Abstract
Purpose
Geographic access to care may be associated with receipt of chemotherapy but has not been fully examined. This study sought to evaluate the association between density of oncologists and travel distance and receipt of adjuvant chemotherapy for colon cancer within 90 days of colectomy.
Patients and Methods
Patients in the National Cancer Data Base with stage III colon cancer, diagnosed between 2007 and 2010, and age 18 to 80 years were selected. Generalized estimating equation clustering by hospital service area was conducted to examine the association between geographic access and receipt of oncology services, controlling for patient sociodemographic and clinical characteristics.
Results
Of 34,694 patients in the study cohort, 75.7% received adjuvant chemotherapy within 90 days of colectomy. Compared with travel distance less than 12.5 miles, patients who traveled 50 to 249 miles (odds ratio [OR], 0.87; P = .009) or ≥ 250 miles (OR, 0.36; P < .001) had decreased likelihood of receiving adjuvant chemotherapy. Density level of oncologists was not statistically associated with receipt of adjuvant chemotherapy (low v high density: OR, 0.98; P = .77). When stratifying analyses by insurance status, non–privately insured patients who resided in areas with low density of oncologists were less likely to receive adjuvant chemotherapy (OR, 0.85; P = .03).
Conclusion
Increased travel burden was associated with a decreased likelihood of receiving adjuvant chemotherapy, regardless of insurance status. Patients with nonprivate insurance who resided in low-density oncologist areas were less likely to receive adjuvant chemotherapy. If these findings are validated prospectively, interventions to decrease geographic barriers may improve the timeliness and quality of colon cancer treatment.
INTRODUCTION
Evidence-based guidelines1–5 recommend the use of adjuvant chemotherapy in patients with stage III colon cancer within 90 days of colectomy to improve disease-free and overall survival; however, a substantial proportion of patients in the United State do not receive this treatment. Factors associated with not receiving chemotherapy after colectomy include nonwhite race,6,7 older age,6 low socioeconomic status (eg, low income6,7 or lack of insurance7–9), comorbidity,10 and surgical complications.6,11 Geographic access to care is another factor that may also be associated with receipt of chemotherapy. Geographic access to care refers to the availability of oncologists in close proximity and/or travel burden (eg, travel distance, travel time). Studies have indicated that travel burden is associated with a reduction of preventive care services, delay in emergency treatment, and worse health outcomes.12–17 Less is known, however, about the relationship between geographic access and quality of cancer care.
With regard to cancer diagnosis, greater availability of physicians seems to be associated with earlier stage of diagnosis and better survival outcomes.18–21 Studies demonstrate that oncologists are unevenly distributed across the country with low or no representation in rural areas.16,22,23 A previous study using the Surveillance, Epidemiology, and Ends Results–Medicare data set found that patients older than age 65 years with stage III colon cancer who reside in areas with oncologists have a greater likelihood of receiving adjuvant chemotherapy compared with those in areas with no oncologists.10 We sought to evaluate the relationship between geographic access to oncology care and receipt of adjuvant chemotherapy among patients with stage III colon cancer. We evaluate the association between density of oncologists and travel distance and cancer treatment, in particular, receipt of adjuvant colon cancer chemotherapy.
PATIENTS AND METHODS
Data Source
The primary data source for this study was the National Cancer Data Base (NCDB), which is a hospital-based cancer registry that is jointly sponsored by the American College of Surgeons and the American Cancer Society. Data are collected from more than 1,500 Commission on Cancer (CoC) –accredited facilities and capture approximately 70% of newly diagnosed cancer cases in the United States.24 Patient demographic and clinical characteristics captured in the NCDB are comparable to those reported in the Surveillance, Epidemiology, and Ends Results population-based cancer registry.25
To identify number and location of oncologists, the Physician Compare data, created by the Centers for Medicare and Medicaid Services,26 were used. These data provide the physicians and other health care professionals who have filed Medicare claims in the previous 12 months and identify physicians by National Provider Identifier, specialty designation, and multiple practice locations. Physician Compare data were selected because the majority of cancers occur in people older than age 65 years, so virtually all oncologists treat Medicare patients.23 In addition, the data are updated monthly for providers who have practiced and billed to Medicare in the previous 12 months.
Study Population
Patients with first primary invasive American Joint Committee on Cancer stage III node-positive colon cancer (International Classification of Disease for Oncology, Third Edition, site codes: C18.0 to C18.9), diagnosed between 2007 and 2010, who were age 18 to 80 years at diagnosis, who underwent colectomy within 3 months of diagnosis, and who were diagnosed and/or treated at CoC-accredited facilities were selected as the study cohort. Patients with unknown chemotherapy data, or unknown chemotherapy administration date, or missing residence information were excluded. In addition, patients who survived less than 6 months after diagnosis were excluded to limit bias.
Outcomes and Covariates
The primary outcome of the study is receipt of adjuvant chemotherapy within 90 days of colectomy. Receipt of colectomy was identified from surgical procedure field in the NCDB (site-specific surgery codes: 30 to 80), including partial and total colectomy. Initiation of chemotherapy was defined from chemotherapy treatment field in the NCDB, including administration of single- or multiple-agent chemotherapy. Time to initiation of chemotherapy was calculated from the date of colectomy to the chemotherapy start date.
Geographic access to cancer care was defined as the density level of oncologists in a patient's area of residence and travel distance to cancer treatment. Through the Physician Compare data, oncologists were identified if their primary or secondary specialty was medical oncology, hematology/oncology, or hematology. Although Physician Compare data identify all locations from which a physician files Medicare claims, nearly one fourth (22%) of oncologists have submitted claims from multiple office locations. Each oncologist was counted only once per hospital service area (HSA). HSAs, defined by the Dartmouth Atlas of Health Care,27 are geographic areas covering one or more zip codes where medical resources are distributed and used based on the analysis of travel patterns for routine hospital care. The density of oncologists was calculated by the number of unique oncologists available per 100,000 residents in each HSA to determine the ratio of oncologists to the population. A high density level of oncologists was defined as higher than the 95th percentile. The 95th percentile was selected because sensitivity analyses at multiple other cut points did not yield treatment variation. Each patient was assigned oncologist density according to the HSA in which the patient resided.
Travel distance to cancer treatment was defined as the driving distance between the geographic centroid of zip codes of the patient residence at diagnosis and the reporting facility by Google Maps and was categorized, based on literature,16,28,29 as 0 to 12.49, 12.5 to 49.9, 50 to 249, and ≥ 250 miles. For patients who lived outside the U.S. continental 48 states but traveled back to seek cancer treatment, travel distance was calculated using the crow-fly method instead of driving distance. The majority of patients (90%) had surgical treatment in the facility that reported their diagnosis.
