Abstract
Purpose
Myelofibrosis is a myeloid malignancy associated with anemia, splenomegaly, and constitutional symptoms. Patients frequently harbor JAK-STAT activating mutations that are sensitive to TG101348, a selective small-molecule Janus kinase 2 (JAK2) inhibitor.
Patients and Methods
In a multicenter phase I trial, oral TG101348 was administered once a day to patients with high- or intermediate-risk primary or post–polycythemia vera/essential thrombocythemia myelofibrosis.
Results
Fifty-nine patients were treated, including 28 in the dose-escalation phase. The maximum-tolerated dose was 680 mg/d, and dose-limiting toxicity was a reversible and asymptomatic increase in the serum amylase level. Forty-three patients (73%) continued treatment beyond six cycles; the median cumulative exposure to TG101348 was 380 days. Adverse events included nausea, vomiting, diarrhea, anemia, and thrombocytopenia; corresponding grades 3 to 4 incidence rates were 3%, 3%, 10%, 35%, and 24%. TG101348 treatment had modest effect on serum cytokine levels, but greater than half of the patients with early satiety, night sweats, fatigue, pruritus, and cough achieved rapid and durable improvement in these symptoms. By six and 12 cycles of treatment, 39% and 47% of patients, respectively, had achieved a spleen response per International Working Group criteria. The majority of patients with leukocytosis or thrombocytosis at baseline (n = 28 and n = 10, respectively) achieved normalization of blood counts after six (57% and 90%, respectively) and 12 (56% and 88%, respectively) cycles. A significant decrease in JAK2 V617F allele burden was observed at 6 months in mutation-positive patients (n = 51; P = .04), particularly in the subgroup with allele burden greater than 20% (n = 23; P < .01); the decrease was durable at 12 months.
Conclusion
TG101348 is well tolerated and produces significant reduction in disease burden and durable clinical benefit in patients with myelofibrosis.
INTRODUCTION
Myelofibrosis (MF) is a BCR-ABL1–negative myeloproliferative neoplasm (MPN) that presents de novo (ie, primary) or may be preceded by polycythemia vera (PV) or essential thrombocythemia (ET). Clinical features include progressive anemia, marked splenomegaly, constitutional symptoms (eg, fatigue, night sweats, bone pain, pruritus, and cough) and weight loss.1 Median survival ranges from less than 2 years to greater than 15 years on the basis of currently identified prognostic factors.2–4 Mutations involving JAK2,5,6 MPL,7 TET2,8 ASXL1,9 IDH1/IDH2,10,11 CBL,12 IKZF1,13 LNK,14 or EZH215 have been described in patients with MPN, including in those with MF. Some mutations occur at high frequency in MF (eg, JAK2 mutations in approximately 50% of patients), and they either directly (eg, JAK2 or MPL mutations) or indirectly (eg, LNK or CBL mutations) induce JAK-STAT hyperactivation. We hypothesized that selective JAK2 inhibition may be of value in a significant proportion of patients with MF. In support of this hypothesis, preclinical studies with TG101348, a potent and selective catalytic site JAK2 inhibitor, have shown potent anti-JAK2 V617F and anti-MPL W515L activity in cell lines, primary cells, and murine disease models.16–19 Here, we report on safety and efficacy data for TG101348 observed in a phase I study of patients with MF.
PATIENTS AND METHODS
Study Design
The study was registered at ClinicalTrials.gov and constituted a phase I, dose-escalation trial (MF-TG101348-001). Study eligible patients were 18 years of age or older and had high- or intermediate-risk primary myelofibrosis (PMF), post-PV MF, or post-ET MF.20 Additional eligibility criteria and participating centers are listed in the Data Supplement (online only). All patients provided written informed consent. The primary end points were determination of safety and tolerability, dose-limiting toxicity (DLT), maximum-tolerated dose (MTD), and pharmacokinetic (PK) behavior of TG101348. The secondary end point was assessment of therapeutic activity.
Patients were successively assigned to one of eight dose cohorts, which ranged from 30 to 800 mg per day, according to a standard 3 + 3 cohort design. TG101348 was administered orally once daily, with a treatment plan for continuous daily therapy for 24 weeks (six × 28-day cycles). Intrapatient dose escalation was permitted after completion of at least three cycles of treatment at the starting dose. Once DLT was identified, a dose-confirmation cohort initiated treatment at the MTD. Treatment beyond six cycles was allowed on an extension study (MF-TG101348-002; NCT00724334) if deemed beneficial to the patient and if well tolerated.
