Abstract
Purpose
We have demonstrated that patients with HER2-amplified tumors derive more benefit from higher doses of doxorubicin-containing chemotherapy (cyclophosphamide, doxorubicin, and fluorouracil [CAF]). Because topoisomerase IIα (Topo-IIα) is a target for doxorubicin and is coamplified in 20% to 50% of HER2-amplified tumors, we postulated that Topo-IIα copy number might account for the benefit from CAF dose escalation in HER2-positive tumors. To address this hypothesis, we examined Topo-IIα and HER2 copy number, CAF dose, and clinical outcomes in Cancer and Leukemia Group B (CALGB) 8541.
Patients and Methods
Topo-IIα and HER2 copy number were measured by fluorescent in situ hybridization (FISH) using a triple-probe system, which includes Topo-IIα, HER2, and chromosome 17 (CEP17). Topo-IIα and/or HER2 were classified as amplified (≥ two copies/CEP17, deleted (≤ 0.67 copies/CEP17) and normal copy number (> .67 to < 2.0 copies/CEP17).
Results
Topo-IIα/HER2/CEP17 measurement was successful in 624 of 687 cases. HER2 was amplified in 117 cases (19%). Topo-IIα was amplified in 41 cases (7%) and deleted in 69 cases (11%). Topo-IIα amplification was highly correlated with HER2 amplification (39 of 41; P < .0001), HER2 by immunohistochemistry, and by dual-probe FISH. Topo-IIα was deleted in both the HER2-amplified (30 of 69; 43%), normal (22 of 69; 32%) and HER2-deleted tumors (17 of 69; 25%). Although Topo-IIα–amplified tumors were nearly always HER2 amplified, these tumors did not receive benefit from increasing the dose of CAF (P = .15).
Conclusion
The correlative companion study CALGB 8541-150013 does not support the hypothesis that Topo-IIα amplification is the mechanism behind benefit from increased dose of anthracyclines in HER2-positive breast cancer.
INTRODUCTION
The use of chemotherapy to treat early-stage breast cancer is proven to extend survival1; however, the best chemotherapeutic regimen has not been determined. Although doxorubicin-containing regimens are standard for higher-risk patients, they tend to have higher toxicity, in particular, a 1% to 3% risk of congestive heart failure in patients who receive doses of more than 300 mg/m2.2 In addition, newer nonanthracycline treatment options are available; however, methods to select which patients are most likely to benefit from anthracyclines are not.3,4 Better understanding of the predictive value of certain biologic markers should allow us to identify chemotherapy regimens that are most likely to be effective with the least amount of toxicity.
The HER2 or ErbB2 oncogene is overexpressed in approximately 25% to 30% of human breast cancer specimens and is associated with a worse outcome in many studies.5,6 Accumulating evidence has shown that HER2 expression is associated with improved outcome after doxorubicin-based therapy.7,8 The Cancer and Leukemia Group B (CALGB) has retrospectively evaluated HER2 expression using various methods and has shown that patients with tumors that overexpress HER2 who received high-dose cyclophosphamide, doxorubicin, and fluorouracil (CAF) had a better outcome compared with patients with HER2-positive tumors who received moderate or low-dose CAF.7,9 In National Surgical Adjuvant Breast and Bowel Project B11, patients with estrogen receptor (ER) or progesterone receptor (PR) –negative disease were randomly assigned between melphalan and fluorouracil versus melphalan, doxorubicin, and fluorouracil.10 Only patients whose tumors were HER2 positive by immunohistochemistry were found to benefit from the addition of doxorubicin (hazard ratio = 0.60; 95% CI, 0.47 to 0.92). Pritchard et al11 evaluated the role of HER2, measured by fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) in premenopausal women with node-positive breast cancer who had received either adjuvant cyclophosphamide, epirubicin, and fluorouracil (CEF) or cyclophosphamide, methotrexate, and fluorouracil (CMF). In patients whose tumors showed amplification of HER2, CEF was superior to CMF on the basis of relapse-free survival, whereas tumors that lacked amplification of HER2 saw no such incremental benefit.