Other variables of interest included patient demographics, comorbidity, socioeconomic status, insurance status, facility type, and clinical characteristics (number of positive lymph nodes, tumor grade, and surgical margin). Patient insurance status was defined as private, uninsured, Medicaid, Medicare (age 18 to 64 years), and Medicare (age ≥ 65 years). Medicare (age ≥ 65 years) was further divided into Medicare with supplemental policy, Medicare without supplemental policy, Medicare Advantage, and Medicare-Medicaid. Race/ethnicity was categorized as non-Hispanic white, black, Hispanic, other, and missing. Comorbidity was measured using the Charlson-Deyo30–34 comorbidity score, categorized as 0, 1, or ≥ 2. Facility type was assigned by CoC accreditation program as community cancer program, comprehensive community cancer program, teaching/research center, National Cancer Institute–designated program/network, and other. Median income level in the neighborhood of a patient's residence was derived from 2000 U.S. Census data (to match the study cohort diagnosed from 2007 to 2010) and categorized based on national quartiles by zip code level.
Statistical Analyses
Geographic distribution of oncologists nationwide was determined using ArcGIS software (version 10.2.2; esri, Redlands, CA). Descriptive analyses were performed to summarize patient characteristics. χ2 tests were used to determine significance of differences in receipt of oncology services by density level of oncologists and by travel distance at P = .05 levels. Generalized estimating equations clustering by HSA was conducted to examine the association between geographic access and receipt of oncology services, controlling for patient sociodemographic and clinical characteristics. Two-sided P values were reported and were considered significant at P = .05.
We investigated the relationship between geographic access and receipt of chemotherapy by insurance status. Insurance status was collapsed into patients with private insurance and without private insurance (including uninsured, Medicaid, and Medicare). A sensitivity analysis was conducted including only Medicaid and Medicare in the nonprivate subset and another sensitivity analysis was conducted grouping Medicare with supplemental policy in the private subset to examine the relationship. In addition, to test whether the relationship between density level of oncologists and receipt of adjuvant chemotherapy is not affected by choice of clustering unit, sensitivity analyses were performed using Health Service Area as an alternative clustering unit. Health Service Areas are defined by the National Center for Health Statistics to include a single county or cluster of contiguous counties that are self-contained with respect to hospital care. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Geographic Distribution of Oncologists Nationwide
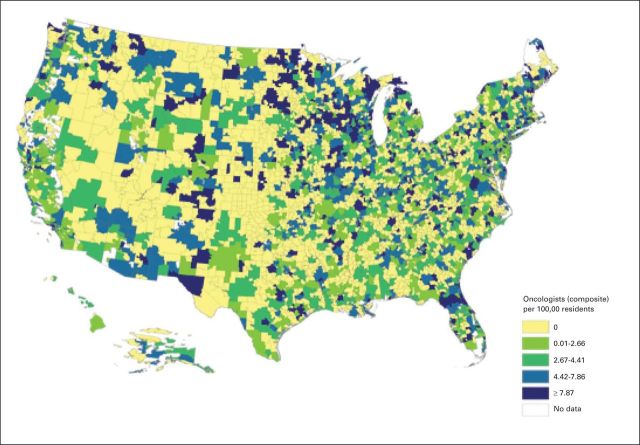
In total, 12,198 unique oncologists were identified through the Physician Compare data. The map in Figure 1 shows the geographic distribution of oncologists in quartiles at HSA level. Of 3,436 HSAs in the United States, 1,469 HSAs (42.75%) have at least one oncologist. The average density is 3.32 oncologists (interquartile range, 3.74 oncologists) per 100,000 residents. The 95th percentile of density is 12.65 oncologists per 100,000 residents. The HSAs with no oncologist were more likely to have a smaller population (average 32,000 residents) and be in the Midwest or South regions.
Fig 1.
Oncologists per 100,000 residents by hospital service area.
To understand whether the Physician Compare file is a reliable data source to identify oncologists, sensitivity analyses were conducted using deidentified American Society of Clinical Oncology (ASCO) membership data aggregated by zip code to identify oncologists and measure the density level of oncologists in each HSA. Overall, geographic distribution of ASCO-member oncologists was similar to that based on the Physician Compare data. However, the Physician Compare data captured higher numbers of oncologists in a majority of the HSAs.
Patient Characteristics
Of 34,694 patients with stage III node-positive colon cancer selected in the study, 75.7% of patients received adjuvant chemotherapy within 90 days of colectomy (Table 1). Median age was 63 years. Most patients were non-Hispanic white (68.6%) and had private insurance (44.9%) or Medicare (42.9%), a comorbidity score of 0 (70.0%), and less than four positive lymph nodes (63.2%). Overall, 44.9% of patients had private insurance; however, among patients younger than age 65 years, 71.9% had private insurance. Approximately half of the patients were diagnosed or treated at a comprehensive community cancer center and traveled less than 12.5 miles to the reporting facility for their cancer diagnosis and/or treatment, whereas 10.2% of patients traveled more than 50 miles. Approximately 7.2% of patients resided in areas with a high density level of oncologists. The proportion of patients receiving adjuvant chemotherapy was not significantly different between areas with different density levels of oncologists but was much lower among patients who traveled ≥ 250 miles to their reporting facility (0 to 12.5 miles v ≥ 250 miles: 75.3% v 59.4%, respectively; P < .001). Patients residing in low-density HSAs traveled a greater distance for medical services. One tenth of patients who resided in a low-density HSA traveled more than 50 miles for their medical services, whereas only 6% of patients residing in a high-density HSA traveled more than 50 miles (P < .001).
Table 1.
Characteristics of Patients With Stage III Colon Cancer
| Characteristic | No. of Patients (N = 34,694) (%)* |
|---|---|
| Receipt of adjuvant chemotherapy | |
| No | 8,422 (24.3) |
| Yes | 26,272 (75.7) |
| Age at diagnosis, years | |
| 18-50 | 6,018 (17.4) |
| 51-64 | 12,441 (35.9) |
| 65-70 | 6,324 (18.2) |
| 71-75 | 5,104 (14.7) |
| 76-80 | 4,807 (13.9) |
| Race/ethnicity | |
| Non-Hispanic white | 23,815 (68.6) |
| Hispanic | 1,954 (5.6) |
| Black | 5,053 (14.6) |
| Other | 1,424 (4.1) |
| Unknown | 2,448 (7.1) |
| Sex | |
| Male | 17,488 (50.4) |
| Female | 17,206 (49.6) |
| Insurance | |
| Uninsured | 1,709 (4.9) |
| Medicaid | 1,963 (5.7) |
| Medicare (age 18-64 years) | 1,566 (4.5) |
| Medicare with supplement | 8,140 (23.5) |
| Medicare without supplement | 2,627 (7.6) |
| Medicare Advantage | 1,600 (4.6) |
| Medicare/Medicaid | 979 (2.8) |
| Private | 15,578 (44.9) |
| Missing | 532 (1.5) |
| Diagnosis year | |
| 2007 | 8,826 (25.4) |
| 2008 | 8,911 (25.7) |
| 2009 | 8,486 (24.5) |
| 2010 | 8,471 (24.4) |
| Median income level | |
| < $30,000 | 5,178 (14.9) |
| $30,000-$34,999 | 6,572 (18.9) |
| $35,000-$45,999 | 9,710 (28.0) |
| ≥ $46,000 | 12,750 (36.8) |
| Missing | 484 (1.4) |
| Facility type | |
| Community cancer program† | 5,184 (14.9) |
| Comprehensive community cancer program‡ | 17,296 (49.9) |
| Teaching/research center§ | 6,676 (19.2) |
| NCI program/network‖ | 2,023 (5.8) |
| Other¶ | 3,515 (10.1) |
| Charlson-Deyo comorbidity score# | |
| 0 | 24,288 (70.0) |
| 1 | 7,285 (21.0) |
| 2+ | 3,121 (9.0) |
| Travel distance, miles | |
| 0-12.49 | 18,563 (53.5) |
| 12.5-49.9 | 12,748 (36.7) |
| 50-249 | 3,026 (8.7) |
| ≥ 250 | 357 (1.0) |
| Density level of medical oncologist | |
| High | 2,479 (7.2) |
| Low | 32,215 (92.9) |
| No. of positive lymph nodes | |
| < 4 | 21,913 (63.2) |
| ≥ 4 | 12,641 (36.4) |
| Unknown | 140 (0.4) |
| Tumor grade | |
| Well differentiated | 2,135 (6.2) |
| Moderately differentiated | 22,996 (66.3) |
| Poorly differentiated | 7,933 (22.9) |
| Undifferentiated | 804 (2.3) |
| Unknown | 826 (2.4) |
| Surgical margin | |
| Negative | 31,502 (90.8) |
| Positive | 2,708 (7.8) |
| Unknown | 484 (1.4) |
Abbreviation: NCI, National Cancer Institute.