Assessment of Toxicity and Response
Safety assessments were performed weekly during cycle 1, every other week during cycles 2 and 3, and every 4 weeks thereafter. Toxicity was graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
Responses were measured every 4 weeks per International Working Group for MPN Research and Treatment (IWG-MRT) criteria.21 Assessment of bone marrow histology was performed at baseline and every 24 weeks of therapy. Changes in JAK2 V617F allele burden in the granulocyte fraction of peripheral blood were measured as previously described22; the assessments were at baseline and every 4 weeks during the first 6 cycles and at every sixth cycle of therapy in the extension study.
Pharmacokinetics
The concentration-time curves of TG101348 in plasma were evaluated by a noncompartmental analysis with the use of WINNonlin (Scientific Consultant, Apex, NC) software, version 5.2.
Cytokine Assessment
Samples for cytokine measurement were collected at baseline and every 4 weeks thereafter. Cytokine levels were measured by using multiplexed sandwich ELISAs (Millipore, St Charles, MO).
RESULTS
Enrollment of Patients
A total of 59 patients were enrolled; 28 were in the dose-escalation phase, and 31 were in the dose-confirmation phase (Table 1). Forty-four patients had PMF, 12 had post-PV MF, and three had post-ET MF; 86% were JAK2 V617F positive. The median duration of disease was 3.4 years (range, 0.06 to 25.8 years). At study enrollment, the median palpable spleen size was 18 cm below the left costal margin (83% had a palpable spleen size > 10 cm), median hemoglobin level was 9.2 g/dL (range, 6.6 to 15.2 g/dL), and 21 patients (36%) were red cell transfusion dependent by IWG-MRT criteria.
Table 1.
Clinical and Demographic Patient Characteristics
| Characteristic | TG101348 Starting Dose (mg/d) |
MTD Cohort (n = 40) |
All Doses (n = 59) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 (n = 4) | 60 (n = 3) | 120 (n = 3) | 240 (n = 3) | 360 (n = 3) | 520 (n = 3) | 680 (n = 34) | 800 (n = 6) | No. | % | No. | % | |
| Age, years | 63.5 | 64.0 | 63.0 | 68.0 | 66.0 | 57.0 | 63.5 | 69.0 | ||||
| Range | 55-76 | 56-66 | 53-71 | 55-79 | 61-71 | 50-66 | 43-83 | 50-85 | 43-85 | 43-85 | ||
| Mean | 65.1 | 64.5 | ||||||||||
| SD | 10.47 | 9.70 | ||||||||||
| Sex | ||||||||||||
| Male | 2 | 3 | 1 | 2 | 2 | 2 | 18 | 4 | 22 | 55.0 | 34 | 57.6 |
| Female | 2 | 0 | 2 | 1 | 1 | 1 | 16 | 2 | 18 | 45.0 | 25 | 42.4 |
| Ethnicity | ||||||||||||
| White | 3 | 2 | 3 | 3 | 3 | 2 | 29 | 6 | 35 | 87.5 | 51 | 86.4 |
| African American | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2.5 | 1 | 1.7 |
| Asian | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 7.5 | 5 | 8.5 |
| Other | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 2.5 | 2 | 3.4 |
| Diagnosis | ||||||||||||
| PMF | 3 | 2 | 1 | 3 | 2 | 2 | 27 | 4 | 31 | 77.5 | 44 | 74.6 |
| Post-PV MF | 1 | 1 | 2 | 0 | 1 | 1 | 6 | 0 | 6 | 15.0 | 12 | 20.3 |
| Post-ET MF | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 7.5 | 3 | 5.1 |
| Mayo PSS risk category | ||||||||||||
| High | 0 | 0 | 1 | 2 | 0 | 3 | 14 | 6 | 20 | 50 | 26 | 44.1 |
| Not high* | 4 | 3 | 2 | 1 | 3 | 0 | 20 | 0 | 20 | 50.0 | 33 | 55.9 |
| JAK2V617F positive | 3 | 3 | 3 | 2 | 3 | 2 | 29 | 6 | 35 | 87.5 | 51 | 86.4 |
| Transfusion dependent | 1 | 1 | 0 | 1 | 0 | 2 | 13 | 3 | 16 | 40.0 | 21 | 35.6 |
| Spleen size > 10 cm | 3 | 3 | 3 | 2 | 3 | 2 | 28 | 5 | 33 | 82.5 | 49 | 83.1 |
Abbreviations: MTD, maximum-tolerated dose; SD, standard deviation; PMF, primary myelofibrosis; PV, polycythemia vera; MF, myelofibrosis; ET, essential thrombocythemia; PSS, prognostic scoring system; JAK, Janus kinase.
Equivalent to symptomatic/treatment refractory intermediate-risk disease.
