Abstract
Human papillomavirus (HPV) is now established as the principal cause of an increase in incidence of a subset of head and neck squamous cell cancers (HNCs) in numerous geographic regions around the world. Further study of the epidemiology of HPV-positive HNC will be critical to the development and implementation of public health interventions to reverse these global incidence trends. Here, recent data are reviewed to provide insight into several topics, including incidence trends and projections for HPV-positive HNC; the worldwide HPV-attributable fraction; sex disparities in cancer risk; the epidemiology of oral HPV infection; the latency period between infection and cancer; the potential impact of prophylactic HPV vaccination; and prospects for secondary prevention through screening for oral HPV infection or seroreactivity to viral antigens. The identification of a single necessary cause for any cancer provides a rare and perhaps extraordinary opportunity for cancer prevention.
INTRODUCTION
It has been nearly a decade since the WHO first concluded that human papillomavirus (HPV) type 16 is a cause of oropharyngeal cancer (OPC) and oral cavity cancer.1 The molecular and epidemiologic evidence in support of this conclusion has recently been reviewed.2,3 This causal link between a sexually transmitted infection and head and neck squamous cell carcinomas (HNCs) has led to a paradigm shift in our understanding of HNC risk, with important implications for global cancer prevention. Here, we summarize recent data on the epidemiology of HPV-positive HNC to provide insight into several topics, including worldwide incidence trends and projections, the global HPV-attributable fraction, sex disparities in risk and their behavioral origins, the latency period, the potential effectiveness of HPV prophylactic vaccines, and prospects for secondary prevention or screening with either oral HPV detection or HPV serology.
GLOBAL INCIDENCE TRENDS
Over the last few years, evidence has accumulated to support a global trend in increased OPC incidence, perhaps most striking in North America and northern Europe.4–11 Even in Taiwan, a geographic region with one of the highest incidence rates for HNC in the world, OPC rates (particularly for tonsil) increased more sharply from 1995 to 2009 than any other anatomic site.12 To further elucidate global incidence trends, worldwide cancer registry data (Cancer Incidence in Five Continents) were used to compare incidence trends from 1983 to 2002 for upper aerodigestive tract malignancies etiologically associated with HPV infection (ie, OPC) versus tobacco smoking (ie, oral cavity and lung squamous cell carcinomas).13 In general, OPC incidence rates were higher and increased more sharply among men than women. Furthermore, OPC incidence increased among young men (< 60 years old) in several economically developed countries, despite concomitant declines in incidence for oral cavity and lung squamous cell carcinomas. These contrasts suggest a role of HPV infection in increasing OPC incidence rates among men. However, among women, incidence increased for all three cancers in tandem, supporting a dominant effect of smoking on increasing incidence rates. These data are consistent with a hypothesis of a greater impact of HPV on OPC incidence trends for men over the last several decades, in contrast to smoking for women.
Several molecular studies have demonstrated HPV to be responsible for observed OPC incidence trends.4,14,15 In these studies, analysis of archived specimens demonstrated significant increases in incidence for HPV-positive OPC in contrast to declines in HPV-negative OPC with or without oral cavity cancer. Recent data further support these observations of increased HPV prevalence in tumors over time. A meta-analysis of 2,099 OPCs evaluated in the US literature observed HPV DNA prevalence by polymerase chain reaction (PCR) to significantly increase from 20.9% before 1990 to 65.4% after 2000.16 A separate meta-analysis including 5,396 OPCs observed increases from 40.5% before 2000 to 72.2% after 2005, with significant increases observed in North America and Europe.17 An increase in HPV DNA–positive OPC from 1995 to 2010 of 20.2% to 63.5% was reported in eight centers in Australia.5 In the Netherlands, a referral center observed an increase from 5.1% to 29.0% during 1990 to 2010 based on dual positivity for p16 expression and HPV DNA.18 Even in geographic regions with a small proportion of OPCs testing positive for HPV (eg, Spain), the proportion positive seems to be increasing over time.19 It is important to note, however, that studies based on proportional estimates alone cannot discriminate between a true increase in HPV-positive OPC incidence versus a relative reduction in incidence of HPV-negative cancers in a population. However, when combined with the population-based incidence data noted earlier, evidence is accumulating to support oral HPV infection as the underlying cause of an ongoing global increase in OPC incidence.