Because of rounding, percentages presented throughout this table may not add up to precisely 100%.
Facilities report 101 to 499 patients with newly diagnosed cancer each year. A full range of diagnostic and treatment services is provided, but referral for a portion of diagnosis or treatment may occur.
Facilities report ≥ 500 patients with newly diagnosed cancer each year. A full range of diagnostic and treatment services is provided either on site or by referral.
Facilities report ≥ 500 patients with newly diagnosed cancer each year and provide postgraduate medical education in at least four program areas. A full range of diagnostic and treatment services is provided either on site or by referral.
Facilities are designated a comprehensive cancer center by the NCI, with an NCI peer-reviewed cancer center support grant. A full range of diagnostic and treatment services is provided. No minimum caseload is required.
Facilities that are accredited as Integrated Network Cancer Program, Hospital Associate Cancer Program, or Free-Standing Cancer Center Program. Integrated Network Cancer Program is for facilities that are owned or operated by multiple facilities providing integrated cancer care. Hospital Associate Cancer Program is for facilities that report ≤ 100 patients with newly diagnosed cancer each year with limited range of diagnostic and treatment services. Free-Standing Cancer Center Program is for non–hospital-based facilities that offer at least one cancer-related treatment.
Charlson-Deyo comorbidity score is a weighted score evaluating severe comorbidities that increase the risk of 1-year mortality. Fifteen noncancer comorbid conditions were identified and weighted to calculate Charlson-Deyo comorbidity scores.
Factors Associated With Receipt of Adjuvant Chemotherapy
The adjusted associations between geographic access and receipt of adjuvant chemotherapy are listed in Table 2. Compared with travel distance less than 12.5 miles, patients who traveled 50 to 249 miles to the reporting facility had a lower likelihood of receipt of adjuvant chemotherapy (odds ratio [OR], 0.87; P = .009), as did patients who traveled more than 250 miles (OR, 0.36; P < .001). However, the density level of oncologists was not significantly associated with receipt of adjuvant chemotherapy (low density v high density: OR, 0.98; P = .77). Patients who were older than 50 years; were African American; were uninsured or insured by Medicaid, Medicare (age 18 to 64 years), Medicare without supplemental policy, or Medicare-Medicaid; and had one or more comorbidities were less likely to receive adjuvant chemotherapy.
Table 2.
Likelihood of Receipt of Adjuvant Chemotherapy Among Patients With Stage III Colon Cancer
| Factor | OR (95% CI) | P |
|---|---|---|
| Travel distance, miles | ||
| 0-12.49 | 1 | |
| 12.5-49.9 | 0.99 (0.92 to 1.06) | .73 |
| 50-249 | 0.87 (0.78 to 0.96) | .009 |
| ≥ 250 | 0.36 (0.28 to 0.45) | < .001 |
| Density level of MO | ||
| High | 1 | |
| Low | 0.98 (0.84 to 1.14) | .77 |
| Age at diagnosis, years | ||
| 18-50 | 1 | |
| 51-64 | 0.75 (0.69 to 0.82) | < .001 |
| 65-70 | 0.50 (0.44 to 0.56) | < .001 |
| 71-75 | 0.35 (0.31 to 0.39) | < .001 |
| 76-80 | 0.20 (0.17 to 0.22) | < .001 |
| Race/ethnicity | ||
| Non-Hispanic white | 1 | |
| Hispanic | 0.94 (0.84 to 1.06) | .32 |
| Black | 0.79 (0.73 to 0.85) | < .001 |
| Other | 0.96 (0.84 to 1.11) | .62 |
| Unknown | 1.10 (0.99 to 1.23) | .07 |
| Sex | ||
| Male | 1 | |
| Female | 1.08 (1.02 to 1.14) | .007 |
| Insurance | ||
| Private | 1 | |
| Uninsured | 0.46 (0.41 to 0.52) | < .001 |
| Medicaid | 0.50 (0.44 to 0.57) | < .001 |
| Medicare (age 18-64 years) | 0.45 (0.40 to 0.51) | < .001 |
| Medicare with supplement | 1.10 (1.00 to 1.22) | .05 |
| Medicare without supplement | 0.76 (0.67 to 0.86) | < .001 |
| Medicare Advantage | 0.98 (0.84 to 1.13) | .75 |
| Medicare/Medicaid | 0.53 (0.45 to 0.63) | < .001 |
| Missing | 0.52 (0.39 to 0.69) | < .001 |
| Diagnosis year | ||
| 2007 | 1 | |
| 2008 | 1.03 (0.96 to 1.12) | .39 |
| 2009 | 0.99 (0.92 to 1.08) | .90 |
| 2010 | 1.00 (0.92 to 1.08) | .92 |
| Median income level | ||
| < $30,000 | 1 | |
| $30,000-$34,999 | 0.99 (0.91 to 1.09) | .91 |
| $35,000-$45,999 | 1.05 (0.96 to 1.15) | .29 |
| ≥ $46,000 | 1.08 (0.99 to 1.19) | .10 |
| Missing | 0.97 (0.77 to 1.23) | .81 |
| Facility type | ||
| Community cancer program | 0.96 (0.87 to 1.05) | .36 |
| Comprehensive community cancer program | 1 | |
| Teaching/research center | 0.92 (0.85 to 1.01) | .08 |
| NCI program/network | 1.32 (1.15 to 1.51) | < .001 |
| Other | 0.98 (0.88 to 1.09) | .73 |
| Charlson-Deyo comorbidity score | ||
| 0 | 1 | |
| 1 | 0.88 (0.82 to 0.94) | < .001 |
| ≥ 2 | 0.59 (0.55 to 0.65) | < .001 |
| No. of positive lymph nodes | ||
| < 4 | 1 | |
| ≥ 4 | 1.21 (1.15 to 1.28) | < .001 |
| Unknown | 1.09 (0.70 to 1.68) | .70 |
| Tumor grade | ||
| Well differentiated | 1 | |
| Moderately differentiated | 1.15 (1.03 to 1.27) | .009 |
| Poorly differentiated | 1.19 (1.06 to 1.34) | .003 |
| Undifferentiated | 1.32 (1.06 to 1.63) | .01 |
| Unknown | 0.94 (0.77 to 1.13) | .50 |
| Surgical margin | ||
| Negative | 1 | |
| Positive | 0.90 (0.82 to 1.00) | .05 |
| Unknown | 1.25 (0.96 to 1.64) | .10 |
| Region | ||
| Northeast | 1 | |
| Midwest | 1.28 (1.16 to 1.42) | < .001 |
| South | 1.03 (0.93 to 1.13) | .58 |
| West | 0.74 (0.66 to 0.83) | < .001 |
Abbreviations: MO, medical oncologist; NCI, National Cancer Institute; OR, odds ratio.
