mothers with previous preterm births and no previous spontaneous abortions. This result replicates other findings[14,41], indicating that the strongest risk factor for delivering a preterm neonate is a history of a prior preterm delivery. Unfortunately we had a small number of mothers who experienced both adverse outcomes, making it difficult to come to a definitive conclusion. We think this strategy of classifying mothers according to their reproductive history (presence/absence of previous miscarriage) could be more informative to estimate risks of recurrence according to clinical subtype and to identify differential risk factors or specific gene susceptibility in each group.
4.5 Newborn outcomes
Differences were observed in neonatal morbidity, SGA and mechanical ventilation requirement according to clinical subtypes.
PTB-I was characterized by greater frequency of patent ductus arteriosus, intercranial hemorrhage. When the comparisons were matched by gestational age, significant differences were observed only for a higher frequency of patent ductus arteriosus. Meanwhile PTB-PPROM had lower frequency of patent ductus arteriosus and mechanical ventilation requirement. These neonatal outcomes are consistent with previous studies[42,43], that found the use of corticosteroid therapy during pregnancy is highly efficient in reducing respiratory distress syndrome, perinatal mortality, intraventricular hemorrhage, hemodynamic failure, patent ductus arteriosus and necrotizing enterocolitis. Therefore, our findings could be explained by the higher frequency of corticosteroids in PTB-PPROM mothers.
PTB-M showed a higher frequency of small for gestational age (<p10), very low birth weight, mechanical ventilation requirement and necrotizing enterocolitis. When the comparisons were matched for gestational age, significant differences were observed for very low birth-weigh, mechanical ventilation requirement, and small for gestational age (<p10) in relation to PTB-I and PTB-PPROM. In a cohort study, Ananth et al.[34] showed that ischemic placental disease comprised by fetal distress, placental abruption, preeclampsia and SGA are associated with medically indicated preterm births (PTB-M). We believe that these findings are important and offer new insights toward understanding the etiology of medically indicated preterm births.
Strengths and Weaknesses
Several methodological limitations should be considered when interpreting the current findings. The results are restricted to singleton, non-malformed, preterm births in a single maternity hospital. A sample obtained from a single hospital favors the social and ethnic homogeneity in the population, but adversely affects any extrapolation of its results to other populations.
This study does not include a full-term control group. Its objective, however, was to determine whether particular environmental and genetics factors could explain the epidemiological differences between the clinical subtypes. Thus, the goal of the study was to have a large sample size and numerous variables.
The probability of missing or misclassified data is low. Our study data were collected at a single center over a 5-year period. Personal interviews were conducted by a qualified and experienced team from a large South American hospital-based network[16]. This design permitted a better assessment of maternal and neonatal characteristics, outcomes, and complications by minimizing the impact of temporal changes, especially those changes related to obstetric interventions[44]. Nevertheless, we cannot rule out maternal recall bias, especially in miscarriage reports.
5. Conclusion
Over 50 maternal variables and ten newborn variables were compared between the three clinical subtypes over 1.291 cases, but few showed significant differences. The significant differences observed were consistent with those reported in the literature. The PTB-I subtype was characterized by younger mothers of lower socio-economic status, PTB-PPROM was characterized by environmental factors resulting from inflammatory processes, and PTB-M was characterized by increased maternal or fetal risk pregnancies. The main risk factor for PTB-I and PTB-M was to have a prior preterm delivery, however previous spontaneous abortion was not a risk factor, suggesting a reproductive selection mechanism. The characterization of preterm subtypes is important not only to elucidate the heterogeneity in the etio-pathogenesis, but also to implement measures that are more efficient in the treatment of preterm birth.
Acknowledgments
The authors would like to thank the support and hard work of the health care team at Maternidad Nuestra Señora de la Merced, in Tucumán, Argentina. Also, Gladys Mirta Leguizamón; Mercedes Elizabeth López; Marta Inés Gonzales de Padilla, and all the preterm infants’ families. Nancy Weathers at the University of Iowa provided database and informatic support.
Footnotes
7-Disclosure Statements
7.1 Financial Support
This work was supported by the March of Dimes grant #6-FY08-260 and NIH grant 1R01 HD-52953 and by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. Grant Number: PICT 2008-0429.