Given the historical increases in OPC incidence over the last several decades, it is of public health interest to clarify to what extent the burden of OPC may continue to increase and for how long. In England, HNC incidence increased by 58% from 1995 to 2011, most sharply for OPC. In contrast, rates for lung cancer, strongly associated with smoking, remained stable.20 Notably, OPC incidence trends paralleled increased rates for genital warts and genital herpes in England from 1995 to 2011.20 OPC incidence was projected to further increase in England by 239% from 2011 to 2025, at which point OPC would compose 35% of all HNCs.20
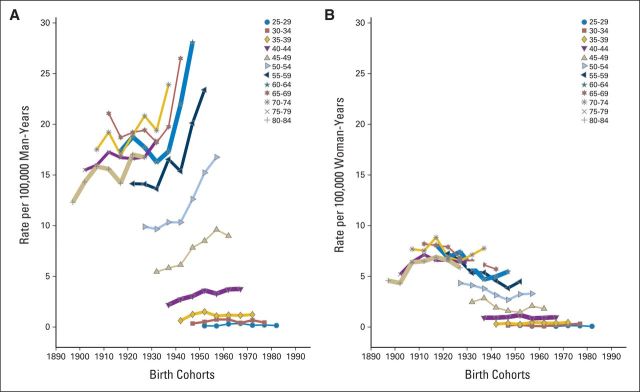
In the United States, the Centers for Disease Control and Prevention noted that OPCs were one of only five cancer types that increased in incidence from 1975 to 2009.11 A previous analysis of Surveillance, Epidemiology, and End Results (SEER) data from 1973 to 2004 attributed these incidence trends to a strong birth cohort effect.21 For all age groups between 40 and 70 years, OPC risk significantly increased for each successive birth cohort born after 1940. An updated analysis including new data from 2005 to 2011 (Fig 1) revealed continued, alarming increases in age-specific incidence rates by birth cohort among all men born after 1940, age 40 to 70 years. Notably, no evidence of a plateau in incidence rates was observed in men age 50 years and older. The broad age range (age 40 to 70 years) affected, as well as the increased incidence observed in the birth cohort age 40 to 44 years (1967 birth cohort), indicate that incidence rates for OPC would continue to increase for at least 30 years in the United States. Previous estimates of projected trends through 2030 appear accurate.14 Importantly, opposite trends were observed among birth cohorts of women (Fig 1), leading to an increase in the relative rates for OPC in men versus women by age and calendar period (Fig 2), reinforcing a dominant effect of HPV on increasing incidence rates exclusively among men in the United States.
Fig 1.
Shown are incidence rates for oropharyngeal cancers among (A) men and (B) women, stratified by cohort year of birth (in 10-year overlapping groups) and age (in 5-years groups). Data were derived from nine cancer registries covered by the National Cancer Institute's SEER program (1973 to 2011). Oropharyngeal cancers include the base of tongue, lingual tonsil, soft palate, uvula, tonsil, oropharynx, and Waldeyer's ring.
Fig 2.
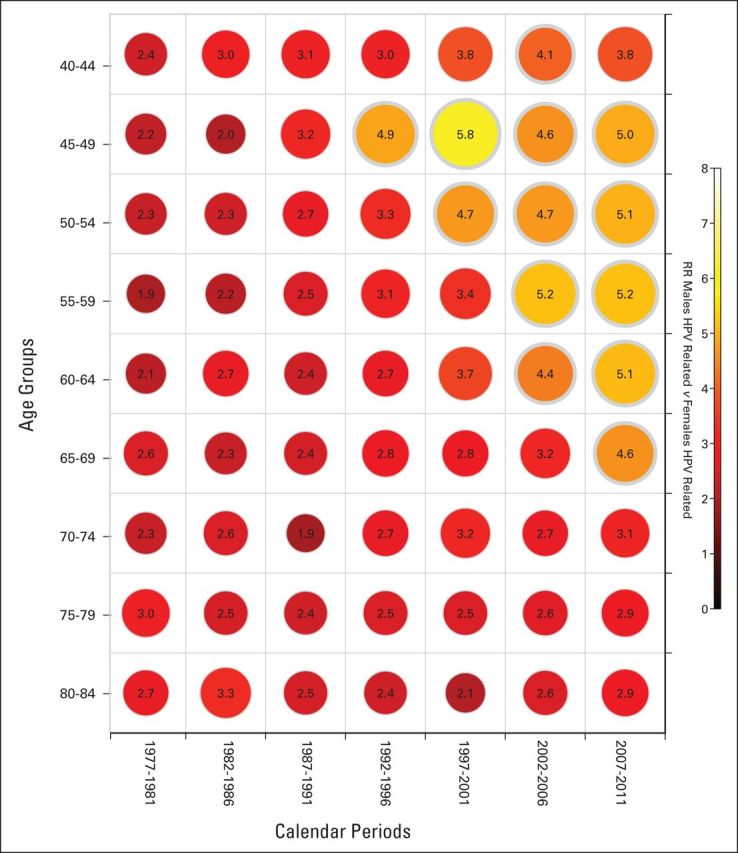
Shown are male-female incidence rate ratios (RRs) for oropharynx cancers, stratified by age in rows (5-year groups) and calendar periods in columns (5-year groups). The diagonals across the age groups and calendar years represent birth cohorts. The scale for the color-coded RRs is also shown on the right. Data were derived from nine cancer registries covered by the National Cancer Institute's SEER program (1973 to 2011). Oropharyngeal cancers include the base of tongue, lingual tonsil, soft palate, uvula, tonsil, oropharynx, and Waldeyer's ring. HPV, human papillomavirus.
Of particular interest is the contribution of declines in tonsillectomy rates to increased incidence of HPV-positive OPC. In the United States, tonsillectomy rates declined from 1965 through 1986 from 63.4 to 11.7 per 10,000 in all ages.22 Analysis of population-level data in Denmark observed an approximately 60% reduction in tonsil cancer risk associated with tonsillectomy but no effect on risk of base of tongue tumors.23,24 Therefore, tonsillectomy trends are unlikely to account for the dramatic increase in rates for all OPCs.