Clinical factors that were associated with receipt of chemotherapy included ≥ four positive lymph nodes (OR, 1.21; P < .001) and high tumor grade (OR, 1.32; P = .01). In addition, female patients (OR, 1.08; P = .007) and patients treated in National Cancer Institute–designated facilities (OR, 1.32; P < .001) had increased likelihood of receiving adjuvant chemotherapy.
Factors Associated With Receipt of Adjuvant Chemotherapy by Insurance Status
Table 3 lists results of the analysis stratified by insurance status (private v without private insurance). Among privately insured patients, there was no significant association between oncologist density and receipt of adjuvant chemotherapy, but travel distance was linearly and negatively associated with the likelihood of receiving adjuvant chemotherapy. Among patients without private insurance, traveling ≥ 250 miles significantly reduced the likelihood of receipt of adjuvant chemotherapy (OR, 0.43; P < .001). Also in this subset of patients, those who resided in areas with a low density of oncologists were less likely to receive adjuvant chemotherapy within 90 days of colectomy (OR, 0.85; P = .03), compared with patients who lived in areas of high oncologist density.
Table 3.
Likelihood of Receipt of Adjuvant Chemotherapy Among Patients With Stage III Colon Cancer by Insurance Status
| Factor | Privately Insured |
Without Private Insurance |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Travel distance, miles | ||||
| 0-12.49 | 1 | 1 | ||
| 12.5-49.9 | 0.93 (0.84 to 1.03) | .18 | 1.01 (0.94 to 1.09) | .77 |
| 50-249 | 0.68 (0.57 to 0.82) | < .001 | 0.97 (0.86 to 1.10) | .61 |
| ≥ 250 | 0.26 (0.18 to 0.36) | < .001 | 0.43 (0.30 to 0.61) | < .001 |
| Density level of MO | ||||
| High | 1 | 1 | ||
| Low | 1.26 (0.97 to 1.63) | .09 | 0.85 (0.73 to 0.98) | .03 |
| Age at diagnosis, years | ||||
| 18-50 | 1 | 1 | ||
| 51-64 | 0.73 (0.66 to 0.81) | < .001 | 0.77 (0.67 to 0.90) | < .001 |
| 65-70 | 0.49 (0.41 to 0.58) | < .001 | 0.65 (0.50 to 0.84) | .001 |
| 71-75 | 0.30 (0.25 to 0.37) | < .001 | 0.46 (0.36 to 0.61) | < .001 |
| 76-80 | 0.16 (0.13 to 0.20) | < .001 | 0.26 (0.20 to 0.35) | < .001 |
| Race/ethnicity | ||||
| Non-Hispanic white | 1 | 1 | ||
| Hispanic | 0.81 (0.66 to 1.00) | .04 | 0.95 (0.83 to 1.10) | .51 |
| Black | 0.77 (0.67 to 0.87) | < .001 | 0.81 (0.74 to 0.90) | < .001 |
| Other | 0.78 (0.62 to 0.97) | .02 | 1.07 (0.90 to 1.29) | .44 |
| Unknown | 1.13 (0.93 to 1.37) | .20 | 1.10 (0.96 to 1.25) | .16 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.12 (1.02 to 1.24) | .03 | 1.05 (0.99 to 1.12) | .13 |
| Insurance | ||||
| Uninsured | 0.51 (0.40 to 0.66) | < .001 | ||
| Medicaid | 0.55 (0.42 to 0.70) | < .001 | ||
| Medicare (age 18-64 years) | 0.52 (0.40 to 0.68) | < .001 | ||
| Medicare with supplement | 1 | |||
| Medicare without supplement | 0.68 (0.61 to 0.75) | < .001 | ||
| Medicare Advantage | 0.88 (0.77 to 1.00) | .05 | ||
| Medicare/Medicaid | 0.47 (0.41 to 0.55) | < .001 | ||
| Diagnosis year | ||||
| 2007 | 1 | 1 | ||
| 2008 | 1.05 (0.92 to 1.20) | .44 | 1.01 (0.92 to 1.11) | .82 |
| 2009 | 0.94 (0.82 to 1.08) | .39 | 1.02 (0.93 to 1.12) | .65 |
| 2010 | 0.95 (0.83 to 1.08) | .44 | 1.03 (0.93 to 1.13) | .59 |
| Median income level | ||||
| < $30,000 | 1 | 1 | ||
| $30,000-$34,999 | 1.08 (0.91 to 1.28) | .38 | 0.96 (0.86 to 1.08) | .52 |
| $35,000-$45,999 | 1.05 (0.90 to 1.24) | .53 | 1.05 (0.94 to 1.17) | .37 |
| ≥ $46,000 | 1.14 (0.96 to 1.35) | .13 | 1.04 (0.92 to 1.16) | .55 |
| Missing | 0.94 (0.65 to 1.37) | .76 | 1.00 (0.73 to 1.38) | .98 |
| Facility type | ||||
| Community cancer program | 1.01 (0.86 to 1.17) | .95 | 0.95 (0.86 to 1.06) | .36 |
| Comprehensive community cancer program | 1 | 1 | ||
| Teaching/research center | 0.90 (0.78 to 1.03) | .13 | 0.93 (0.84 to 1.03) | .17 |
| NCI program/network | 1.53 (1.23 to 1.90) | < .001 | 1.05 (0.89 to 1.22) | .58 |
| Other | 1.18 (0.96 to 1.46) | .12 | 0.90 (0.80 to 1.01) | .08 |
| Charlson-Deyo comorbidity score | ||||
| 0 | 1 | 1 | ||
| 1 | 0.88 (0.78 to 0.99) | .04 | 0.88 (0.81 to 0.95) | < .001 |
| ≥ 2 | 0.67 (0.56 to 0.81) | < .001 | 0.58 (0.52 to 0.64) | < .001 |
| No. of positive lymph nodes | ||||
| < 4 | 1 | 1 | ||
| ≥ 4 | 1.10 (1.00 to 1.21) | .04 | 1.28 (1.19 to 1.38) | < .001 |
| Unknown | 0.61 (0.34 to 1.09) | .10 | 1.63 (0.93 to 2.85) | .08 |
| Tumor grade | ||||
| Well differentiated | 1 | 1 | ||
| Moderately differentiated | 1.12 (0.93 to 1.34) | .24 | 1.18 (1.03 to 1.34) | .01 |
| Poorly differentiated | 1.14 (0.94 to 1.39) | .19 | 1.25 (1.09 to 1.44) | .002 |
| Undifferentiated | 1.29 (0.91 to 1.82) | .15 | 1.36 (1.03 to 1.79) | .03 |
| Unknown | 0.68 (0.51 to 0.93) | .01 | 1.24 (0.96 to 1.59) | .09 |
| Surgical margin | ||||
| Negative | 1 | 1 | ||
| Positive | 0.91 (0.77 to 1.08) | .29 | 0.90 (0.80 to 1.02) | .11 |
| Unknown | 1.20 (0.79 to 1.81) | .40 | 0.81 (0.60 to 1.09) | .16 |
| Region | ||||
| Northeast | 1 | 1 | ||
| Midwest | 1.36 (1.15 to 1.61) | < .001 | 1.25 (1.12 to 1.40) | < .001 |
| South | 1.01 (0.86 to 1.19) | .89 | 1.01 (0.91 to 1.12) | .88 |
| West | 0.76 (0.63 to 0.92) | .005 | 0.74 (0.64 to 0.84) | < .001 |
Abbreviations: MO, medical oncologist; NCI, National Cancer Institute; OR, odds ratio.
