Abstract
Purpose
New treatments are needed for patients with fludarabine- and alemtuzumab-refractory (FA-ref) chronic lymphocytic leukemia (CLL) or patients with fludarabine-refractory CLL with bulky (> 5 cm) lymphadenopathy (BF-ref) who are less suitable for alemtuzumab treatment; these groups have poor outcomes with available salvage regimens. Ofatumumab (HuMax-CD20) is a human monoclonal antibody targeting a distinct small-loop epitope on the CD20 molecule. We conducted an international clinical study to evaluate the efficacy and safety of ofatumumab in patients with FA-ref and BF-ref CLL.
Patients and Methods
Patients received eight weekly infusions of ofatumumab followed by four monthly infusions during a 24-week period (dose 1 = 300 mg; doses 2 to 12 = 2,000 mg); response by an independent review committee (1996 National Cancer Institute Working Group criteria) was assessed every 4 weeks until week 24 and then every 3 months until month 24.
Results
This planned interim analysis included 138 treated patients with FA-ref (n = 59) and BF-ref (n = 79) CLL. The overall response rates (primary end point) were 58% and 47% in the FA-ref and BF-ref groups, respectively. Complete resolution of constitutional symptoms and improved performance status occurred in 57% and 48% of patients, respectively. Median progression-free survival and overall survival times were 5.7 and 13.7 months in the FA-ref group, respectively, and 5.9 and 15.4 months in the BF-ref group, respectively. The most common adverse events during treatment were infusion reactions and infections, which were primarily grade 1 or 2 events. Hematologic events during treatment included anemia and neutropenia.
Conclusion
Ofatumumab is an active, well-tolerated treatment providing clear clinical improvements for fludarabine-refractory patients with very poor-prognosis CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is characterized by progressive accumulation of mature B cells in the blood, lymph nodes, spleen, liver, and bone marrow and remains incurable with standard therapies. Fludarabine is a cornerstone of treatment and is most effective in combination regimens.1–5 Patients who become refractory to fludarabine-based regimens have low response rates to salvage therapy and poor survival outcomes.6,7 The CD52 monoclonal antibody (mAb) alemtuzumab is indicated as a single-agent therapy in CLL, producing a 33% response rate in fludarabine-refractory patients.8 However, low response rates are generally seen with alemtuzumab monotherapy in relapsed/refractory patients with bulky (> 5 cm) lymph node involvement.8–13 Patients with fludarabine-refractory CLL also refractory to alemtuzumab (FA-ref) or less suitable for alemtuzumab as a result of bulky lymphadenopathy (BF-ref) have a poor prognosis.6,7 Therefore, new effective and well-tolerated treatments are needed for these patients.
The CD20 mAb rituximab, combined with fludarabine and cyclophosphamide, has substantially improved outcomes for patients with CLL.2,5,14,15 However, single-agent, standard-dose rituximab has limited activity in relapsed/refractory CLL.16,17 Higher response rates were seen with dose-intense rituximab (up to 2,250 mg/m2), but refractoriness to fludarabine was associated with a low response rate (20% in fludarabine-refractory patients v 56% in fludarabine-sensitive patients; P = .02).18
Ofatumumab (HuMax-CD20) is a human mAb that binds a distinct epitope composed of both small and large loops on the CD20 molecule.19 Ofatumumab induces killing of a panel of tumor B-cell lines and primary tumor cells via activation of complement- and antibody-dependent, cell-mediated cytotoxicity in vitro.20,21 Ofatumumab demonstrates increased binding of C1q and more potent complement-dependent cytotoxicity than rituximab, even in cells with low CD20 expression levels, including freshly isolated CLL cells and complement-resistant B-cell lines. The potent complement-dependent cytotoxicity with ofatumumab may be a result of the close proximity of the small-loop binding site to the cell surface, potentially leading to more effective deposition of complement on the cell surface.19–22 In a phase I/II study, patients with relapsed or refractory CLL were treated with four weekly doses of single-agent ofatumumab (dose 1 = 500 mg; doses 2 to 4 = 2,000 mg). The overall response rate (ORR) was 50%, median duration of response was 3.7 months, median time to next CLL therapy was 12 months, and treatment was well tolerated.23,24
We conducted an international, multicenter study of ofatumumab in patients with FA-ref and BF-ref CLL. Here we report a planned interim analysis demonstrating efficacy, clinical improvement, and safety of single-agent ofatumumab.