Topoisomerase IIα (Topo-IIα) is a DNA-modifying enzyme that binds to the double helix to release torsional stress and create double-strand breaks that allow replication to occur. Drugs that interfere with Topo-IIα include the anthracyclines (doxorubicin, daunorubicin), etoposide, teniposide, and amsacrine. These agents act by binding covalently with Topo-IIα after double-strand breaks have occurred, inducing lethal cellular damage by inhibition of religation. Increase in Topo-IIα expression is associated with sensitivity to these agents, both in cell lines and tumors, as a result of increased substrate on which the drug may act.12,13 Topo-IIα occurs in the same amplicon on chromosome 17 (CEP17) as HER2, and Jarvinen et al14 have shown in breast cancer cell lines and primary tumors that amplification or deletion of the Topo-IIα gene is frequent in HER2 tumors. Of 57 HER2-amplified primary breast carcinomas, 25 (44%) showed ErbB2-topoIIα coamplification, and 24 (42%) showed a physical deletion of the Topo-II α gene. These studies showed a consistent relationship between deletion of the gene and decreased protein expression.15
Our previous work has shown that activation of the HER family of receptors is associated with upregulation of Topo-IIα and increase in sensitivity to doxorubicin.16 This suggests an alternative mechanism of sensitivity to anthracyclines, which involves receptor activation rather than coamplification. In an attempt to understand the mechanism of this observation, we evaluated Topo-IIα activity on activation of the HER2 receptor using a chimeric receptor model.17 We showed that the increase in sensitivity to doxorubicin can be explained by increased enzymatic activity of Topo-IIα as measured by decatenating and cleavage assays.18 In addition, this effect could be reversed by the anti-HER2 antibody, trastuzumab, in breast cancer cells that overexpress HER2 (BT474). Chimeric cells were also more sensitive to etoposide; therefore, it is likely that doxorubicin sensitivity is mediated by Topo-IIα.
Between January 1985 and May 1991, CALGB 8541 randomly assigned patients with node-positive breast cancer to three treatment arms evaluating different dose and scheduling of CAF: arm 1 (low-dose), 300 mg/m2 of cyclophosphamide, 30 mg/m2 of doxorubicin, and 300 mg/m2 of fluorouracil every 28 days for four cycles; arm 2 (moderate dose), 400 mg/m2 of cyclophosphamide, 40 mg/m2 of doxorubicin, and 400 mg/m2 of fluorouracil every 28 days for six cycles, and arm 3, 600 mg/m2 of cyclophosphamide, 60 mg/m2 of doxorubicin, and 600 mg/m2 of fluorouracil every 28 days for four cycles.19 At a median follow-up of 9 years, disease-free survival (DFS) and overall survival (OS) for patients on the moderate- and high-dose arms are superior to the corresponding survival measures for patients on the low-dose arm (two-sided P < .0001 and two-sided P = .004, respectively), with no difference in DFS or OS between the moderate- and the high-dose arms. This study attempted to address whether Topo-IIα amplification is responsible for the benefit seen with increasing the dose of anthracycline in CALGB 8541.
PATIENTS AND METHODS
Patients
A subgroup of patients registered to CALGB 8541 who received adjuvant doxorubicin and whose tumors had been previously evaluated for HER2 by PathVysion FISH (Abbott Molecular; Abbott Park, IL) were included in this retrospective study, provided that sufficient invasive cancer remained in the block to provide representative sections of the primary tumor for assay. Six hundred eighty-seven patient samples matching these criteria were available from the CALGB Pathology Coordinating Office. Topo-IIα/HER2/CEP17 measurement was successful in 624 (91%) of 687 available cases. There was no significant difference in patient characteristics between the 624 patients assessable for Topo-IIα compared with the entire 1,572 patients registered to CALGB 8541. Age, tumor size, and number of positive nodes were compared using the Wilcoxon Rank-Sum test. The χ2 test was used to test for differences in menopausal status, receptor status, and the three dose levels of CAF (Table 1). There was no significant difference in patient characteristics between patients assessable for Topo-IIα across the three CAF dose arms. Age, tumor size, and number of positive nodes were compared using the Wilcoxon rank-sum test. The χ2 test was used to test for differences in menopausal status, receptor status, and the three dose levels of CAF (Table 2). There was no significant difference in patient characteristics between patients assessable for Topo-IIα across the three CAF dose arms. Age, tumor size, and number of positive nodes were compared using the Wilcoxon rank-sum test. The χ2 test was used to test for differences in menopausal status, receptor status, and the three dose levels of CAF (Table 2).
Table 1.
Patient Characteristics
| Characteristic | Patients With Topoisomerase IIα Assessment (n = 624) | All Treated Patients (n = 1572) | P (assessed to all treated) |
|---|---|---|---|
| Age, years | |||
| Median | 50 | 49 | .13 |
| Range | 22-77 | 22-81 | |
| Tumor size, cm | |||
| Median | 2.6 | 2.6 | .26 |
| Range | 0.3-12.0 | 0.1-12.0 | |
| No. of positive nodes | |||
| Median | 3.0 | 3.0 | .17 |
| Range | 1-54 | 1-54 | |
| Premenopausal, % | 39.7 | 43 | .12 |
| Receptor positive, % | 72.3 | 72 | .86 |
| Dose of CAF, % | |||
| Low | 32 | 33 | |
| Moderate | 33 | 33 | .82 |
| High | 35 | 34 |
NOTE. Age, tumor size and No. of positive nodes were compared using the Wilcoxon rank–sum test. The χ2 test was used to test for differences in percentage premenopausal, receptor positive, and in the three dose levels of CAF.