The following three sensitivity analyses were conducted: separating uninsured patients from patients without private insurance; including Medicare with supplemental policy in the private subset; and replacing HSA with Health Service Area as the clustering unit to aggregate number of oncologists. In those sensitivity analyses, the results were similar to those from primary analyses (data not shown).
DISCUSSION
Using the NCDB, we found an association between distance to the initial treatment center and receipt of adjuvant colon cancer chemotherapy, but we did not observe an association between oncologist density and receipt of adjuvant treatment. However, in a subset analysis of patients without private insurance, patients who lived in areas with low oncologist density were less likely to receive adjuvant chemotherapy.
The majority of patients in this cohort received adjuvant chemotherapy within 90 days of colectomy.1–5 The CoC requires that accredited facilities report concordance with this metric as a quality standard. We found that patients traveling more than 50 miles to their reporting facility were less likely to receive chemotherapy treatment compared with those traveling shorter distance.
We observed substantial variation in the density of oncologists across the country, which is consistent with the literature.16,22,23 Onega et al16 used the American Medical Association Masterfile to identify oncologists and their practice location. They reported that density of oncologists varied regionally and that density was greater in urban or suburban areas compared with rural areas.22 In the attempt to improve the accuracy of this relationship, Kirkwood et al23 examined the Masterfile database, American Board of Internal Medicine certification reports, the National Provider Identifier database, and Physician Compare data. In this analysis, the distribution of oncologists was skewed toward urban areas, whereas some rural areas had no oncologist at all.23 In our study, we used Physician Compare data to identify oncologists and validated its accuracy through a comparison with ASCO membership data. Consistently, a substantial proportion of the population resides in areas without oncologists. Oncologists were more likely to cluster in areas with greater population density. Patients who reside in low-density HSAs might need to wait longer or travel farther to be seen by oncologists.
We found that patients without private insurance were more likely to receive chemotherapy closer to their area of residence compared with privately insured patients. This may be a result of a lack of resources available to travel a longer distance for cancer care. Therefore, accessibility to local oncologists has much more impact among patients without private insurance. Expanded coverage alone might not fully address the barriers to receive guideline-recommended treatment because patients who traveled ≥ 50 miles to medical services were less likely to receive adjuvant chemotherapy than those who traveled shorter distance. Rural states and regions extend care through visiting consulting clinics, as have been used in Iowa to improve access.35 Additional research should examine how to extend care to areas that require long travel distances.
Despite whether cancer care is outpatient or inpatient, previous studies found that transportation and distance to medical care are factors in forgoing needed treatment, especially among underinsured minorities.36,37 Depending on the regimen oncologists prescribe, adjuvant chemotherapy may involve visits every other week for 24 weeks.38–40 Alternative approaches, such as telemedicine for cancer care consultations, have been developed, but the extent of their use in treatment delivery and impact on outcomes is uncertain. Volunteer-based transportation assistance (eg, the American Cancer Society Road to Recovery program) is another alternative to provide transportation. Also, enhancing patient navigators in facilities can help improve coordination of cancer services.41
We found that travel burden, as opposed to oncologist density, is a potentially modifiable factor that decreases utilization of adjuvant chemotherapy in patients with stage III colon cancer. Studies have reported that many rural patients travel great distances to receive their cancer care, even if providers are nearby.15 It is possible that patients bypass local providers because of concerns about the quality of care42 or experience level of providers43 or because they need to see providers who have coverage in an insurance network. Although the oncologists we identified through Physician Compare data were actively engaged in clinical care, we have no information of their capability to accept new patients or accommodate patient demand.
Our study has several limitations inherent to observational registry studies. First, the NCDB is a hospital-based cancer registry that captures only patients who are diagnosed or treated in CoC-accredited facilities. Even though the NCDB captures approximately 70% of incident cancers each year, our results may not be fully generalizable. Second, the NCDB does not indicate location of chemotherapy treatment, if it is delivered outside of the COC facility; however, the majority of oncologists (approximately 90%) saw their patients at the same hospital where they had surgery.6 Third, the Physician Compare data exclude oncologists who have not billed Medicare in the previous 12 months and do not provide information on the primary office location. However, we have previously shown that Physician Compare data provide better representation of the total number of oncologists than other databases.23 Fourth, social support could also be associated with adherence to treatment guidelines; however, NCDB does not collect information on social support (eg, caregiver, spouse, or number of people in the household). Fifth, travel distance might be underestimated. However, further detail on location of patients or facilities is not available because of Health Insurance Portability and Accountability Act regulations for privacy. Sixth, the majority of patients in our study did not have severe comorbidities. Even though this is consistent with the literature,44,45our results may not be generalizable to patients with more severe comorbid conditions. Finally, this study does not include information on physician or patient preferences that might influence decisions regarding whether and where to initiate chemotherapy.
In summary, this study observed that increased travel burden was associated with a decreased likelihood of receiving adjuvant chemotherapy for patients with stage III colon cancer, regardless of insurance status. We also found that patients who were not privately insured and resided in areas with a low density of oncologists were less likely to receive adjuvant chemotherapy. More in-depth analysis to focus on low-density areas would help analyze how interventions to decrease geographic barriers may improve the access to colon cancer treatment. Understanding how insurance can be a barrier to quality cancer care is increasingly important because the number of people with Medicaid coverage has expanded under the Affordable Care Act.