References
- 1.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 2.Barth WH., Jr Familial spontaneous preterm birth: further evidence of a complex genetic disease. Obstet Gynecol. 2010;115:1114–1115. doi: 10.1097/AOG.0b013e3181e15e1f. [DOI] [PubMed] [Google Scholar]
- 3.DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007;25:040–051. doi: 10.1055/s-2006-956774. [DOI] [PubMed] [Google Scholar]
- 4.Plunkett J, Borecki I, Morgan T, Stamilio D, Muglia LJ. Population-based estimate of sibling risk for preterm birth, preterm premature rupture of membranes, placental abruption and pre-eclampsia. BMC Genet. 2008;9:44. doi: 10.1186/1471-2156-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moutquin JM. Classification and heterogeneity of preterm birth. British Journal of Obstet Gynecol. 2003;110(Suppl 20):30–33. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, Kramer M, Culhane JF, Barros FC, Conde-Agudelo A, Bhutta ZA, Goldenberg RL. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012 Feb;206(2):119–23. doi: 10.1016/j.ajog.2011.10.866. [DOI] [PubMed] [Google Scholar]
- 7.Savitz DA, Dole N, Herring AH, Kaczor D, Murphy J, Siega-Riz AM, Thorp JM, Jr, MacDonald TL. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19:97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 8.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–93. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 9.Merenstein GB, Weisman LE. Premature rupture of the membranes: neonatal consequences. Semin Perinatol. 1996;20:375–80. doi: 10.1016/s0146-0005(96)80004-8. [DOI] [PubMed] [Google Scholar]
- 10.Kamath-Rayne BD, DeFranco EA, Chung E, Chen A. Subtypes of preterm birth and the risk of postneonatal death. J Pediatr. 2013 Jan;162(1):28–34. e2. doi: 10.1016/j.jpeds.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barros FC, Velez MP. Temporal trends of preterm birth subtypes and neonatal outcomes. Obstet Gynecol. 2006;107:1035–41. doi: 10.1097/01.AOG.0000215984.36989.5e. [DOI] [PubMed] [Google Scholar]
- 12.Carnero AM, Mejía CR, García PJ. Rate of gestational weight gain, pre-pregnancy body mass index and preterm birth subtypes: a retrospective cohort study from Peru. BJOG. 2012 Jul;119(8):924–35. doi: 10.1111/j.1471-0528.2012.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Feenstra B, Melbye M. Maternal Contributions to Preterm Delivery. Am J Epidemiol. 2009;170:1358–1364. doi: 10.1093/aje/kwp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupitzki HB, Gadow EC, Gili JA, Comas B, Cosentino VR, Saleme C, Murray JC, Lopez Camelo JS. Environmental risk factors and perinatal outcomes in preterm newborns, according to family recurrence of prematurity. Am J Perinatol. 2013 Jun;30(6):451–61. doi: 10.1055/s-0032-1326990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann PC, Cooper ME, Ryckman KK, Comas B, Gili J, Crumley S, Bream ENA, Byers HM, Piester T, Schaefer A, Christine PJ, Lawrence A, Schaa KL, Kelsey KJP, Berends SK, Momany AM, Gadow E, Cosentino V, Castilla EE, López Camelo J, Saleme C, Day LJ, England SK, Marazita, Dagle JM, Murray JC. Polymorphisms in the fetal progesterone receptor and a calcium-activated potassium channel isoform are associatedwith preterm birth in an Argentinian population. J Perinatol. 2013 May;33(5):336–40. doi: 10.1038/jp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castilla EE, Orioli IM. ECLAMC: The Latin-American collaborative study of congenital malformations. Community genet. 2004;7:76–94. doi: 10.1159/000080776. [DOI] [PubMed] [Google Scholar]
- 17.Manetti G, Rodas E, Morici P, Fernandez RG, Tonietto A, Oliveri N. Continuous quality improvement through use of perinatal clinical record and AGUSTINA database. Stud Health Technol Inform. 1998;52(Pt 2):824–826. [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 5. Elk Grove Village, IL: American Academy of Pediatrics; 2005. [Google Scholar]
- 19.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Obstet Gynecol. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 20.Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol. 1991;164:467–71. doi: 10.1016/s0002-9378(11)80001-3. [DOI] [PubMed] [Google Scholar]