THE HPV-ETIOLOGIC FRACTION
Of considerable interest from a public health perspective is the proportion of all HNCs worldwide attributable to HPV infection and thus potentially preventable by HPV vaccination. Recent meta-analyses have been published with a goal of providing such estimates and have observed significant heterogeneity in the HPV-attributable proportion (as measured by HPV DNA detection) by cancer anatomic site, geographic location, and calendar time.17,25–27 Generally, meta-analyses have found HPV DNA presence to be highest for OPC in North America and northern Europe and in recent calendar periods. For example, a recent meta-analysis summarizing data from 12,263 patients with HNC from 44 countries estimated the HPV-attributable proportion to be 45.8% for OPC, 24.2% for oral cavity, and 22.1% for larynx based on HPV DNA detection by PCR. In a subset of patients with OPC, dual positive testing for either p16 expression (by immunohistochemistry [IHC]) or HPV E6/E7 mRNA expression, considered a more accurate assessment of causality, was observed in approximately 87% of HPV DNA–positive patients, whereas dual testing for oral cavity and larynx sites was rarely performed. With regard to HPV type distribution, HPV16 prevalence accounted for 82.2% of all HPV DNA–positive cases and was higher for OPC (> 90%) than for other cancer types. In agreement with other meta-analyses,17,25 the highest proportions were observed in tonsil and base of tongue cancers (subsites of the oropharynx), and the proportion of OPCs that tested positive for HPV increased over calendar time.
Although a useful summary of the literature, limitations of meta-analyses include nonuniform HPV testing and reliance on HPV DNA testing alone, likely resulting in overestimates of the proportion of HNCs caused by HPV infection. Importantly, preliminary data have been reported by Castellsague et al28 regarding worldwide HPV DNA prevalence estimates from 3,741 HNCs diagnosed after 1990 and collected from 32 countries. The strength of this study is its uniform and extensive testing of paraffin materials for sample quality and HPV DNA, E6/E7 mRNA expression, and p16 IHC. Estimates for the proportion causally associated with HPV decreased from 9.3% of oral cavity, 27.6% of oropharynx, and 7.2% of larynx cancers based on HPV DNA testing alone to 3.0%, 18.3%, and 1.6%, respectively, based on cotesting for HPV DNA, mRNA, and p16 IHC. Notably, this difference may be accounted for by confounding of the virus-tumor association by smoking. For example, 20% of current smokers in the United States were found to have an oral HPV infection,29 and therefore, detection of pathophysiologically unrelated HPV infections by PCR would be expected among current smokers diagnosed with HNC.30
It is important to note that a small etiologic fraction for HPV in oral cavity and laryngeal cancers nevertheless significantly increases the burden of HPV-positive cancers worldwide. For example, a 3.0% prevalence among 300,400 incident oral cavity cancers and a 1.6% prevalence among 157,000 larynx cancers diagnosed worldwide in 201231 are equivalent to 11,542 cases, adding considerably to the estimated 22,000 OPCs attributed to HPV infection worldwide as of 2008.32
Importantly, the 18% to 28% worldwide estimate for the HPV-attributable fraction for OPC would be inappropriate to apply to a specific population for determination of disease burden and associated costs. For example, this estimate is inappropriate for the United States, where considerable data support an HPV-etiologic fraction for OPC of at least 72% after 2000.14,33 Disease burden is also strongly dependent on incidence. A population with high incidence but low HPV-attributable proportion may nevertheless have a higher burden of HPV-attributable disease compared with a population with a low incidence but high HPV-attributable proportion. Similarly, women may have a higher HPV-attributable proportion than men in some populations, but disease burden would still be less for women as a result of the uniformly lower incidence rates globally.
RISK FACTORS FOR HPV-POSITIVE OPC
Heterogeneous patterns in incidence rates, trends, and etiologic fractions for HPV across geographic regions would originate from distributions for the principal risk factors for HNC across populations. HNCs are etiologically heterogeneous, with a majority caused by tobacco and/or alcohol use and a minority caused by HPV infection.2,34 In 2012, prevalence rates of tobacco smoking varied considerably across 187 countries worldwide, from 5.0% to 61.1% among men and 0.8% to 34.7% among women.35 Smoking prevalence trends from 1980 to 2012 also differed significantly across geographic regions, with notable decreases observed for men and women in North America and northern Europe, in contrast to significant increases in southern and eastern Europe.35
Sexual behavior is now established as a risk factor for HNC2 and is most strong and consistent as a risk factor for OPC. Lifetime number of oral sexual partners seems to be the behavioral measure most strongly, consistently, and specifically associated with OPC (tonsil and base of tongue).2,36 As noted for smoking, sexual behavior also dramatically differs across geographic regions, as observed among control populations enrolled onto case-control studies summarized by the International Head and Neck Cancer Epidemiology consortium.36 The proportion of controls who reported ever having had oral sex varied among men from 9% in India to 78% in the United States and among women from 12% in Brazil to 66% in the United States. Notably, the proportion of men and women reporting a history of oral sex also significantly increased among recent birth cohorts in comparison to distant birth cohorts (15% to 54% of men and 13% to 69% of women born before 1930 v after 1960, respectively).36 Changes in smoking and sexual behaviors across calendar time thus likely underlie the incidence trends noted earlier.