In the dose-escalation phase, the starting dose of TG101348 was 30 mg/d and subsequent dose levels were 60, 120, 240, 360, 520, 680, and 800 mg/d (Table 1). At 800 mg/d, two of six patients experienced DLT; consequently, the MTD was declared at 680 mg/d. In the dose-confirmation phase, all patients started treatment at the MTD. The MTD cohort (n = 40; Table 1) included patients who received 680 mg/d as their starting dose (dose-escalation cohort, n = 3; dose-confirmation cohort, n = 31) and those whose drug dose was decreased from 800 mg/d (n = 6) to 680 mg/d after MTD was declared.
The median exposures to TG101348 for the overall (n = 59) and MTD (n = 40) cohorts were 155 days (range, 2 to 172 days) and 147 days (range, 8 to 171 days), respectively. TG101348 doses at the end of each cycle per dose cohort are illustrated in the Data Supplement (online only). In the MTD cohort, 28 patients (70%) required dose-reduction during the first six cycles; the primary reasons were as follows: cytopenias (20%), gastrointestinal adverse events (12.5%), amylase/lipase elevation (10%), ALT elevation (7.5%), investigator discretion (7.5%), or other adverse events (12.5%). The median cycle at dose-reduction for the MTD cohort was cycle 3 (range, 1 to 7); the median dose at the end of cycle 3 was 680 mg/d (range, 360 to 680 mg/d), and it was 520 mg/d (range, 360 to 680 mg/d) at the end of cycle 6.
Forty three patients (73%), including 28 (70%) from the MTD cohort, continued treatment on the extension study; at entry into the extension study, 31 patients (72%) were receiving less than 680 mg/d of the drug (median, 520 mg/d; range, 120 to 680 mg/d). At data cutoff, the median cumulative exposure to TG101348 for the 43 patients was 380 days (range, 170 to 767 days). The number of treatment cycles completed ranged from 7 to 29; 39 patients (66%), including 27 (68%) from the MTD cohort completed 12 treatment cycles. At data cutoff, 28%, and 14% of patients who entered the extension study had completed 18 and 24 treatment cycles, respectively. The median treatment dose during the extension phase was 440 mg/d (range, 120 to 680 mg/d).
Pharmacokinetics
Peak plasma concentration of TG101348 was achieved 1 to 4 hours after dosing. TG101348 showed greater than dose-proportional increases in plasma PK parameters (maximum plasma concentration [Cmax] and area under the concentration-time curve [AUC0-t]; Data Supplement). Mean steady-state Cmax and AUC0-t values increased approximately 54- and 88-fold, respectively, over a 27-fold increase in dose. The terminal phase half-life at steady-state remained similar across all doses (16 to 34 hours), consistent with linear drug elimination.
Safety Profile
The DLT in two of six patients treated at 800 mg/d was asymptomatic grade 3 or 4 hyperamylasemia (with or without hyperlipasemia) that was reversible. The most common nonhematologic adverse events at least possibly related to TG101348 included predominantly grade 1 nausea, diarrhea, and vomiting; grade 3 events were reported overall and in the MTD cohort for 3% and 5%, 10% and 13%, and 3% and 3% of patients, respectively, and there were no grade 4 events (Table 2). These adverse events were dose dependent, and grade 3 occurrences were observed almost exclusively with a TG101348 starting dose of 680 mg/d or greater. The gastrointestinal symptoms were largely self limited or controlled by symptomatic treatment and/or dose reduction. Other adverse events (grades 3 to 4; overall and MTD cohort) included asymptomatic increases in serum lipase (10% and 15%), AST (2% and 3%), ALT (7% and 8%), creatinine (0% and 0%), and alkaline phosphatase (0% and 0%; Table 2).
Table 2.