Acknowledgment
We thank the American College of Surgeons and the American Cancer Society for their efforts in the creation of the National Cancer Data Base. We also thank the American Society of Clinical Oncology and American Society for Radiation Oncology staff who provided in-kind analysis and administrative support.
Appendix
Table A1.
Characteristics of Patients With Stage III Colon Cancer by Density Level of Oncologists
| Characteristic | No. of Patients (%)* |
P | |
|---|---|---|---|
| Low Density Level of MO | High Density Level of MO | ||
| Total | 32,215 (92.9) | 2,479 (7.2) | |
| Receipt of adjuvant chemotherapy | .44 | ||
| No | 7,836 (24.3) | 586 (23.6) | |
| Yes | 24,379 (75.7) | 1,893 (76.4) | |
| Age at diagnosis, years | .35 | ||
| 18-50 | 5,597 (17.4) | 421 (17.0) | |
| 51-64 | 11,579 (35.9) | 862 (34.8) | |
| 65-70 | 5,850 (18.2) | 474 (19.1) | |
| 71-75 | 4,750 (14.7) | 354 (14.3) | |
| 76-80 | 4,439 (13.8) | 368 (14.8) | |
| Race/ethnicity | < .001 | ||
| Non-Hispanic white | 22,311 (69.3) | 1,504 (60.7) | |
| Hispanic | 1,791 (5.6) | 163 (6.6) | |
| Black | 4,558 (14.2) | 495 (20.0) | |
| Other | 1,292 (4.0) | 132 (5.3) | |
| Unknown | 2,263 (7.0) | 185 (7.5) | |
| Sex | .99 | ||
| Male | 16,238 (50.4) | 1,250 (50.4) | |
| Female | 15,977 (49.6) | 1,229 (49.6) | |
| Insurance | .02 | ||
| Uninsured | 1,603 (5.0) | 106 (4.3) | |
| Medicaid | 1,800 (5.6) | 163 (6.6) | |
| Medicare (age 18-64 years) | 1,460 (4.5) | 106 (4.3) | |
| Medicare with supplement | 7,545 (23.4) | 595 (24.0) | |
| Medicare without supplement | 2,424 (7.5) | 203 (8.2) | |
| Medicare Advantage | 1,506 (4.7) | 94 (3.8) | |
| Medicare/Medicaid | 894 (2.8) | 85 (3.4) | |
| Private | 14,480 (45.0) | 1,098 (44.3) | |
| Missing | 503 (1.6) | 29 (1.2) | |
| Diagnosis year | .13 | ||
| 2007 | 8,186 (25.4) | 640 (25.8) | |
| 2008 | 8,323 (25.8) | 588 (23.8) | |
| 2009 | 7,855 (24.4) | 631 (25.5) | |
| 2010 | 7,851 (24.4) | 620 (25.0) | |
| Median income level | < .001 | ||
| < $30,000 | 4,755 (14.8) | 423 (17.1) | |
| $30,000-$34,999 | 6,227 (19.3) | 345 (13.9) | |
| $35,000-$45,999 | 9,024 (28.0) | 686 (27.7) | |
| ≥ $46,000 | 11,743 (36.5) | 1,007 (40.6) | |
| Missing | 466 (1.5) | 18 (0.7) | |
| Facility type | < .001 | ||
| Community cancer program | 4,890 (15.2) | 294 (11.9) | |
| Comprehensive community cancer program | 16,370 (50.8) | 926 (37.4) | |
| Teaching/research center | 6,084 (18.9) | 592 (23.9) | |
| NCI program/network | 1,647 (5.1) | 376 (15.2) | |
| Other | 3,224 (10.0) | 291 (11.7) | |
| Charlson-Deyo comorbidity score | .54 | ||
| 0 | 22,568 (70.1) | 1,720 (69.4) | |
| 1 | 6,764 (21.0) | 521 (21.0) | |
| ≥ 2 | 2,883 (9.0) | 238 (9.6) | |
| Travel distance, miles | < .001 | ||
| 0-12.49 | 16,880 (52.4) | 1,683 (67.9) | |
| 12.5-49.9 | 12,101 (37.6) | 647 (26.1) | |
| 50-249 | 2,904 (9.0) | 122 (4.9) | |
| ≥ 250 | 330 (1.0) | 27 (1.1) | |
| No. of positive lymph nodes | .08 | ||
| < 4 | 20,318 (63.1) | 1,595 (64.3) | |
| ≥ 4 | 11,761 (36.5) | 880 (35.5) | |
| Unknown | 136 (0.4) | 4 (0.2) | |
| Tumor grade | < .001 | ||
| Well differentiated | 1,996 (6.2) | 139 (5.6) | |
| Moderately differentiated | 21,435 (66.5) | 1,561 (63.0) | |
| Poorly differentiated | 7,309 (22.7) | 624 (25.2) | |
| Undifferentiated | 715 (2.2) | 89 (3.6) | |
| Unknown | 760 (2.4) | 66 (2.7) | |
| Surgical margin | .05 | ||
| Negative | 29,270 (90.9) | 2,232 (90.0) | |
| Positive | 2,488 (7.7) | 220 (8.9) | |
| Unknown | 457 (1.4) | 27 (1.1) | |
Abbreviations: MO, medical oncologist; NCI, National Cancer Institute.
Because of rounding, percentages presented throughout this table may not add up to precisely 100%.
Table A2.