Case-control studies have established oral HPV infection as the principal risk factor for HPV-positive OPC,2 and the overwhelming majority (≥ 90%) of oral HPV infections are sexually acquired.29 In particular, consistent associations between oral sexual behavior (eg, number of partners) and prevalent oral HPV infection are emerging across studies conducted in heterogeneous patient populations.37–41 Notably, partner studies have found type-specific oral HPV infection to be associated with the same type detected in the genital tract or mouth of a sexual partner.42,43 Importantly, oral sexual behaviors (including oral-oral, oral-genital, and oral-anal) have also been associated with risk of acquiring a new oral HPV infection.44–47
Considerable insights into heterogeneity in risk for HPV-positive OPC by age, sex, race, geography, and calendar time can thus be gained through the study of the epidemiology of oral HPV infection. To date, parallels between oral HPV infection prevalence and OPC incidence are best explored for the U.S. population.29 In the United States from 2000 to 2009, OPC incidence rates were four-fold higher and increased more sharply among men than women.11 HPV prevalence in OPCs collected from SEER registries from 1984 to 2004 in the United States was higher in men than women and increased significantly among men but not women over this calendar period, thus implicating HPV as a factor in these sex differences.14
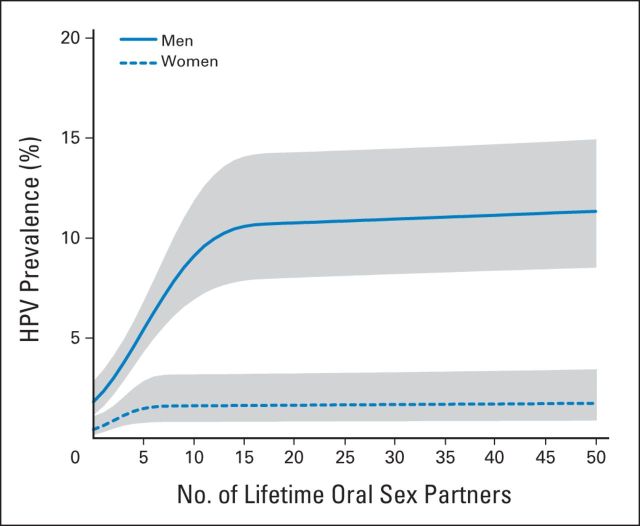
The four-fold higher OPC rates among men versus women are likely explained in part by the three- to five-fold higher prevalence of oral high-risk and type 16 HPV infections in men versus women in the United States in 2009 to 2010.29 High-risk oral HPV infection was most prevalent among men age 55 to 64 years, who also experienced the highest uptick in OPC incidence from 2000 to 2009.11 Recent data provide insight for the male predominance of oral HPV infection in the United States. Although men have a higher average number of lifetime oral sexual partners than women in the United States, this does not entirely explain the sex difference in prevalence.48,49 A recent analysis of the National Health and Nutrition Examination Survey data (2009 to 2012) demonstrated the per sexual partner increase in high-risk oral HPV prevalence to be three-fold greater for men than for women, consistent with reported higher transmission rates for HPV from female to male than vice versa.50 Also noted was a plateau in prevalence among men at approximately 15 oral sexual partners in contrast to approximately five partners among women (Fig 3). Thus, the prevalence of oral HPV infection continues to increase among men with more than five partners but not among women. This sex difference may reflect reduced seroconversion rates among men versus women after genital HPV infection,50 resulting in greater protection against subsequent oral infections among women. Natural seroconversion to genital HPV16 infection reduces risk of subsequent infection among women by approximately 50%.51–53 Thus, increased oral sexual behaviors among recent birth cohorts of men and women would result in greater prevalence increases for oral HPV infection and consequent accelerated rates for HPV-positive OPC in men versus women over the last several decades in the United States. This likely explains the trends in rate ratios observed in Figure 2.
Fig 3.
Shown are the associations of lifetime number of oral sex partners with oral human papillomavirus (HPV) prevalence among men (solid line) and women (dashed line) in the US population age 14 to 69 years. Data are based on the National Health and Nutrition Examination Survey (NHANES) 2009 to 2010 and 2011 to 2012 cycles. The shaded areas represent the 95% CIs. Reprinted by permission from the American Association for Cancer Research: Chaturvedi A, Graubard B, Broutian T, et al: NHANES 2009 to 2012 findings: Association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res [epub ahead of print on April 14, 2015].
For cervical cancer, the latency period between HPV infection and cancer development has been estimated by comparing the age at peak prevalence (approximately 20 years) for infection to median age at diagnosis (approximately 49 years).54 Figure 4 shows oral high-risk HPV prevalence in the US population (age 14 to 69 years) per the National Health and Nutrition Examination Survey (Fig 4A) and OPC incidence per SEER as a function of age (Fig 4C). Oral oncogenic HPV infection prevalence peaked at ages 25 to 30 years and 55 to 60 years, and median age at OPC diagnosis was 63 years21 (58 years for HPV-positive OPC).14 From this, we estimate an average latency period for HPV-positive OPC of approximately 10 to 30 years, assuming either peak in prevalence could contribute to risk. The disease relevance of either of the two peaks for oral oncogenic HPV infection, however, remains an important unaddressed question.