Treatment-Emergent Nonhematologic and Hematologic Adverse Events Considered At Least Possibly Related to TG101348 and Reported for ≥ 10% of Patients
| Adverse Event | MTD Cohort (n = 40) |
All Patients (n = 59) |
||||||
|---|---|---|---|---|---|---|---|---|
| Severity Grade1 to 2 |
Severity Grade3 to 4 |
Severity Grade1 to 2 |
Severity Grade3 to 4 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Gastrointestinal | ||||||||
| Nausea | 31 | 77.5 | 2 | 5.0 | 39 | 66.1 | 2 | 3.4 |
| Diarrhea | 25 | 62.5 | 5 | 12.5 | 32 | 54.2 | 6 | 10.2 |
| Vomiting | 27 | 67.5 | 1 | 2.5 | 32 | 54.2 | 2 | 3.4 |
| Abdominal pain | 4 | 10.0 | 0 | 6 | 10.2 | 0 | ||
| General | ||||||||
| Anorexia | 6 | 15.0 | 0 | 8 | 13.6 | 0 | ||
| Edema peripheral | 4 | 10.0 | 0 | 6 | 10.2 | 0 | ||
| Abnormal laboratory values | ||||||||
| Hyperlipasemia | 9 | 22.5 | 6 | 15.0 | 10 | 16.9 | 6 | 10.2 |
| Alanine aminotransferase increased | 9 | 22.5 | 3 | 7.5 | 11 | 18.6 | 4 | 6.8 |
| Aspartate aminotransferase increased | 13 | 32.5 | 1 | 2.5 | 15 | 25.4 | 1 | 1.7 |
| Blood creatinine increased | 11 | 27.5 | 0 | 14 | 23.7 | 0 | ||
| Blood alkaline phosphatase increased | 9 | 22.5 | 0 | 10 | 16.9 | 0 | ||
| Hypocalcemia | 6 | 15.0 | 1 | 2.5 | 7 | 11.9 | 1 | 1.7 |
| Skin and subcutaneous tissue disorders | ||||||||
| Skin exfoliation | 8 | 20.0 | 0 | 8 | 13.6 | 0 | ||
| Dry skin | 6 | 15 | 0 | 6 | 10.2 | 0 | ||
| Hematologic | ||||||||
| Anemia* | 2 | 8.3 | 13 | 54.2 | 3 | 8.1 | 13 | 35.1 |
| Thrombocytopenia | 8 | 20.0 | 11 | 27.5 | 10 | 17.0 | 14 | 23.7 |
| Neutropenia | 2 | 5.0 | 4 | 10.0 | 2 | 3.4 | 6 | 1.02 |
Abbreviation: MTD, maximum-tolerated dose.
Events reported only for patients who were not transfusion dependent at study entry (MTD cohort, n = 24; all patients, n = 37) are presented.
Grades 3 to 4 hematologic adverse events considered related to TG101348 included anemia (35% of 37 patients who were not transfusion dependent at baseline), thrombocytopenia (24%) and/or neutropenia (10%; Table 2). The majority of treatment-emergent cytopenias were noted in the first three cycles of treatment. Of the 13 patients who developed grades 3 to 4 anemia (all in the MTD cohort), 67% entered the study with grade 2 anemia. Emergence of transfusion requirement was significantly lower for patients who initiated treatment at 240 to 520 mg/d (33%) as opposed to 680 mg/d (72%). Of the 14 patients with grades 3 to 4 thrombocytopenia, four and five patients entered the study with grade 1 and 2 thrombocytopenia, respectively.
At data cutoff, no unique safety findings have emerged with continued dosing of TG101348 beyond six cycles of therapy. Serious adverse events considered at least possibly related to TG101348 occurred in eight patients and included asymptomatic hyperlipasemia, thrombocytopenia/neutropenia, depression, tumor lysis syndrome, cerebrovascular accident, and dehydration (Data Supplement). One event of cardiac arrest was fatal, and one patient committed suicide 12 weeks after discontinuing study treatment for severe depression; one patient discontinued treatment because of grade 4 thrombocytopenia; all other events were reversible, and patients were able to resume treatment at a lower dose after resolution of the adverse event. There were two other fatalities deemed unrelated to TG101348, including one that was a result of progressive disease and another that resulted from complications of systemic bleeding associated with an acquired factor VIII inhibitor.
Fifteen patients (25%) discontinued treatment during the first six cycles of therapy (Data Supplement). Reasons for discontinuation included treatment-related adverse events (n = 6), investigator decision/intercurrent illness (n = 3), or withdrawal of consent (n = 6). Eight (19%) of 43 patients discontinued treatment during the extension study; the eight patients included three who discontinued treatment because of adverse events after a total of 24 to 46 weeks on therapy (Data Supplement).
Three patients had disease progression (doses at study start and discontinuation are indicated): one each with progressive hepatosplenomegaly and ascites with superimposed endocarditis (cycle 2; 680 and 520 mg/d), accelerated myelofibrosis (cycle 13; 520 and 200 mg/d), and leukemic transformation (cycle 2; 520 and 520 mg/d).
Responses
Splenomegaly.
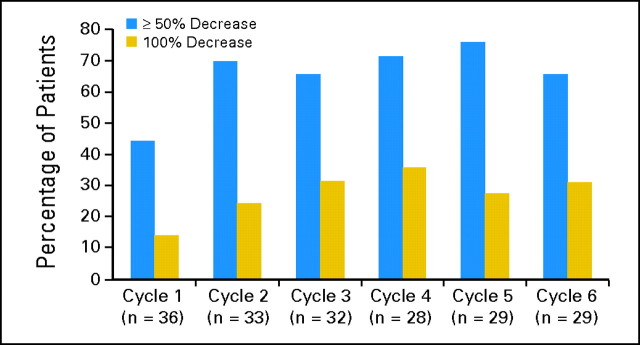
The onset of spleen response was rapid and generally seen within the first two cycles. By cycle 6, 36 patients (61%) had experienced a minimum 25% decrease in palpable spleen size, including 65% in the MTD cohort (intent-to-treat analysis). By this time point, a ≥ 50% decrease in palpable spleen size persistent for at least 8 weeks (ie, clinical improvement [CI] per IWG-MRT criteria) had been observed in 39% and 45% of patients overall and in the MTD cohort, respectively. Spleen responses per treatment cycle for the MTD cohort are shown in Figure 1. Three (75%) of four patients with JAK2 V617F–negative MF who completed six cycles of treatment achieved CI. The lowest starting dose at which CI was observed was 240 mg/d. The median time to CI across doses was 141 days (range, 41 to 171 days), and it was 113 days (range, 41 to 170 days) for the MTD cohort. By cycle 12, spleen responses (CI) were observed in 47% and 50% of patients for the overall and MTD cohorts, respectively. The mean duration of spleen response per IWG-MRT criteria was 315 days (standard deviation, ± 129 days) and 288 days (± 76 days) for the overall and MTD cohorts, respectively.