PATIENTS AND METHODS
Patients
Patients (age ≥ 18 years) with active CLL (1996 National Cancer Institute Working Group [NCI-WG] criteria)25 indicated for treatment, tumor immunophenotype of CD5+/20+/23+, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and life expectancy of ≥ 6 months were eligible for enrollment. There were no restrictions based on blood counts or transfusion requirements. Patients were required to be refractory to at least one fludarabine-containing regimen and either refractory to at least one alemtuzumab-containing regimen (FA-ref) or considered less suitable for alemtuzumab as a result of bulky (> 5 cm) lymphadenopathy (BF-ref). Bulky lymphadenopathy was confirmed either by physical examination or computed tomography scan at screening. Refractoriness to fludarabine (at least two cycles) and alemtuzumab (at least 12 doses) was defined as failure to achieve at least partial response (PR) by 1996 NCI-WG criteria or disease progression during treatment or within 6 months of the last dose of each agent.
Exclusion criteria included CLL therapy within 4 weeks or autologous stem-cell transplantation within 6 months of study initiation, allogeneic stem-cell transplantation, Richter's transformation or CNS involvement, active infectious disease requiring systemic treatment, clinically significant cardiac disease, or positive hepatitis B serology. All patients provided signed informed consent at enrollment. Protocol, amendments, consent forms, and patient information were approved by health authorities and local independent ethics committees or institutional review boards. The study was conducted in accordance with the Guidelines for Good Clinical Practice and the ethical principles of the Declaration of Helsinki. This study is registered at ClinicalTrials.gov (NCT00349349).
Study Design and Treatment
This is an international, single-arm study. Patients received eight weekly intravenous infusions of ofatumumab, followed by four monthly infusions (dose 1 = 300 mg; doses 2 to 12 = 2,000 mg). Patients received acetaminophen 1,000 mg and cetirizine 10 mg (or equivalent) before infusions. Patients also received glucocorticoid (prednisolone 100 mg or equivalent) before infusions 1, 2, and 9; if initial infusions were well tolerated, the glucocorticoid dose could be reduced to less than 100 mg for other infusions. Anti-infective prophylaxis was not mandated.
Baseline assessments included physical examination, hematology, biochemistry, evaluation of constitutional symptoms, ECOG performance status, prior treatments, and prognostic factors. Disease status and response were assessed (physical examination and blood counts) every 4 weeks until week 28 and every 3 months thereafter until month 24. After month 24, patients were monitored at 3-month intervals for survival and B-cell counts. Monitoring continued until the B-cell counts reached baseline level or above, alternative CLL therapy was initiated, or month 48 was reached.
Efficacy
The primary end point was ORR based on objective response (including complete response, nodular PR, and PR, defined by the 1996 NCI-WG criteria25) during the 24-week period from the start of treatment. Responses were assessed by an independent review committee (IRC). In accordance with the 1996 NCI-WG criteria, responses must have been maintained for ≥ 2 months, and computed tomography scans were not included for response assessment. Secondary end points included duration of response (time from the initial response to progression or death) and the following events calculated from time of first ofatumumab infusion: time to response, progression-free survival (PFS), and overall survival (OS).
Safety Evaluations
Severity of adverse events (AEs) was graded by investigators according to the NCI Common Terminology Criteria for Adverse Events (version 3.0). Serious AEs were monitored from the time informed consent was given until month 48 or until alternative CLL therapy was initiated. Major infections were defined as those requiring hospitalization for at least 48 hours and occurring during or within 4 weeks of completing treatment. Early deaths were defined as those occurring within 8 weeks from the start of treatment.
Blood samples were drawn at screening and at all visits during the study period (screening to month 24) for blood chemistry and hematology and at screening, week 12, and months 9, 12, 18, and 24 for evaluation of human antihuman antibodies (HAHAs). Blood chemistry and hematology samples were analyzed at central laboratories (Bio-Analytical Research Corporation, Lake Success, NY) in the United States and in Europe, and HAHA was analyzed at Charles River Laboratories (Margate, United Kingdom).