Fig 4.
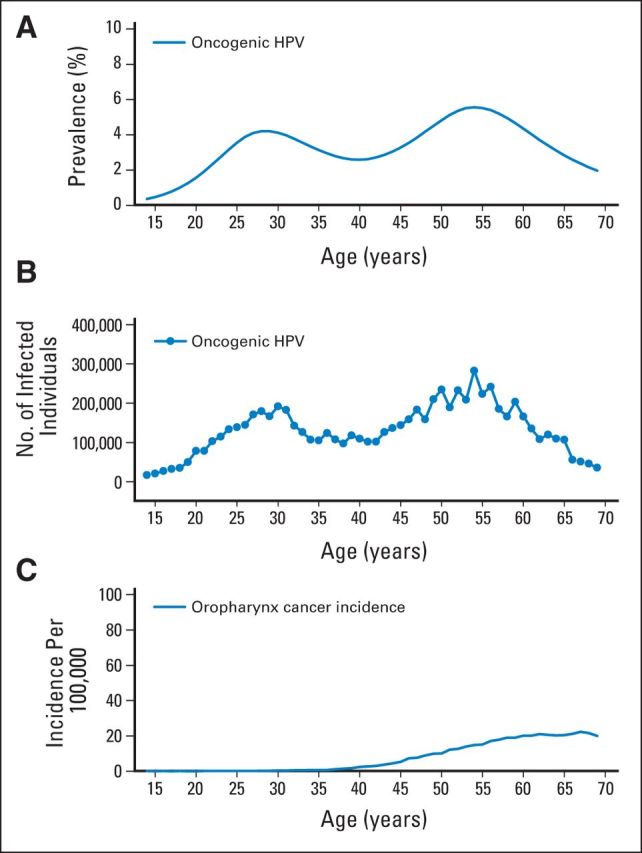
Shown are (A) the prevalence of 12 oral oncogenic human papillomavirus (HPV) types, (B) the number of individuals with prevalent infection with 12 oral oncogenic HPV types, and (C) the incidence rate per 100,000 of oropharyngeal cancer for ages 14 to 69 years in the US population. Oral HPV data are based on the National Health and Nutrition Examination Survey 2009 to 2010 and 2011 to 2012 cycles. Cancer incidence data are based on incidence rates in the year 2011 from nine cancer registries covered by the National Cancer Institute's SEER program (1973 to 2011). Oropharyngeal cancers include the base of tongue, lingual tonsil, soft palate, uvula, tonsil, oropharynx, and Waldeyer's ring.
PROSPECTS FOR PRIMARY PREVENTION
Prophylactic HPV vaccines are 90% to 100% effective in preventing HPV infections and associated anogenital precancerous lesions55 and are projected to dramatically reduce global cervical cancer burden after 2050.56 In contrast, vaccine efficacy against oral HPV infection and related diseases is unknown. To date, regulatory agencies have required a clinical disease end point (eg, precancer) for trials of HPV vaccine efficacy, and the inability to detect such lesions for HPV-positive OPC thus precluded clinical trials. In 2014, the WHO recommended that regulatory agencies accept efficacy against incident and persistent HPV infection as an acceptable end point for HPV vaccine trials, because this is now an established surrogate for disease risk.57 A single study has observed point prevalence for oral HPV16/18 infection to be lower 4 years after vaccination among women who receive HPV vaccination versus placebo.58 Although considered by many to be sufficient evidence for efficacy given precedence at anogenital sites, these data do not meet the new WHO standards of prevention of an incident and persistent infection that would be required in a prospective clinical trial to establish vaccine policy recommendations.
Natural history studies of HPV infection are beginning to reveal significant differences by sex and anatomic site50 that may have future implications for HPV vaccine policy. Men seem to remain at high risk of incident infection regardless of age.50 Moreover, oral infections are less likely to clear among older men.44 Assuming high vaccine efficacy against oral HPV infections that lead to cancer, older men (> 26 years of age) may thus derive protective benefit from vaccination. Even assuming high HPV vaccine efficacy and high population coverage, OPC incidence rates would not be expected to decline until after cervical cancer, given higher median age at diagnosis for OPC than cervical cancer. This also assumes long-lasting protection and no contribution of the second peak in high-risk oral HPV infection prevalence to disease risk. Therefore, current incidence trends for OPC would not reverse as a result of vaccination until after 2060.
SECONDARY PREVENTION BY SCREENING
At this time, screening for HPV-positive OPC is infeasible as a result of our current inability to detect precursor lesions and subclinical or early-stage cancers, as well as the absence of an established intervention to reduce cancer incidence or cancer mortality. However, recent provocative data indicate it may be possible to identify high-risk individuals for whom management algorithms could be developed. For example, advances in imaging59,60 may identify oropharyngeal lesions amenable to biopsy61 should high-risk individuals otherwise be identified.