Fig 1.
Splenomegaly response to TG101348 therapy. Decrease in palpable spleen size from baseline by cycle for patients in the maximum-tolerated dose cohort (n = 37). The proportion of patients with 50% or greater and 100% decrease in palpable splenomegaly is displayed. For patients who completed six cycles of treatment, 90% had a 25% or greater reduction in palpable spleen size, 66% had a 50% or greater reduction, and the spleen became nonpalpable in 31%.
Constitutional symptoms.
Thirty-five patients in the MTD cohort endorsed the presence and severity of early satiety, fatigue, night sweats, cough, and pruritus on an 11-point scale (0 = absence of symptoms to 10 = worst imaginable symptoms) at baseline and at the end of at least one cycle. Symptoms were categorized as absent (score = 0), mild (score = 1 to 3), moderate (score = 4 to 7), or severe (score =8 to 10).
Early satiety was reported by 29 patients (85%) at baseline. After two cycles of treatment (n = 27), 56% reported complete resolution of this symptom (Fig 2A). Fatigue was reported at baseline by 26 patients (76%). After six cycles (n = 16), 63% reported improvement, and 25% reported complete resolution, of this symptom (Fig 2B). Night sweats were reported at baseline by 14 patients (40%). After one cycle, 64% of patients had complete resolution of this symptom; after six cycles, this proportion had increased to 89% (n = 9; Fig 2C). Cough was reported at baseline by 13 patients (37%). After one cycle (n = 12), 75% reported improvement, and 67% reported complete resolution, of this symptom. Pruritus was reported by eight patients (23%) at baseline. After one cycle, 75% had improvement, and 50% reported complete resolution. Responses in constitutional symptoms were durable in most instances.
Fig 2.
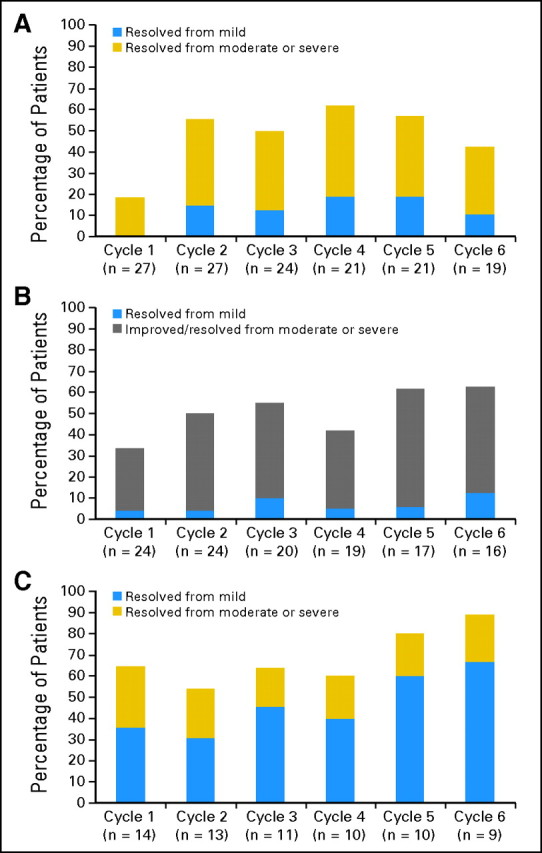
Effects of TG101348 on symptoms of myelofibrosis. (A) Proportion of patients in maximum-tolerated dose cohort with complete resolution of early satiety by cycle from a baseline symptom score of‘mild (score = 1 to 3), moderate (score = 4 to 7), or severe (score = 8 to 10). Twenty-seven patients (79%) and 19 patients (56%) were evaluable for improvement in early satiety at the end of 1 and 6 cycles, respectively. After two cycles of treatment, 56% reported complete resolution of this symptom with durable benefit. (B) Proportion of patients in maximum-tolerated dose cohort with complete resolution of fatigue by cycle from a baseline symptom score of mild (score = 1 to 3), or improvement in or complete resolution of fatigue from a baseline score of moderate (score = 4 to 7) or severe (score = 8 to 10). Twenty-four patients (71%) and 16 patients (47%) were evaluable for improvement in fatigue at the end of one and six cycles, respectively. After six cycles, 63% reported improvement, and 25% had complete resolution of this symptom. (C) Proportion of patients in maximum-tolerated dose cohort with complete resolution of night sweats by cycle from a baseline symptom score of mild (score = 1 to 3), moderate (score = 4 to 7), or severe (score = 8 to 10). Fourteen patients (40%) and nine patients (26%) were evaluable for improvement in night sweats at the end of one and six cycles, respectively. After one cycle, 64% of patients had complete resolution of this symptom; after six cycles, this proportion had increased to 89%.