Characteristics of Patients With Stage III Colon Cancer by Travel Distance
| Characteristic | No. of Patients (%)* |
P | |||
|---|---|---|---|---|---|
| 0-12.49 Miles | 12.5-49.9 Miles | 50-249 Miles | ≥ 250 Miles | ||
| Total | 18,563 (53.5) | 12,748 (36.7) | 3,026 (8.7) | 357 (1.0) | |
| Receipt of adjuvant chemotherapy | < .001 | ||||
| No | 4,589 (24.7) | 2,919 (22.9) | 769 (25.4) | 145 (40.6) | |
| Yes | 13,974 (75.3) | 9,829 (77.1) | 2,257 (74.6) | 210 (59.4) | |
| Age at diagnosis, years | < .001 | ||||
| 18-50 | 3,125 (16.8) | 2,316 (18.2) | 512 (16.9) | 65 (18.2) | |
| 51-64 | 6,531 (35.2) | 4,735 (37.1) | 1,033 (34.1) | 142 (39.8) | |
| 65-70 | 3,255 (17.5) | 2,398 (18.8) | 612 (20.2) | 59 (16.5) | |
| 71-75 | 2,841 (15.3) | 1,739 (13.6) | 476 (15.7) | 48 (13.5) | |
| 76-80 | 2,811 (15.1) | 1,560 (12.2) | 393 (13.0) | 43 (12.0) | |
| Race/ethnicity | < .001 | ||||
| Non-Hispanic white | 11,968 (64.5) | 9,254 (72.6) | 2,330 (77.0) | 263 (73.7) | |
| Hispanic | 1,196 (6.4) | 639 (5.0) | 102 (3.4) | 17 (4.8) | |
| Black | 3,377 (18.2) | 1,383 (10.9) | 264 (8.7) | 29 (8.1) | |
| Other | 917 (4.9) | 417 (3.3) | 72 (2.4) | 18 (5.0) | |
| Unknown | 1,105 (6.0) | 1,055 (8.3) | 258 (8.5) | 30 (8.4) | |
| Sex | < .001 | ||||
| Male | 9,134 (49.2) | 6,572 (51.6) | 1,593 (52.6) | 189 (52.9) | |
| Female | 9,429 (50.8) | 6,176 (48.5) | 1,433 (47.4) | 168 (47.1) | |
| Insurance | < .001 | ||||
| Uninsured | 917 (4.9) | 636 (5.0) | 143 (4.7) | 13 (3.6) | |
| Medicaid | 1,216 (6.6) | 590 (4.6) | 150 (5.0) | 7 (2.0) | |
| Medicare (age 18-64 years) | 826 (4.5) | 574 (4.5) | 157 (5.2) | 9 (2.5) | |
| Medicare with supplement | 4,257 (22.9) | 2,958 (23.2) | 845 (27.9) | 80 (22.4) | |
| Medicare without supplement | 1,459 (7.9) | 893 (7.0) | 249 (8.2) | 26 (7.3) | |
| Medicare Advantage | 944 (5.1) | 532 (4.2) | 118 (3.9) | 6 (1.7) | |
| Medicare/Medicaid | 560 (3.0) | 326 (2.6) | 90 (3.0) | 3 (0.8) | |
| Private | 8,122 (43.8) | 6,053 (47.5) | 1,226 (40.5) | 177 (49.6) | |
| Missing | 262 (1.4) | 186 (1.5) | 48 (1.6) | 36 (10.1) | |
| Diagnosis year | .300 | ||||
| 2007 | 4,797 (25.8) | 3,210 (25.2) | 749 (24.8) | 70 (19.6) | |
| 2008 | 4,764 (25.7) | 3,270 (25.7) | 781 (25.8) | 96 (26.9) | |
| 2009 | 4,513 (24.3) | 3,146 (24.7) | 736 (24.3) | 91 (25.5) | |
| 2010 | 4,489 (24.2) | 3,122 (24.5) | 760 (25.1) | 100 (28.0) | |
| Median income level | < .001 | ||||
| < $30,000 | 2,673 (14.4) | 1,449 (11.4) | 1,006 (33.3) | 50 (14.0) | |
| $30,000-$34,999 | 3,037 (16.4) | 2,391 (18.8) | 1,053 (34.8) | 91 (25.5) | |
| $35,000-$45,999 | 5,378 (29.0) | 3,629 (28.5) | 597 (19.7) | 106 (29.7) | |
| ≥ $46,000 | 7,216 (38.9) | 5,105 (40.1) | 330 (10.9) | 99 (27.7) | |
| Missing | 259 (1.4) | 174 (1.4) | 40 (1.3) | 11 (3.1) | |
| Facility type | < .001 | ||||
| Community cancer program | 3,132 (16.9) | 1,806 (14.2) | 212 (7.0) | 34 (9.5) | |
| Comprehensive community cancer program | 9,007 (48.5) | 6,526 (51.2) | 1,635 (54.0) | 128 (35.9) | |
| Teaching/research center | 3,690 (19.9) | 2,337 (18.3) | 596 (19.7) | 53 (14.9) | |
| NCI program/network | 664 (3.6) | 826 (6.5) | 412 (13.6) | 121 (33.9) | |
| Other | 2,070 (11.2) | 1,253 (9.8) | 171 (5.7) | 21 (5.9) | |
| Charlson-Deyo comorbidity score | < .001 | ||||
| 0 | 12,854 (69.3) | 9,024 (70.8) | 2,129 (70.4) | 281 (78.7) | |
| 1 | 3,941 (21.2) | 2,654 (20.8) | 633 (20.9) | 57 (16.0) | |
| ≥ 2 | 1,768 (9.5) | 1,070 (8.4) | 264 (8.7) | 19 (5.3) | |
| Density level of MO | < .001 | ||||
| Low | 16,880 (90.9) | 12,101 (94.9) | 2,904 (96.0) | 330 (92.4) | |
| High | 1,683 (9.1) | 647 (5.1) | 122 (4.0) | 27 (7.6) | |
| No. of positive lymph nodes | .330 | ||||
| < 4 | 11,786 (63.5) | 8,026 (63.0) | 1,892 (62.5) | 209 (58.5) | |
| ≥ 4 | 6,708 (36.1) | 4,663 (36.6) | 1,124 (37.1) | 146 (40.9) | |
| Unknown | 69 (0.4) | 59 (0.5) | 10 (0.3) | 2 (0.6) | |
| Tumor grade | < .001 | ||||
| Well differentiated | 1,171 (6.3) | 783 (6.1) | 169 (5.6) | 12 (3.4) | |
| Moderately differentiated | 12,268 (66.1) | 8,536 (67.0) | 1,991 (65.8) | 201 (56.3) | |
| Poorly differentiated | 4,296 (23.1) | 2,807 (22.0) | 707 (23.4) | 123 (34.5) | |
| Undifferentiated | 407 (2.2) | 307 (2.4) | 79 (2.6) | 11 (3.1) | |
| Unknown | 421 (2.3) | 315 (2.5) | 80 (2.6) | 10 (2.8) | |
| Surgical margin | < .001 | ||||
| Negative | 16,879 (90.9) | 11,556 (90.7) | 2,763 (91.3) | 304 (85.2) | |
| Positive | 1,498 (8.1) | 996 (7.8) | 195 (6.4) | 19 (5.3) | |
| Unknown | 186 (1.0) | 196 (1.5) | 68 (2.3) | 34 (9.5) | |
Abbreviations: MO, medical oncologist; NCI, National Cancer Institute.
Because of rounding, percentages presented throughout this table may not add up to precisely 100%.