Markers of HPV exposure may provide an opportunity to identify high-risk individuals. In case-control studies, the presence of oral HPV infection (overall, oncogenic, or HPV16) or HPV serum antibodies (L1, E6, or E7) was strongly associated with OPC. For example, oral HPV16 infection has been estimated to confer a three to 230 times increased risk and HPV16 E6/E7 seropositivity a nine to 231 times increased risk for OPC.2 These robust associations were driven in part by the low prevalence of oral HPV16 infection (approximately 1.0%) and serum HPV16 E6/E7 seropositivity (0.5% to 5%) in healthy individuals. However, strong estimates of relative risk do not always underscore clinical utility.62 The potential use of these assays for either detection of subclinical disease or identification of high-risk populations depends on assay sensitivity and predictive value, respectively. In the literature, sensitivity for HPV16 DNA–positive OPC of oral HPV16 infection has ranged from approximately 50% to 80%63–65 and of HPV16 E6/E7 seropositivity from approximately 50% to 90%.66–69 Specificity of both assays seems to be higher than sensitivity. These assays may be of insufficient sensitivity to serve as diagnostic tests. A recent study observed HPV16 E6 seropositivity to predate the development of OPC by as long as 10 years, indicating that seroreactivity may identify individuals at higher risk than the general population.70 By analogy to the cervical literature,71 individuals with a persistent oral HPV16 infection would likely be at similar risk.
Despite the impressive specificity of oral HPV16 infection or E6/E7 antibodies, the rarity of both markers combined with the low incidence of HPV-positive OPC in the general population make screening based on a single time point of marker positivity among the general population a challenging prospect. Shown in Figure 4B are the number of individuals in the US population with a prevalent oral HPV16 infection as a function of age as well as corresponding incidence rates. In the US population age 40 to 69 years, approximately 1.3% of individuals (1.4 million) have a prevalent oral HPV16 infection. Although not US population based, approximately 0.7% would be expected to be HPV16 E6 seropositive.72 These data, along with current incidence rates for OPC in the US population, allow us to make several approximations (Table 1). Among men and women age 40 to 69 years in the United States, one in 77 would have an oral HPV16 infection and one in 143 would be HPV16 E6 seropositive. Assuming all OPCs are attributable to HPV16 infection, approximately 0.7% of individuals with oral HPV16 infection and approximately 1.25% of HPV16 E6 seropositive individuals would also have concurrent OPC. It is important to note that this is an estimate of concurrent cancer among biomarker-positive individuals and does not estimate per-year cumulative risk. These numbers would indicate that approximately 10,500 individuals would need to be screened to detect one cancer. However, ongoing studies are evaluating whether it is possible to improve on these approximations through risk stratification and/or combined or repeated measures of risk.
Table 1.
Potential Screening Scenarios in the US Population Based on Single Time Point Detection of Oral HPV16 Infection, HPV16 E6 Seropositivity, and Incidence of Oropharyngeal Cancer
| Scenario | Men and Women, Age 40-69 Years With Oral HPV16 Infection | Men and Women, Age 40 to 69 Years With HPV16 E6 Seropositivity |
|---|---|---|
| No. of individuals in the US population* | 112 million | 112 million |
| Prevalence of marker, %* | 1.3 | 0.7 |
| No. of individuals with marker positivity* | 1.4 million | 784,000 |
| Incidence rate of oropharyngeal cancer† | 9.5 per 100,000 | 9.5 per 100,000 |
| No. of incident oropharyngeal cancers‡ | ∼9,800 | ∼9,800 |
| NNS to identify one individual with marker positivity§ | 77 | 143 |
| Probability of concurrent cancer given infection, %‖ | 0.70 | 1.25 |
| NNS for prevalent oropharynx cancer¶ | ∼10,500 | ∼10,500 |
Abbreviations: HPV, human papillomavirus; NNS, number needed to screen.
Derived from National Health and Nutrition Examination Survey 2009-2012 data.
Derived from nine cancer registries in the National Cancer Institute's SEER program for the year 2011. Oropharyngeal cancers include the base of tongue, lingual tonsil, soft palate, uvula, tonsil, oropharynx, and Waldeyer's ring. The estimates indicate incidence of all oropharyngeal cancers, both HPV positive and HPV negative.
Estimated based on the number of individuals in the US population and incidence rate of oropharyngeal cancers. The number of cancers for ages 40 to 69 years was based on the proportion of cancers in ages 14 to 69 that occur in individuals age 40 to 69 years.
Denotes the number needed to be screened to detect one individual with prevalent oral HPV16 infection or HPV16 E6 seropositivity. For example, estimated as 100 divided by the prevalence of oral HPV16 infection.
Estimated as the number of oropharynx cancers per year divided by the number of individuals with prevalent oral HPV16 infection or HPV16 E6 seropositivity.
Estimated as the number of oropharynx cancers per year divided by the total number of individuals in the US population.