Body weight.
At the end of six and 12 cycles, the median body weight was stable relative to baseline for the overall and MTD cohorts (Data Supplement).
Leukocytosis and thrombocytosis.
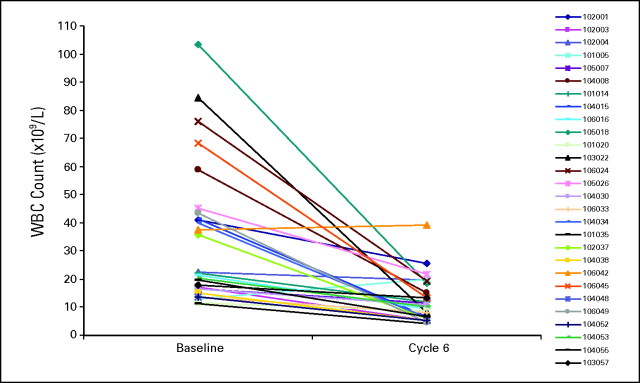
Leukocytosis (WBC > 11 × 109/L) was present at baseline in 33 patients (56%), 28 of whom completed six cycles of treatment; of these, 18 were in the MTD cohort. After six cycles, 16 patients across doses (57%) and 13 patients in the MTD cohort (72%) achieved a normal WBC (Fig 3); after 12 cycles, 14 (56%) of 25 patients across doses and 10 (59%) of 17 in the MTD cohort had normal WBC.
Fig 3.
Response of leukocytosis to TG101348 therapy. Changes in WBC after six cycles for patients who entered the study with leukocytosis (WBC > 11 × 109/L). After six cycles, 16 patients across doses (57%) and 13 patients in the MTD cohort (72%) achieved a normal WBC, with durable benefit.
Thrombocytosis (platelet count > 450 × 109/L) was noted at baseline for 10 patients (17%) across doses and for seven patients (19%) in the MTD cohort (n = 37), all of whom completed six cycles of therapy. At this time point, 90% and 100% of patients across doses and in the MTD cohort, respectively, achieved a normal platelet count; after 12 cycles, seven (88%) of eight patients across doses and all six patients in the MTD cohort had a normal platelet count.
JAK2 V617F allele burden.
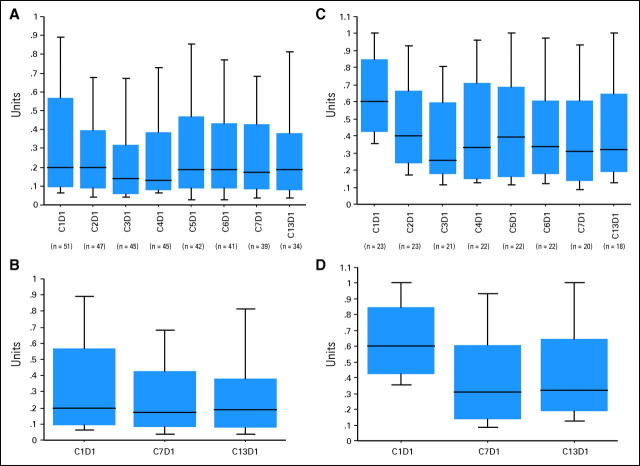
Fifty-one patients (86%) were JAK2 V617F positive and had a median allele burden of 20% (range, 3% to 100%); of these, 23 (45%) had a significant allele burden (defined as > 20% at baseline) with a median of 60% (range, 23% to 100%). For the overall mutation-positive patients, there was a significant decrease in the JAK2 V617F allele burden after six cycles (P = .04) and 12 cycles of treatment (P = .01; Figs 4A and 4B). After six and 12 cycles of treatment, the median allele burdens were 17% (range, 0% to 100%) and 19% (range, 0% to 100%), respectively. Similarly, for the 23 patients with baseline JAK2 V617F allele burden of greater than 20%, there was a significant and even more pronounced decrease in the JAK2 V617F allele burden after six cycles (P = .002) and 12 cycles of treatment (P = .002) (Figs 4C and 4D). After six and 12 cycles of treatment, the median allele burdens were 31% (range, 4% to 100%) and 32% (range, 7% to 100%), respectively. After six cycles, 16 (80%) of 20 patients with baseline allele burden greater than 20% who reached this time point exhibited a median 61% (range, 6% to 96%) decrease, and nine patients (45%) had a ≥ 50% decrease in JAK2 V617F allele burden. In contrast, four patients (20%) exhibited an increase (one each with increase of 18%, 21%, 30%, and 58%). Eighteen patients (78%) of the group with allele burden greater than 20% completed 12 cycles of treatment with a median 50% (range, 29% to 82%) decrease, and seven (39%) patients had a ≥ 50% decrease in JAK2 V617F. Three patients (17%) exhibited an increase in allele burden (one each with increase of 7%, 18%, and 22%), and two others with 100% allele burden at baseline exhibited no change.