Abbreviation: CAF, cyclophosphamide, doxorubicin, and fluorouracil.
Table 2.
Dose of CAF Effect in the Analyzed Subset (n = 624)
| Characteristic | Dose of CAF |
P | ||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Age, years | ||||
| Median | 51 | 50 | 49 | .62 |
| Range | 22-76 | 26-75 | 29-77 | |
| Tumor size, cm | ||||
| Median | 3.0 | 3.0 | 2.5 | .24 |
| Range | 0.4-11.5 | 0.5-12.0 | 0.3-9.5 | |
| No. of positive nodes | ||||
| Median | 3.0 | 3.0 | 3.0 | .83 |
| Range | 1-29 | 1-54 | 1-38 | |
| Premenopausal, % | 39 | 43 | 38 | .58 |
| Receptor positive, % | 74 | 70 | 73 | .64 |
NOTE. Age, tumor size, and No. of positive nodes were compared using the Kruskal–Wallis test. The χ2 test was used to test for differences in percentage premenopausal and receptor positive.
Abbreviation: CAF, cyclophosphamide, doxorubicin, and fluorouracil.
As only existing pathologic specimens were studied that were not identifiable to the investigators on this study, additional informed consent was not required. Samples provided to laboratory investigators were stripped of identifiers, and relinking of clinical outcome with laboratory findings was performed at the CALGB Statistical Center. This study was reviewed and approved by the institutional review board at the institutions where the laboratory work was performed.
HER2 by CB11 immunohistochemistry has been previously performed and reported. PathVysion FISH for HER2(10) and S-phase by flow cytometry have been previously reported.20–22
FISH for HER2 and Topo-IIα
FISH for HER2 and Topo-IIα was performed using the Vysis LSI FISH Probe, Topo-IIα(17.21-22)/HER2(17q11.2-q12/)CEP17(17p11.1-q11.1) Tricolor Probe in the laboratory of L.D. The system is similar to that used for the VYSIS PathVysion kit for HER2, which is approved by the United states Food and Drug Administration. We used the VP2000 automated tissue processor and HYBRITE denaturation hybridization system from Abbott Molecular for all assays.23 Acid pretreatment and protease digestion to breakdown formaldehyde cross-links was performed (Vysis Paraffin pretreatment kit), followed by sodium chloride-sodium citrate (SSC) and formamide denaturation (72°C, 5 minutes). After dehydration, the HER2/CEP17/Topo-IIα probe cocktail was added, and coverslips were applied and sealed with rubber cement. Slides were incubated in a humidity chamber overnight for 18 hours at 37°C. On the following day, slides were washed in a stringency buffer (SSC, NP40), dried on a slide warming tray and incubated with 4'-6-diamidino-2-phenylindole (DAPI) for nuclear identification. Slides were stored in the dark, at −20°C. Nonamplified and amplified control slides (fixed cell lines embedded in paraffin, MDA-MB-231 and Hs578T, respectively) were analyzed with each assay, provided in the Vysis kit. Twenty nuclei were scored individually for Topo-IIα, HER2, and CEP17 probes. By convention, Topo-IIα and/or HER2 were considered amplified when the ratio of Topo-IIα/CEP17 signals or HER2/CEP17 signals was ≥ 2.00. A case was considered deleted when the ratio of signals was ≤ 0.67. Cases with signal ratios between 0.67 and 2.00 were considered normal copy number in this study.15
Statistical Methods
All FISH data were submitted to the CALGB Statistical Center as a continuous variable for analysis. Statistical analyses were performed by CALGB statisticians using SAS 9.1 (SAS Institute, Cary, NC). We used a multivariate Cox proportional hazards regression model to test the interaction of treatment arm and Topo-IIα while adjusting for important covariates. The relationship between Topo-IIα amplification with HER2 amplification by FISH was determined by contingency tables and test for association with the χ2 test. The χ2 test was also used to test for associations between Topo-IIα amplification and deletion with ER/PR status. The Kruskal-Wallis test was used to evaluate for differences in the mean rank values for HER2 IHC, FISH ratio, S phase, and ER-positive status in the three Topo-IIα categories (amplified, deleted, and normal). Kaplan-Meier estimates were used to graphically display outcomes for DFS and OS by dose (low, moderate, high) and by marker/method for amplified versus nonamplified HER2 or Topo-IIα amplified/deleted/diploid.
RESULTS
We performed FISH for HER2, Topo-IIα, and CEP17 using the triple-probe system on 687 of 687 available cases. Topo-IIα/HER2/CEP17 measurement was successful in 624 (91%) of 687 available cases. HER2 was amplified in 117 cases (19%) and deleted in 18 cases (3%). Topo-IIα was amplified in 41 cases (7%) and deleted in 69 cases (11%). A comparison of HER2 FISH status in CALGB 8869 using PathVysion compared with HER2 FISH using tricolor system (Vysis) in the CALGB 9344 study showed the sensitivity and specificity of 95% and 97% for Topo-IIα by triple probe and for Topo-IIα by dual probe (L.D.), respectivelyl. The Pearson correlation coefficient of FISH dual probe to tricolor probe is 0.88 (P < .001).