CONCLUSION
It is now possible to state that oral HPV infection is a necessary cause of a dramatic and ongoing increase in OPC incidence largely among men in numerous countries worldwide. Given that incidence rates continue to accelerate, experts in the field now debate as to whether this constitutes an epidemic. Regardless, the identification of a single necessary cause for this cancer provides a rare and perhaps extraordinary opportunity for the development of public health interventions. Prophylactic HPV vaccination holds considerable promise in reversing these incidence trends after 2060. However, it is difficult for public health officials and physicians to advocate HPV vaccination among men for the prevention of HPV-positive OPC in the absence of clinical trial evidence. Additional epidemiologic study of the natural history of oral HPV infection and clinical trials designed to develop and evaluate strategies for secondary prevention and early detection of this malignancy are important alternative strategies to combat this disease for the next four decades.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Maura L. Gillison, Anil K. Chaturvedi, Carole Fakhry
Administrative support: Maura L. Gillison
Data analysis and interpretation: Anil K. Chaturvedi, William F. Anderson, Carole Fakhry
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Maura L. Gillison
Honoraria: BMS
Travel, Accommodations, Expenses: Merck, Sanofi
Anil K. Chaturvedi
No relationship to disclose
William F. Anderson
No relationship to disclose
Carole Fakhry
No relationship to disclose
REFERENCES
- 1.International Agency for Research on Cancer (ed) Human Papillomaviruses. Lyon, France: World Health Organization; 2007. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; p. 670. [Google Scholar]
- 2.Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(suppl 5):F34–F54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Castellsagué X, Chaturvedi A, et al. Eurogin Roadmap: Comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134:497–507. doi: 10.1002/ijc.28201. [DOI] [PubMed] [Google Scholar]
- 4.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 5.Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus related oropharyngeal cancer in Australia over the last two decades. Head Neck. doi: 10.1002/hed.23942. [epub ahead of print on December 18, 2014] [DOI] [PubMed] [Google Scholar]
- 6.Forte T, Niu J, Lockwood GA, et al. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992-2009. Cancer Causes Control. 2012;23:1343–1348. doi: 10.1007/s10552-012-0013-z. [DOI] [PubMed] [Google Scholar]
- 7.Blomberg M, Nielsen A, Munk C, et al. Trends in head and neck cancer incidence in Denmark, 1978-2007: Focus on human papillomavirus associated sites. Int J Cancer. 2010;129:733–741. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 8.Reddy VM, Cundall-Curry D, Bridger MW. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985-2006. Ann R Coll Surg Engl. 2010;92:655–659. doi: 10.1308/003588410X12699663904871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braakhuis BJ, Visser O, Leemans CR. Oral and oropharyngeal cancer in the Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;45:e85–e89. doi: 10.1016/j.oraloncology.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Mork J, Møller B, Dahl T, et al. Time trends in pharyngeal cancer incidence in Norway 1981-2005: A subsite analysis based on a reabstraction and recoding of registered cases. Cancer Causes Control. 2010;21:1397–1405. doi: 10.1007/s10552-010-9567-9. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang TZ, Hsiao JR, Tsai CR, et al. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int J Cancer. 2015;137:395–408. doi: 10.1002/ijc.29330. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus. Vaccine. 2010;28:3269–3272. doi: 10.1016/j.vaccine.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 16.Stein AP, Saha S, Yu M, et al. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27:462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehanna H, Beech T, Nicholson T, et al. The prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer: Systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 18.Rietbergen MM, Leemans CR, Bloemena E, et al. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int J Cancer. 2013;132:1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigo JP, Heideman DA, García-Pedrero JM, et al. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009) Int J Cancer. 2014;134:487–492. doi: 10.1002/ijc.28355. [DOI] [PubMed] [Google Scholar]
- 20.Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncol. 2015;51:341–348. doi: 10.1016/j.oraloncology.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services. National Center for Health Statistics. Hyattsville, MD: Department of Health and Human Services Publication; 1989. Trends in Hospital Utilization: United States, 1965-1986. [Google Scholar]
- 23.Fakhry C, Andersen K, Christensen J, et al. The impact of tonsillectomy upon the risk of oropharyngeal carcinoma diagnosis and prognosis in the Danish Cancer Registry. Cancer Prev Res (Phila) doi: 10.1158/1940-6207.CAPR-15-0101. [epub ahead of print on April 20, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaturvedi A. Tonsillectomy and risk of oropharyngeal cancer. Cancer Prev Res (Phila) doi: 10.1158/1940-6207.CAPR-15-0135. [epub ahead of print on April 20, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abogunrin S, Di Tanna GL, Keeping S, et al. Prevalence of human papillomavirus in head and neck cancers in European populations: A meta-analysis. BMC Cancer. 2014;14:968. doi: 10.1186/1471-2407-14-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 27.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50:370–379. doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Castellsague X, Alemany L, Holzinger D, et al. Estimation of the HPV etiological fraction in over 4,000 head and neck cancers worldwide. Presented at the International Human Papillomavirus Conference; August 24, 2014; Seattle, WA. [Google Scholar]
- 29.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 32.de Martel C, Ferlay J, Franceschi S, et al. The global burden of cancers attributable to infections in the year 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 33.Steinau M, Saraiya M, Goodman MT, et al. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis. 2014;20:822–828. doi: 10.3201/eid2005.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 35.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 36.Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendes BL, Wang SJ, Groppo ER, et al. Oral human papillomavirus infection in men who have sex with men with anal squamous intraepithelial lesions. Head Neck. doi: 10.1002/hed.24006. [epub ahead of print on January 12, 2015] [DOI] [PubMed] [Google Scholar]