Fig 4.
Effect of TG101348 therapy on JAK2 V617F allele burden. Box plot representation of JAK2 V617F allele burden data (A,B) for all mutation-positive patients (n = 51) and (C,D) for the subgroup with baseline allele burden greater than 20% (n = 23). The y-axis represents the JAK2 V617F allele burden from 1.0 (100%) to 0.0 (0%).The change in JAK2 V617F allele burden per cycle of treatment (up to end of cycle 12; ie, C13D1) compared with prestudy baseline is shown (A,C) for the two groups. (B,D) The change at the end of cycle 6 (ie, C7D1) and cycle 12. A significant decrease in JAK2 V617F allele burden compared with prestudy baseline was observed at the end of cycle 6 (B) for the mutation-positive group (P = .04) and (D) for the subgroup with baseline allele burden greater than 20% (P = .002); a similar significant decrease was seen at the end of cycle 12 (B) for the former (P = .01) and (D) latter (P = .002) groups. The Wilcoxon matched-pair signed-rank test was used to compare the median JAK2 V617F allele burden for the comparisons.
DISCUSSION
The current study provides proof-of-concept for selective JAK2 inhibition with TG101348 as a therapeutic strategy in myelofibrosis. A significant proportion of patients experienced rapid, substantial, and durable control of symptomatic splenomegaly, leukocytosis, thrombocytosis, and constitutional symptoms. In addition, there was also evidence for a significant reduction in genomic disease burden that indicates potential for disease-modifying activity. We also observed responses in patients with MF who were JAK2 V617F negative that may plausibly be attributed to other mutations in the JAK-STAT signal transduction pathway in these patients, such as MPL, LNK, or as yet unknown alleles.23–25
Of note, among small molecule inhibitors of the JAK-STAT pathway in MF, TG101348 appears to be unique in its ability to induce a significant and sustained decrease in JAK2 V617F mutant allele burden. We surmise that the effect of JAK2 inhibition on disease burden is the basis for evidence of clinical efficacy in MF with TG101348, as opposed to an indirect anticytokine effect that may play a major role in responses to JAK family antagonists that have off-target activity for JAK1 as well as for JAK2. In support of this hypothesis, we observed no consistent changes in levels of proinflammatory cytokines (interleukin [IL] -6, IL-2, IL-8, and TNF-α) relative to baseline during the course of TG101348 treatment (Data Supplement). In contrast, and consistent with the on-target activity of TG101348 for JAK2, increases in serum erythropoietin and, to a lesser extent thrombopoietin, levels relative to baseline were observed after treatment initiation (data not shown).
The DLT (asymptomatic hyperamylasemia, sometimes with hyperlipasemia) for TG101348 has also been observed with other small molecule inhibitors that target BCR-ABL.26 Gastrointestinal adverse events were frequent in the current study but accounted for treatment discontinuation in only one patient. These symptoms occurred as early as after the first administered dose and demonstrated a clear dose-dependent relationship. The myelosuppressive effects of TG101348 were also dose dependent and consistent with the expected on-target activity and JAK2 selectivity of this agent.
Although the MTD (680 mg/d) of TG101348 was the most efficacious dose, it was also associated with the highest incidence of adverse events. Therefore, a lower starting dose (eg, 400 to 500 mg/d) may provide an optimal risk/benefit balance. Furthermore, because MF is a heterogeneous disease, a dynamic dosing schedule may maximize the opportunity for identifying a patient-specific optimal dose.
The number of patients in the current study was too small to correlate clinical responses with mutant allele reduction. Unlike chronic myelogenous leukemia, MF is a molecularly heterogeneous disease in which many patients harbor greater than 1 mutation of potential pathogenetic relevance. Similarly, JAK2 V617F allele burden can vary from the limit-of-detection (approximately 1%) to 100%, which likely reflects distinct biologic phenotypes. Therefore, we are hesitant to interpret treatment-related changes in JAK2 V617F burden as a primary response criterion; instead, we would suggest that this illustrates activity of TG101348 against the malignant clone.