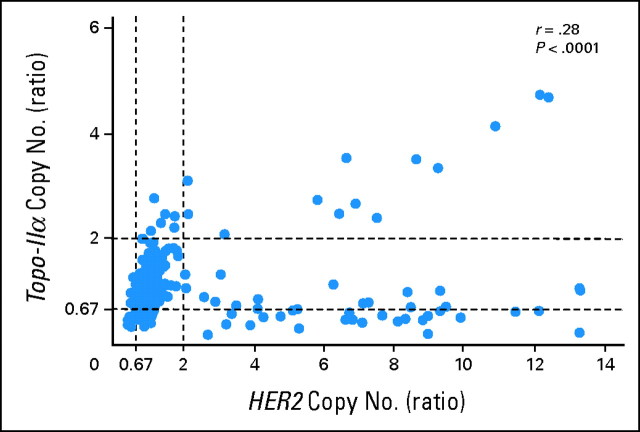
Topo-IIα was deleted in both the HER2-amplified (30 of 69; 43%), normal (22 of 69; 32%), and HER2-deleted tumors (17 of 69; 25%). A scatterplot of HER2 versus Topo-IIα copy number reveals a biphasic relationship between these two amplicons, with HER2-amplified tumors either showing coamplification of Topo-IIα or reduction in copy number of Topo-IIα (median, 0.83) compared with HER2 nonamplified tumor samples (Fig 1). Of note, Topo-IIα was never amplified in more than five copies per cell, despite high levels of HER2 amplification of up to 14 copies: CEP17.
Fig 1.
Topoisomerase II α copy number versus HER2 copy number. Topoisomerase IIα and HER2 copy number was determined using Vysis Triple Probe kit (HER2, TOP2A, CEP17). The topoisomerase IIα ratio was measured by counting the number of TOP2A signals/nucleus in a minimum of 20 nuclei over the number of CEP17 signals/nucleus in a minimum of 20 nuclei. The HER2 ratio was measured by counting the number of HER2 signals/nucleus in a minimum of 20 nuclei over the number of CEP17 signals/nucleus in a minimum of 20 nuclei. Topoisomerase IIα ratio (Y-axis) is plotted against HER2 ratio (X-axis) and the correlation coefficient calculated using SAS 9.1.
Topo-IIα amplification was highly correlated with HER2 amplification (39 of 41; P < .0001), HER2 by IHC (CB11; P < .0001), and by dual-probe FISH (P < .0001; Table 3). The median S phase in the Topo-IIα–nonamplified group was 12, whereas it was 17.5 in the Topo-IIα–amplified group (P = .066). Topo-IIα amplification did not show a statistically significant association with ER and/or PR negativity (P = .15). Topo-IIα deletion was also correlated with HER2 amplification (P < .0001) and overexpression (P < .0001), but not with ER/PR or S phase.
Table 3.
Association of Topo–IIα Amplification With Disease and Treatment Variables
| Variable | Topo–IIα Amplified | Topo–IIα Deleted | Topo–IIα Normal | P |
|---|---|---|---|---|
| HER2 IHC 3+, No. of cases | 90 | 50 | 1.0 | < .0001 |
| FISH, ratio | 4.5 | 1.11 | 1.05 | < .0001 |
| S phase, % nuclei | 17.5 | 14 | 12 | .12 |
| ER, % nuclei | 55 | 61 | 67 | .20 |
NOTE. The values displayed for HER2 IHC, FISH ratio, and S phase are medians and for ER the percentage positive. Differences in the mean ranks or proportions are compared using the Kruskal–Wallis test.
Abbreviations: Topo–IIα, topoisomerase IIα; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; ER, estrogen receptor.
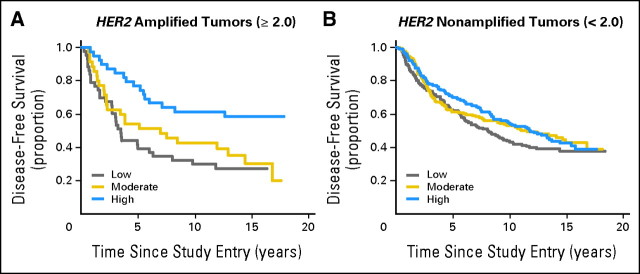
To determine whether HER2 amplification remained a predictor of benefit from anthracycline in this subset of patients evaluated by the triple probe, we performed an interaction analysis of HER2 ratio with CAF dose. HER2-amplified tumors treated with moderate- and higher-dose CAF regimens had an improved DFS and OS compared with those treated with the low-dose CAF regimen in an unadjusted analysis (P = .0032). Although the trend was similar, the interaction between HER2 and CAF in the Cox proportional hazards model adjusted for CAF dose, number of positive nodes, tumor size, and menopausal status was of borderline significance (P = .079; Fig 2).