- 21.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Med. 2006;19:773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 22.Khashan AS, McNamee R, Abel KM, Mortensen PB, Kenny LC, Pedersen MG, Webb RT, Baker PN. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24:429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, Poletti G, Mazzotti G, Hill K, Hurtado AM, Camrena B, Nicolini H, Klitz W, Barrantes R, Molina JA, Freimer NB, Bortolini MC, Salzano FM, Petzl-Erler ML, Tsuneto LT, Dipierri JE, Alfaro EL, Bailliet G, Bianchi NO, Llop E, Rothhammer F, Excoffier L, Ruiz-Linares A. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008 Mar 21;4(3):e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci. 2010 Sep;1205:118–22. doi: 10.1111/j.1749-6632.2010.05684.x. [DOI] [PubMed] [Google Scholar]
- 26.Parker JD, Schoendorf KC, Kiely JL. Associations between measures of soci-economic status and low birthweight, small for gestational age and premature delivery in the United States. Ann Epidemiol. 1994;4:271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MS, Goulet L, Lydon J, Séguin L, McNamara H, Dassa C, Platt RW, Chen MF, Gauthier H, Genest J, Kahn S, Libman M, Rozen R, Masse A, Miner L, Asselin G, Benjamin A, Klein J, Koren G. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediat Perinat Epidemiol. 2001;15(Suppl 2):104–123. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 28.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–50. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Chen A, Feresu SA, Barsoom MJ. Heterogeneity of preterm birth subtypes in relation to neonatal death. Obstet Gynecol. 2009 Sep;114(3):516–22. doi: 10.1097/AOG.0b013e3181b473fc. [DOI] [PubMed] [Google Scholar]
- 30.Erez O, Espinoza J, Chaiworapongsa T, Gotsch F, Kusanovic JP, Than NG, Mazaki-Tovi S, Vaisbuch E, Papp Z, Yoon BH, Han YM, Hoppensteadt D, Fareed J, Hassan SS, Romero R. A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Matern Fetal Neonatal Med. 2008 Oct;21(10):732–44. doi: 10.1080/14767050802361807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina TM, Hill DA. Preterm premature rupture of membranes: diagnosis and management. Am Fam Physician. 2006 Feb 15;73(4):659–64. [PubMed] [Google Scholar]
- 32.Phupong V, Kulmala L. Clinical course of preterm prelabor rupture of membranes in the era of prophylactic antibiotics. BMC Res Notes. 2012 Sep 22;5:515. doi: 10.1186/1756-0500-5-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005 Feb-Mar;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 34.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006 Dec;195(6):1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 35.DeVader SR, Neeley HL, Myles TD, Leet TL. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007 Oct;110(4):745–51. doi: 10.1097/01.AOG.0000284451.37882.85. [DOI] [PubMed] [Google Scholar]
- 36.Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of lowbirth-weight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011 Nov;118(12):1411–21. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aliyu MH, Lynch O, Saidu R, Alio AP, Marty PJ, Salihu HM. Intrauterine exposure to tobacco and risk of medically indicated and spontaneous preterm birth. Am J Perinatol. 2010 May;27(5):405–10. doi: 10.1055/s-0029-1243316. [DOI] [PubMed] [Google Scholar]
- 38.Stone WL, Bailey B, Khraisha N. The pathophysiology of smoking during pregnancy: a systems biology approach. Front Biosci (Elite Ed) 2014 Jun 1;6:318–28. doi: 10.2741/e708. [DOI] [PubMed] [Google Scholar]
- 39.Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol. 2014 Feb;210(2):131e1–8. doi: 10.1016/j.ajog.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ananth CV, Peltier MR, Getahun D, Kirby RS, Vintzileos AM. Primiparity: an ‘intermediate’ risk group for spontaneous and medically indicated preterm birth. J Matern Fetal Neonatal Med. 2007 Aug;20(8):605–11. doi: 10.1080/14767050701451386. [DOI] [PubMed] [Google Scholar]
- 41.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283:1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 42.Magny JF, Rigourd V, Kieffer F, Voyer M. Perinatal corticosteroid therapy: modalities, efficacy, consequences. J Gynecol Obstet Biol Reprod (Paris) 2001 Feb;30(1 Suppl):36–46. [PubMed] [Google Scholar]
- 43.Nayara López C, Mar González A, Laura Álvarez C, Nuria Martínez S, Antonio González G, Félix Omeñaca T, Belén San José VA. Factores obstétricos claves en los resultados neonatales y a los dos años de seguimiento en la prematuridad extrema. REV CHIL OBSTET GINECOL. 2011;76(5):302–310. [Google Scholar]
- 44.Barros FC, Victora CG, Barros AJ, Santos IS, Albernaz E, Matijasevich A, Domingues MR, Sclowitz IK, Hallal PC, Silveira MF, Vaughan JP. The challenge of reducing neonatal mortality in middle-income countries: findings from three Brazilian birth cohorts in 1982, 1993, and 2004. Lancet. 2005;365:847–854. doi: 10.1016/S0140-6736(05)71042-4. [DOI] [PubMed] [Google Scholar]