Footnotes
Listen to the podcast by Dr Onega at www.jco.org/podcasts
Supported by American Cancer Society Intramural Research Funding.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
This study used the National Cancer Data Base (NCDB). The interpretation and reporting of these data are the sole responsibility of the authors. The data used in the study are derived from the limited data set of the NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the authors.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Chun Chieh Lin, Suanna S. Bruinooge, Ahmedin Jemal, Michael Kosty, Dawn L. Hershman
Administrative support: Suanna S. Bruinooge, M. Kelsey Kirkwood
Provision of study materials or patients: Chun Chieh Lin, Suanna S. Bruinooge, M. Kelsey Kirkwood
Collection and assembly of data: Chun Chieh Lin, M. Kelsey Kirkwood
Data analysis and interpretation: Chun Chieh Lin, Suanna S. Bruinooge, M. Kelsey Kirkwood, Christine Olsen, Ahmedin Jemal, Dean Bajorin, Sharon H. Giordano, Michael Goldstein, B. Ashleigh Guadagnolo, Michael Kosty, Shane Hopkins, James B. Yu, Anna Arnone, Amy Hanley, Stephanie Stevens, Dawn L. Hershman
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between Geographic Access to Cancer Care, Insurance, and Receipt of Chemotherapy: Geographic Distribution of Oncologists and Travel Distance
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Chun Chieh Lin
No relationship to disclose
Suanna S. Bruinooge
No relationship to disclose
M. Kelsey Kirkwood
No relationship to disclose
Christine Olsen
No relationship to disclose
Ahmedin Jemal
No relationship to disclose
Dean Bajorin
No relationship to disclose
Sharon H. Giordano
No relationship to disclose
Michael Goldstein
No relationship to disclose
B. Ashleigh Guadagnolo
No relationship to disclose
Michael Kosty
Speakers' Bureau: Astellas Pharma, Genentech/Roche, Sanofi, Lilly, Bayer
Research Funding: Genentech/Roche (Inst), Merck Serono (Inst)
Shane Hopkins
No relationship to disclose
James B. Yu
Research Funding: 21st Century Oncology (Inst)
Anna Arnone
Employment: American Society for Radiation Oncology
Amy Hanley
No relationship to disclose
Stephanie Stevens
Employment: American Society for Radiation Oncology
Dawn L. Hershman
No relationship to disclose
REFERENCES
- 1.André T, Iveson T, Labianca R, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer—Trial design and current status. Curr Colorectal Cancer Rep. 2013;9:261–269. doi: 10.1007/s11888-013-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 3.Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 5.Sargent D, Shi Q, Yothers G, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: Data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;47:990–996. doi: 10.1016/j.ejca.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorey KM, Haji-Jama S, Bartfay E, et al. Lack of access to chemotherapy for colon cancer: Multiplicative disadvantage of being extremely poor, inadequately insured and African American. BMC Health Serv Res. 2014;14:133. doi: 10.1186/1472-6963-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roetzheim RG, Pal N, Gonzalez EC, et al. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90:1746–1754. doi: 10.2105/ajph.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh AA, Robinson J, Zaydfudim VM, et al. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol. 2014;110:227–232. doi: 10.1002/jso.23627. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Virgo KS. Association between the availability of medical oncologists and initiation of chemotherapy for patients with stage III colon cancer. J Oncol Pract. 2013;9:27–33. doi: 10.1200/JOP.2012.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendren S, Birkmeyer JD, Yin H, et al. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53:1587–1593. doi: 10.1007/DCR.0b013e3181f2f202. [DOI] [PubMed] [Google Scholar]
- 12.Chou S, Deily ME, Li S. Travel distance and health outcomes for scheduled surgery. Med Care. 2014;52:250–257. doi: 10.1097/MLR.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 13.Ahamad A. Geographic access to cancer care: A disparity and a solution. Postgrad Med J. 2011;87:585–589. doi: 10.1136/pgmj.2010.111930. [DOI] [PubMed] [Google Scholar]
- 14.Probst JC, Laditka SB, Wang JY, et al. Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res. 2007;7:40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24:390–399. doi: 10.1111/j.1748-0361.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 17.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22:140–146. doi: 10.1111/j.1748-0361.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 18.Ananthakrishnan AN, Hoffmann RG, Saeian K. Higher physician density is associated with lower incidence of late-stage colorectal cancer. J Gen Intern Med. 2010;25:1164–1171. doi: 10.1007/s11606-010-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roetzheim RG, Pal N, van Durme DJ, et al. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol. 2000;43:211–218. doi: 10.1067/mjd.2000.106242. [DOI] [PubMed] [Google Scholar]
- 20.Ferrante JM, Gonzalez EC, Pal N, et al. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13:408–414. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- 21.Fleisher JM, Lou JQ, Farrell M. Relationship between physician supply and breast cancer survival: A geographic approach. J Community Health. 2008;33:179–182. doi: 10.1007/s10900-008-9090-z. [DOI] [PubMed] [Google Scholar]
- 22.Onega T, Duell EJ, Shi X, et al. Influence of place of residence in access to specialized cancer care for African Americans. J Rural Health. 2010;26:12–19. doi: 10.1111/j.1748-0361.2009.00260.x. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood MK, Bruinooge SS, Goldstein MA, et al. Enhancing the American Society of Clinical Oncology workforce information system with geographic distribution of oncologists and comparison of data sources for the number of practicing oncologists. J Oncol Pract. 2014;10:32–38. doi: 10.1200/JOP.2013.001311. [DOI] [PubMed] [Google Scholar]
- 24.Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the National Cancer Data Base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 25.Fedewa SA, Etzioni R, Flanders WD, et al. Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004-2006. Cancer Epidemiol Biomarkers Prev. 2010;19:2437–2444. doi: 10.1158/1055-9965.EPI-10-0299. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. Physician Compare datasets. https://data.medicare.gov/data/physician-compare.
- 27.The Dartmouth Institute. Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org/data/region/
- 28.Massarweh NN, Chiang YJ, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32:942–948. doi: 10.1200/JCO.2013.52.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang EH, Mougalian SS, Soulos PR, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: A National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys. 2014;90:993–1000. doi: 10.1016/j.ijrobp.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, et al. A new method for classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 32.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 33.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: A comparison of hospital records and Medicare claims for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Gruca TS, Nam I, Tracy R. Trends in medical oncology outreach clinics in rural areas. J Oncol Pract. 2014;10:e313–e320. doi: 10.1200/JOP.2013.001350. [DOI] [PubMed] [Google Scholar]
- 36.Guidry JJ, Aday LA, Zhang D, et al. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5:361–366. [PubMed] [Google Scholar]
- 37.Scoggins JF, Fedorenko CR, Donahue SM, et al. Is distance to provider a barrier to care for Medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28:54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 39.Cheeseman SL, Joel SP, Chester JD, et al. A “modified de Gramont” regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87:393–399. doi: 10.1038/sj.bjc.6600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maindrault-Goebel F, de Gramont A, Louvet C, et al. Evaluation of oxaliplatin dose intensity in bimonthly leucovorin and 48-hour 5-fluorouracil continuous infusion regimens (FOLFOX) in pretreated metastatic colorectal cancer: Oncology Multidisciplinary Research Group (GERCOR) Ann Oncol. 2000;11:1477–1483. doi: 10.1023/a:1026520812351. [DOI] [PubMed] [Google Scholar]
- 41.Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32:2758–2764. doi: 10.1200/JCO.2013.53.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieber GM, Benzie D, McMahon S. Why patients bypass rural health care centers. Minn Med. 1996;79:46–50. [PubMed] [Google Scholar]
- 43.Meyerhardt JA, Catalano PJ, Schrag D, et al. Association of hospital procedure volume and outcomes in patients with colon cancer at high risk for recurrence. Ann Intern Med. 2003;139:649–657. doi: 10.7326/0003-4819-139-8-200310210-00008. [DOI] [PubMed] [Google Scholar]
- 44.Lund JL, Stürmer T, Sanoff HK, et al. Determinants of adjuvant oxaliplatin receipt among older stage II and III colorectal cancer patients. Cancer. 2013;119:2038–2047. doi: 10.1002/cncr.27991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624–2634. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]