Fig 2.
Disease-free survival by cyclophosphamide, doxorubicin, and fluorouracil (CAF) dose in HER2 amplified and nonamplified tumors. Kaplan-Meier estimates were calculated using SAS 9.1 for disease-free survival by CAF dose (low, moderate, high) for HER2 amplified versus HER2 nonamplified tumors. P = .079, interaction term for the three arms.
The benefit of CAF dose in Topo-IIα–amplified tumors was then evaluated. Despite the fact that HER2 amplification suggested benefit from CAF dose escalation, Topo-IIα amplification did not account for this benefit, as no interaction of CAF dose was seen in Topo-IIα–amplified cases. An unplanned analysis of Topo-IIα copy number ≤ 0.67 seemed to show more benefit from an increased dose of CAF in the Kaplan-Meier plot. However, the interaction of Topo-IIα and dose of CAF was not statistically significant in the Cox proportional hazards multivariate model. Similarly, Topo-IIα normal cases showed no benefit from increasing CAF dose (Fig 3).
Fig 3.
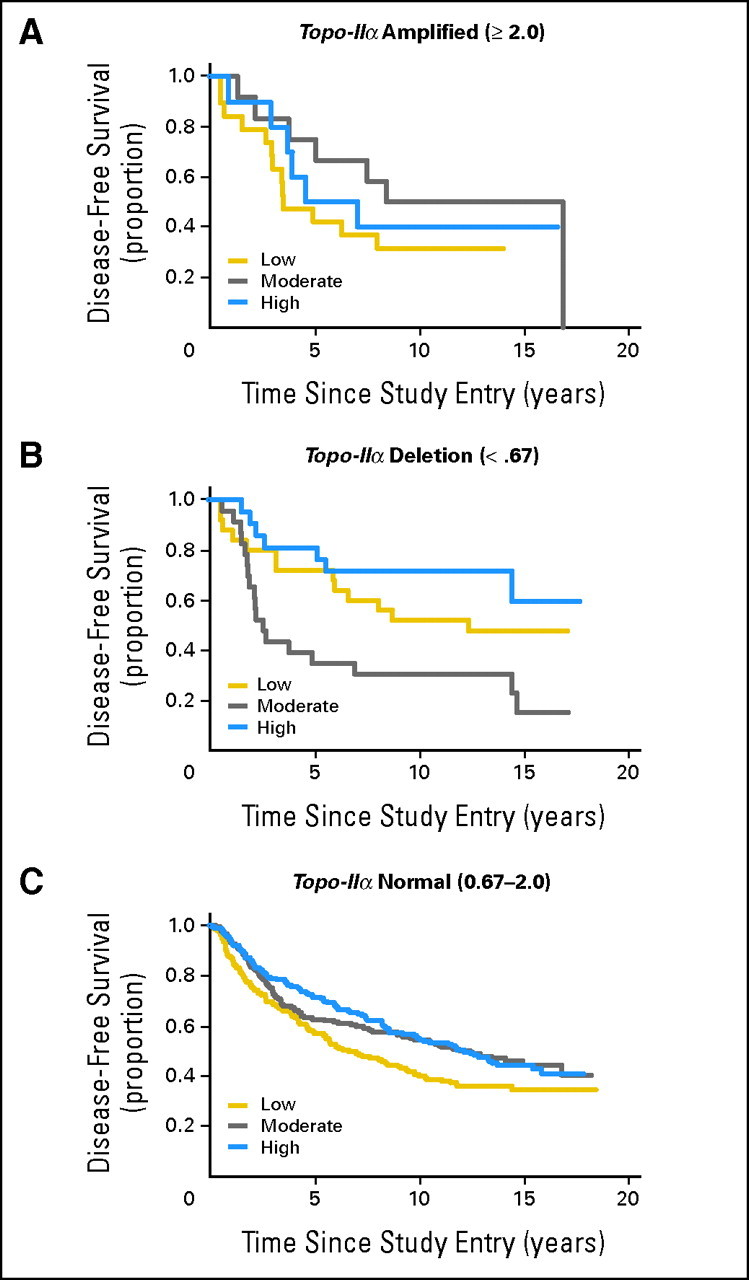
Disease-free survival by cyclophosphamide, doxorubicin, and fluorouracil (CAF) dose in tumors with topoisomerase (topo) II α amplification, deletion and normal copy number. Kaplan-Meier estimates were calculated using SAS 9.1 for disease-free survival by CAF dose (low, moderate, high) for tumors with topo IIα amplification, topo IIα deletion and topo IIα diploid (normal) copy number. P = .15, interaction term for the three arms.