- 38.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–133. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Souza G, Kluz N, Wentz A, et al. Oral human papillomavirus (HPV) infection among unvaccinated high-risk young adults. Cancers (Basel) 2014;6:1691–1704. doi: 10.3390/cancers6031691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahlstrom KR, Burchell AN, Ramanakumar AV, et al. Sexual transmission of oral human papillomavirus infection among men. Cancer Epidemiol Biomarkers Prev. 2014;23:2959–2964. doi: 10.1158/1055-9965.EPI-14-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lang Kuhs KA, Gonzalez P, Struijk L, et al. Prevalence of and risk factors for oral human papillomavirus among young women in Costa Rica. J Infect Dis. 2013;208:1643–1652. doi: 10.1093/infdis/jit369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mbulawa ZZ, Johnson LF, Marais DJ, et al. Risk factors for oral human papillomavirus in heterosexual couples in an African setting. J Infect. 2014;68:185–189. doi: 10.1016/j.jinf.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Vogt SL, Gravitt PE, Martinson NA, et al. Concordant oral-genital HPV infection in South Africa couples: Evidence for transmission. Front Oncol. 2013;3:303. doi: 10.3389/fonc.2013.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beachler DC, Sugar EA, Margolick JB, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol. 2015;181:40–53. doi: 10.1093/aje/kwu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelstein ZR, Schwartz SM, Hawes S, et al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis. 2012;39:860–867. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickard R, Xiao W, Broutian T, et al. The prevalence and incidence of oral human papillomavirus infection among young men and women, age 18-30 years. Sex Transm Dis. 2012;39:559–566. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- 47.Kreimer AR, Pierce Campbell CM, Lin HY, et al. Incidence and clearance of oral human papillomavirus infection in men: The HIM cohort study. Lancet. 2013;382:877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Souza G, Cullen K, Bowie J, et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9:e86023. doi: 10.1371/journal.pone.0086023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaturvedi A, Graubard B, Broutian T, et al. NHANES 2009-2012 findings: Association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. doi: 10.1158/0008-5472.CAN-14-2843. [epub ahead of print on April 14, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 2015;136:2752–2760. doi: 10.1002/ijc.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho GY, Studentsov Y, Hall CB, et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186:737–742. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- 52.Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson L, Pawlita M, Castle PE, et al. Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210:448–455. doi: 10.1093/infdis/jiu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiffman M, Solomon D. Clinical practice: Cervical-cancer screening with human papillomavirus and cytologic cotesting. N Engl J Med. 2013;369:2324–2331. doi: 10.1056/NEJMcp1210379. [DOI] [PubMed] [Google Scholar]
- 55.Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol. 2013;10:400–410. doi: 10.1038/nrclinonc.2013.84. [DOI] [PubMed] [Google Scholar]
- 56.Jit M, Brisson M, Portnoy A, et al. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. Lancet Glob Health. 2014;2:e406–e414. doi: 10.1016/S2214-109X(14)70237-2. [DOI] [PubMed] [Google Scholar]
- 57.International Agency for Research on Cancer (ed) IARC Working Group Reports. Geneva, Switzerland: World Health Organization; 2014. Primary end-points for prophylactic HPV vaccine trials; pp. 1–104. [PubMed] [Google Scholar]
- 58.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fakhry C, Agrawal N, Califano J, et al. The use of ultrasound in the search for the primary site of unknown primary head and neck squamous cell cancers. Oral Oncol. 2014;50:640–645. doi: 10.1016/j.oraloncology.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanco RG, Califano J, Messing B, et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PLoS One. 2014;9:e87565. doi: 10.1371/journal.pone.0087565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fakhry C, Rosenthal BT, Clark DP, et al. Associations between oral HPV16 infection and cytopathology: Evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila) 2011;4:1378–1384. doi: 10.1158/1940-6207.CAPR-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castle PE. Teaching moment: Why promising biomarkers do not always translate into clinically useful tests. J Clin Oncol. 2014;32:359–361. doi: 10.1200/JCO.2013.52.3076. [DOI] [PubMed] [Google Scholar]
- 63.D'Souza G, Gross ND, Pai SI, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408–2415. doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahn SM, Chan JY, Zhang Z, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:846–854. doi: 10.1001/jamaoto.2014.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nordfors C, Vlastos A, Du J, et al. Human papillomavirus prevalence is high in oral samples of patients with tonsillar and base of tongue cancer. Oral Oncol. 2014;50:491–497. doi: 10.1016/j.oraloncology.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: The ARCAGE study. J Natl Cancer Inst. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 67.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 68.Koslabova E, Hamsikova E, Salakova M, et al. Markers of HPV infection and survival in patients with head and neck tumors. Int J Cancer. 2013;133:1832–1839. doi: 10.1002/ijc.28194. [DOI] [PubMed] [Google Scholar]
- 69.Liang C, Marsit CJ, McClean MD, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72:5004–5013. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen HC, Schiffman M, Lin CY, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst. 2011;103:1387–1396. doi: 10.1093/jnci/djr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: A pooled analysis. Cancer Epidemiol Biomarkers Prev. 2015;24:683–689. doi: 10.1158/1055-9965.EPI-14-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]