These observations suggest that, in addition to MF, TG101348 may also have a potential role for the treatment of PV and ET, with the caveat that long-term safety is first demonstrated. In summary, TG101348 appears to have been well tolerated and has shown evidence for efficacy in patients with MF in this phase I study. Additional study is warranted in this clinical context and in others.
Supplementary Material
Acknowledgment
We thank key clinical research personnel, including Ann Engebretson and Kris Djupedal (Mayo Clinic); Andrea Linder and Cheryl Langford (Stanford); Debra Harris Nolan, Kristen Rice, and Tristan Loucks (University of California, San Diego); Maria Gavino and Little Pullokaran (M.D. Anderson Cancer Center); Suzanne Robben (University of Michigan); and Ilene Galinsky and Casey Graziani (Dana-Farber Cancer Institute).
Footnotes
See accompanying editorial on page 781
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00631462.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jolene Shorr, TargeGen (C) Consultant or Advisory Role: Richard M. Stone, Novartis (C), Genzyme; Michael H. Silverman, TargeGen (C) Stock Ownership: Jolene Shorr, TargeGen Honoraria: Catriona Jamieson, TargeGen Research Funding: Animesh Pardanani, TargeGen, Cytopia; Jason R. Gotlib, TargeGen; Catriona Jamieson, TargeGen; Jorge E. Cortes, TargeGen; Moshe Talpaz, TargeGen; Ayalew Tefferi, TargeGen. Expert Testimony: Moshe Talpaz, TargeGen (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Animesh Pardanani, D. Gary Gilliland, Jolene Shorr, Ayalew Tefferi
Provision of study materials or patients: Animesh Pardanani, Jason R. Gotlib, Catriona Jamieson, Jorge E. Cortes, Moshe Talpaz, Richard M. Stone, Ayalew Tefferi
Collection and assembly of data: Animesh Pardanani, Jason R. Gotlib, Catriona Jamieson, Jorge E. Cortes, Moshe Talpaz, Richard M. Stone, Michael H. Silverman, Jolene Shorr, Ayalew Tefferi
Data analysis and interpretation: Animesh Pardanani, Jason R. Gotlib, Catriona Jamieson, Jorge E. Cortes, Moshe Talpaz, Richard M. Stone, Michael H. Silverman, D. Gary Gilliland, Jolene Shorr, Ayalew Tefferi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 2.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 3.Hussein K, Pardanani AD, Van Dyke DL, et al. International Prognostic Scoring System– independent cytogenetic risk categorization in primary myelofibrosis. Blood. 2010;115:496–499. doi: 10.1182/blood-2009-08-240135. [DOI] [PubMed] [Google Scholar]
- 4.Patnaik MM, Caramazza D, Gangat N, et al. Age and platelet count are IPSS-independent prognostic factors in young patients with primary myelofibrosis and complement IPSS in predicting very long or very short survival. Eur J Haematol. 2010;84:105–108. doi: 10.1111/j.1600-0609.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 5.James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 6.Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 9.Carbuccia N, Murati A, Trouplin V, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23:2183–2186. doi: 10.1038/leu.2009.141. [DOI] [PubMed] [Google Scholar]
- 10.Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 2010;362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Lasho TL, Abdel-Wahab O, et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia. 2010;24:1302–1309. doi: 10.1038/leu.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113:6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 13.Jäger R, Gisslinger H, Passamonti F, et al. Deletions of the transcription factor Ikaros in myeloproliferative neoplasms. Leukemia. 2010;24:1290–1298. doi: 10.1038/leu.2010.99. [DOI] [PubMed] [Google Scholar]
- 14.Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116:988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 42:722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 16.Lasho TL, Tefferi A, Hood JD, et al. TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515K or JAK2 exon 12 mutations as well as mutation negative patients. Leukemia. 2008;22:1790–1792. doi: 10.1038/leu.2008.56. [DOI] [PubMed] [Google Scholar]
- 17.Geron I, Abrahamsson AE, Barroga CF, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 21.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 22.Kittur J, Knudson RA, Lasho TL, et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer. 2007;109:2279–2284. doi: 10.1002/cncr.22663. [DOI] [PubMed] [Google Scholar]
- 23.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 24.Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. doi: 10.1182/blood-2010-02-270108. doi: 10.1182/blood.2010.02.270108 [epub on April 19, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardanani A, Lasho TL, Finke C. LNK mutations studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2, and MPL mutations. Leukemia. 2010;24:1713–1718. doi: 10.1038/leu.2010.163. [DOI] [PubMed] [Google Scholar]
- 26.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.