Multivariate Cox proportional hazards models with either DFS or OS as the dependent variable and other independent variables (CAF treatment arm, number of positive nodes [square root transformed], tumor size [≤ 2 v > 2 cm], menopausal status [pre v peri/post], Topo-IIα [deleted v not], and the interaction of Topo-IIα and CAF treatment assignment) resulted in nonstatistically significant interaction (P values of .24 and .21, respectively). No interaction with dose was observed in the HER2 normal/deleted group or Topo-IIα normal/deleted groups.
In view of the biologic connection between HER2 and Topo-IIα and the putative association between Topo-IIα and anthracyclines, we evaluated whether Topo-IIα contributed to the benefit from escalating doses of CAF in the HER2-amplified cases. In a multivariate Cox proportional hazards model adjusting for CAF dose, number of nodes, tumor size, and menopausal status, HER2 amplification predicted benefit from CAF dose escalation, but in the current analysis, Topo-IIα amplification with or without HER2 amplification did not account for this benefit (Table 4).
Table 4.
Variable of Interaction With CAF Dose*
| Variable of Interaction | P |
|---|---|
| HER2 positive v HER2 negative | .079 |
| HER2 positive or Topo–IIα amplified (≥ 2.0) v all others | .34 |
| HER2 positive and Topo–IIα amplified (≥ 2.0) v all others | .77 |
Abbreviations: CAF, cyclophosphamide, doxorubicin, and fluorouracil; Topo–IIα, topoisomerase IIα.
All terms were modeled as dichotomous.
DISCUSSION
The mechanism by which patients with HER2-amplified tumors benefit more from anthracyclines is unknown. We attempted to address one hypothesis, that Topo-IIα amplification was responsible for this effect, with negative findings. It is particularly notable that HER2-amplified tumors continued to show benefit from increasing doses of CAF in this subset tested with the HER2, CEP17, Topo-IIα triple probe, yet Topo-IIα amplification did not stratify the patients in this way. This suggests that an alternative mechanism to coamplification should be considered and perhaps lies in the fact that Topo-IIα gene expression is not well represented by copy number as measured by FISH.
A number of retrospective studies have evaluated the role of Topo-IIα amplification as a predictive marker of response to anthracyclines in breast cancer.26,26a,26b Di Leo et al27 evaluated HER2 and Topo-IIα gene aberrations by FISH in a series of 430 primary breast cancer samples of patients with node-positive breast cancer treated with one of two epirubicin-containing regimens versus CMF. In this study, Topo-IIα evaluation suggested that the superiority of anthracyclines over CMF in HER2-amplified patients could be confined to the subgroup of Topo-IIα–amplified tumors. In contrast, Knoop et al28 retrospectively identified and analyzed tumor tissue for HER2 positivity and for Topo-IIα amplification and deletion from 805 of 980 patients randomly assigned to CMF versus CEF in the Danish Breast Cancer Cooperative Group trial 89D. Topo-IIα changes were identified in 23% of the 773 evaluated tumors: 12% had Topo-IIα amplification and 11% had Topo-IIα deletions. They reported improved recurrence-free survival both in patients with Topo-IIα amplification and in patients with Topo-IIα–deleted tumors who were treated with CEF compared with CMF. Patients with normal Topo-IIα genotype had a similar outcome in both treatment arms. This finding was not expected, as the hypothesis was that the deletions predicted resistance to anthracyclines.
In the Breast Cancer International Research Group 06 trial, correlation between response to anthracyclines and Topo-IIα aberrations was prospectively planned. Patients with Topo-IIα amplification had better DFS after adjuvant therapy in both the trastuzumab-containing arm without anthracycline and in the nontrastuzumab-containing arm that included an anthracycline.30,31 The investigators did not find a significant level of Topo-IIα deletion, and interpreted these data to mean that Topo-IIα amplification was a good surrogate of benefit from anthracycline.
O'Malley et al32 evaluated the predictive value of Topo-IIα in premenopausal women with node-positive breast cancer randomly assigned in the MA.5 trial to CEF versus CMF. Cox model analysis suggested that Topo-IIα protein overexpression was highly predictive of a better DFS with CEF than with CMF. In this study, both Topo-IIα amplification and deletion predicted benefit from CEF versus CMF for both DFS and OS. Analysis from the United Kingdom National Epirubicin Adjuvant Trial (NEAT), which compared CMF with epirubicin, followed by CMF, included HER2, Topo-IIα, Ki67, and chromosome 17 polysomy.33 The results suggest that the most powerful predictor of benefit from adjuvant anthracycline is chromosome 17 polysomy, perhaps as a marker of chromosome instability. No effect was observed for differing Topo-IIα status.
In our current study, Topo-IIα was coamplified in 33% of HER2-amplified tumors and was rarely seen in non–HER2-amplified tumors. Topo-IIα deletion was numerically more common than amplification (43% v 33%) although not statistically different. Of note, HER2-amplified tumors seemed to show two patterns of Topo-IIα copy number, either amplified or reduced in number compared with HER2-nonamplified tumors. This supports the contention that HER2 amplification is associated with Topo-IIα copy number alterations, an indirect indicator of genomic instability. The association with sensitivity to anthracyclines may be explained by the fact that tumors with greatest degree of genomic instability showed the highest levels of Topo-IIα mRNA as shown by Carter et al24 using multiple gene expression data sets.
It is also apparent, from recent high-density genomic mapping, that FISH analysis may not accurately predict amplification at a particular locus. Indeed, Lezon-Geyda et al34 have shown that the Topo-II locus is rarely amplified using Representational Oligonucleotide Microarray Analysis, even when the FISH probe suggests this is the case. Hence it seems more likely that Topo-II copy number alterations may be a surrogate for a more global event (ie, genomic instability).
On the basis of this study and others cited, we do not support the hypothesis that Topo-IIα amplification alone explains the benefit of anthracyclines in HER2 tumors. CALGB 8541 did not include a nonanthracycline arm; however, in this retrospective subset analysis, Topo-IIα does not explain the reported benefit from higher doses of doxorubicin-containing chemotherapy in CALGB 8541. Further studies are required to define the relationship between Topo-IIα copy number, RNA, and protein in data sets with treatment response information.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Paula Friedman, Abbott Laboratories Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lyndsay N. Harris, Donald A. Berry, Daniel F. Hayes, Lynn Dressler
Administrative support: Lyndsay N. Harris, Gloria Broadwater, Clifford A. Hudis, Paula Friedman
Provision of study materials or patients: Daniel Budman
Collection and assembly of data: Lyndsay N. Harris, Gloria Broadwater, David Cowan, Ann D. Thor, Daniel Budman, Constance T. Cirrincione, Lynn Dressler
Data analysis and interpretation: Lyndsay N. Harris, Gloria Broadwater, Maysa Abu-Khalaf, Ann D. Thor, Constance T. Cirrincione, Donald A. Berry, Clifford A. Hudis, Paula Friedman, Matthew Ellis, Lynn Dressler
Manuscript writing: Lyndsay N. Harris, Gloria Broadwater, Maysa Abu-Khalaf, Donald A. Berry, Eric P. Winer, Clifford A. Hudis, Daniel F. Hayes, Paula Friedman, Matthew Ellis, Lynn Dressler
Final approval of manuscript: Lyndsay N. Harris, Gloria Broadwater, Maysa Abu-Khalaf, David Cowan, Ann D. Thor, Daniel Budman, Constance T. Cirrincione, Donald A. Berry, Eric P. Winer, Clifford A. Hudis, Daniel F. Hayes, Paula Friedman, Matthew Ellis, Lynn Dressler
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomized trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 2.Buzdar AU, Kau SW, Smith TL, et al. Ten-year results of FAC adjuvant chemotherapy trial in breast cancer. Am J Clin Oncol. 1989;12:123–128. doi: 10.1097/00000421-198904000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Savin M, Holmes F, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. BCIRG006: 2nd interim analysis phase III randomized trial comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2 positive early breast cancer patients. 29th Annual San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. abstr 52. [Google Scholar]
- 5.Borg A, Baldetorp B, Ferno M, et al. ErbB2 amplification in breast cancer with a high rate of proliferation. Oncogene. 1991;6:137–143. [PubMed] [Google Scholar]
- 6.van de Vijver MJ, Peters JL, Mooi WJ, et al. Neu-protein overexpression in breast cancer: Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319:1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 7.Thor AD, Berry DA, Budman DR, et al. ErbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Can Inst. 1998;90:1346–1360. doi: 10.1093/jnci/90.18.1346. [DOI] [PubMed] [Google Scholar]
- 8.Paik S, Bryant J, Wolmark N. ErbB-2 Overexpression and response to chemotherapy: NSABP study. Breast Cancer Res Treat. 1998;50:3. abstr 19. [Google Scholar]
- 9.Dressler LG, Berry DA, Broadwater G, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23:4287–4297. doi: 10.1200/JCO.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Paik S, Bryant J, Park C, et al. ErbB-2 and response to doxorubicin inpatients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst. 1998;90:1361–1370. doi: 10.1093/jnci/90.18.1361. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard K, Shepherd L, O'Malley F, et al. Her 2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Houlbrook S, Greenall M, et al. Topoisomerase II alpha co-amplification with erbB-2 in human primary breast cancer and breast cancer cell lines: Relationship to m-AMSA and mitoxantrone sensitivity. Oncogene. 1993;8:933–938. [PubMed] [Google Scholar]
- 13.Kim R, Hirabayashi N, Nishiyama M, et al. Expression of MDR1, GST and Topoisomerase II as an indicator of clinical response to Adriamycin. Anticancer Res. 1991;11:429–431. [PubMed] [Google Scholar]
- 14.Järvinen TA, Tanner M, Rantanen V, et al. Amplification and deletion of topoisomerase II alpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Järvinen TA, Tanner M, Barlund M, et al. Characterization of topoisomerase II alpha amplification and deletion in breast cancer. Genes Chromosomes Cancer. 1999;26:142–150. [PubMed] [Google Scholar]
- 16.Harris LN, Yang L, Liotcheva V, et al. Induction of topoisomerase II activity after ErbB2 activation is associated with a differential response to breast cancer chemotherapy. Clin Cancer Res. 2001;7:1497–1504. [PubMed] [Google Scholar]
- 17.Rowe TC, Chen GL, Hsiang Y, et al. DNA damage by antitumor acridines mediated by mammalian topoisomerase II. Cancer Res. 1986;46:2021–2026. [PubMed] [Google Scholar]
- 18.DiGiovanna M, Stern D. Activation state-specific monoclonal antibody detects tyrosine phosphorylated p185 neu/erbB-2 in a subset of human breast tumors overexpressing this receptor. Cancer Res. 1995;55:1946–1955. [PubMed] [Google Scholar]
- 19.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J Natl Cancer Inst. 1998;90:1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 20.DiGiovanna M, Carter D, Flynn S, et al. Functional assay for HER2/neu demonstrates active signaling in a minority of HER2/neu overexpressing invasive human breast tumors. Br J Cancer. 1996;74:802–806. doi: 10.1038/bjc.1996.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thor A, Liu S, Edgerton S, et al. Activation (tyrosine phosphorylation)of Erb B-2(HER2/neu): A study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18:3230–3239. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- 22.Harris L, Tang C, Yang C, et al. Induction of chemotherapy sensitivity in MCF-7 breast cancer cells by heregulin. Clin Cancer Res. 1998;4:1005–1012. [PubMed] [Google Scholar]
- 23.Jacobson K, Thompson A, Browne G, et al. Automation of fluorescence in situ hybridization pretreatment: A comparative study of different sample types. Mol Diagn. 2000;5:209–220. doi: 10.1054/modi.2000.9731. [DOI] [PubMed] [Google Scholar]
- 24.Carter S, Eklund A, Kohane I, et al. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcomes in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson K, Morrison L, Henderson B, et al. Gene copy mapping of the ERBB2/TOP2A region in breast cancer. Genes Chromosomes Cancer. 2004;40:19–31. doi: 10.1002/gcc.20019. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz R, Schnitt S, Rousseau A, et al. Topoisomerase IIa expression and response to anthracycline-based neoadjuvant chemotherapy: Results from a randomized clinical trial. Mod Pathol. 2003;16:195. [Google Scholar]
- 26a.Knoop A, Knudsen H, Balslev E, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Group. J Clin Oncol. 2005;23:7483–7490. doi: 10.1200/JCO.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26b.Tanner M, Isola J, Wiklund T, et al. Topoisomerase IIa gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu amplified breast cancer: Results from the randomized trial SBG 9401. J Clin Oncol. 2006;24:2428–2436. doi: 10.1200/JCO.2005.02.9264. [DOI] [PubMed] [Google Scholar]
- 27.Di Leo A, Gancberg D, Larsimont D, et al. HER-2 amplification and topoisomerase II gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate and 5-fluorouracil. Clin Cancer Res. 2002;8:1107–1116. [PubMed] [Google Scholar]
- 28. Reference deleted.
- 29. Reference deleted.
- 30.Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC –> T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC –> TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat. 2005;94(suppl 1):S5. [Google Scholar]
- 31.Press M, Bernstein L, Sauter G, et al. Topoisomerase II-alpha gene amplification as a predictor of responsiveness to anthracycline containing chemotherapy in the Cancer International Research Group 006 clinical trial of trastuzumab (Herceptin) in the adjuvant setting. San Antonio Breast Cancer Symposium; December 8-11, 2005; San Antonio, TX. abstr 1045. [Google Scholar]
- 32.O'Malley FP, Chia S, Tu D, et al. Topoisomerase II alpha protein overexpression has predictive utility in a randomized trial comparing CMF to CEF in premenopausal women with node positive breast cancer (NCIC CTG MA. 5) Breast Cancer Res Treat. 2006;100(suppl 1):S18. abstr 38. [Google Scholar]
- 33.Bartlett JMS, Munro A, Dunn JA, et al. Chromosome 17 polysomy as a predictor of anthracycline response: Emerging evidence from the UK NEAT adjuvant breast cancer trial. San Antonio Breast Cancer Conference; December 10-14, 2008; San Antonio, TX. abstr 45. [Google Scholar]
- 34.Lezon-Geyda K, Hicks JB, Tuck DP, et al. Genomic rearrangements in breast cancer: Clinical implications. Biol Ther Breast Cancer. 2007;8:7–11. [Google Scholar]