Statistical Analysis
This planned interim analysis was triggered when the primary end point data became available for 66 patients in the FA-ref group. Assuming a 30% ORR, data from 66 patients (per patient group) provide a two-sided exact 99% CI to exclude a 15% ORR (at a significance level of 1%) with 63% power. For the final primary end point analysis, 100 patients correspond to an increase of the statistical power to 92%. In the interim analysis, a superiority analysis (the 99% CI for ORR excludes 15%) and a futility analysis (the conditional power under the alternative hypothesis < 10%) were performed for both patient groups. The independent data monitoring committee (including an independent statistician) notified the sponsor that the criteria for futility or superiority had been met. Evaluation of all end points was based on the full analysis set, including all patients exposed to ofatumumab.
Duration of response, PFS, and OS were evaluated using Kaplan-Meier estimates. An exploratory analysis was conducted to evaluate the association between response and OS using a landmark analysis26 at week 12. AEs and clinical safety data were summarized using descriptive statistics.
RESULTS
Patient Characteristics
Enrollment began on June 13, 2006; patients were enrolled from 41 centers in 10 countries. We report results from a planned interim analysis of data collected through May 19, 2008 (two thirds of planned enrollment). As a result of protocol amendments in the eligibility criteria that defined refractoriness to fludarabine and alemtuzumab therapies, 16 of 154 treated patients with primary end point data did not qualify for either the FA-ref or BF-ref groups based on IRC assessment; data from those patients were analyzed separately. Thus, the current report includes 59 patients with FA-ref CLL and 79 patients with BF-ref CLL (Table 1). Of these patients, 91% received eight or more ofatumumab infusions, and 54% received all 12 infusions.
Table 1.
Baseline Characteristics of Patients With Refractory CLL Treated With Ofatumumab
| Characteristic | FA-ref (n = 59) |
BF-ref (n = 79) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 64 | 62 | ||
| Range | 41-86 | 43-84 | ||
| Sex | ||||
| Male | 44 | 75 | 57 | 72 |
| Female | 15 | 25 | 22 | 28 |
| No. of prior treatments | ||||
| Median | 5 | 4 | ||
| Range | 1-14 | 1-16 | ||
| Duration of CLL, years | ||||
| Median | 6 | 6 | ||
| Range | 1-18.6 | 0.7-18.0 | ||
| Largest lymph node > 5 cm based on clinical evaluation | 11 | 19 | 36 | 46 |
| Largest lymph node > 5 cm by palpation or CT scan | 55 | 93 | 79 | 100 |
| Rai stage at screening | ||||
| 0 | 1 | 2 | 0 | 0 |
| I or II | 26 | 44 | 24 | 30 |
| III or IV | 32 | 54 | 55 | 70 |
| Binet stage at screening | ||||
| A | 6 | 10 | 4 | 5 |
| B | 23 | 39 | 24 | 30 |
| C | 30 | 51 | 51 | 65 |
| ECOG PS | ||||
| 0 | 27 | 46 | 25 | 32 |
| 1-2 | 31* | 53 | 54 | 68 |
| Prior alkylating therapy | 55 | 93 | 73 | 92 |
| Prior rituximab-containing regimen | 35 | 59 | 43 | 54 |
Abbreviations: CLL, chronic lymphocytic leukemia; FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
One patient in the FA-ref group had ECOG PS of 3 at baseline as a result of elbow surgery unrelated to CLL and was allowed to enroll onto the study.
Efficacy
The ORR was 58% (99% CI, 40% to 74%) for the FA-ref group and 47% (99% CI, 32% to 62%) for the BF-ref group, surpassing the 15% criterion for superiority (P < .001 for both groups) and allowing for continued accrual. One complete response was observed in the BF-ref group, and all other responses were PRs. Stable disease was noted in 31% of patients with FA-ref CLL and 41% of patients with BF-ref CLL. Responses by baseline characteristics are listed in Table 2. The ORRs among patients previously treated with a rituximab-containing regimen were 54% and 44% in the FA-ref and BF-ref groups, respectively. The ORRs among patients refractory to fludarabine combined with cyclophosphamide and rituximab were 50% and 44% in the FA-ref and BF-ref groups, respectively. Among all characteristics evaluated, 17p deletion in the BF-ref group was the only factor associated with lower response rate (Table 2).
Table 2.
Response Rates According to Baseline Characteristics for Patients With Refractory CLL Treated With Ofatumumab
| Baseline Characteristic | FA-ref |
BF-ref |
||||
|---|---|---|---|---|---|---|
| No. of Patients | ORR (%) | P* | No. of Patients | ORR (%) | P* | |
| Age, years | ||||||
| < 65 | 32 | 63 | .4400 | 46 | 48 | 1.00 |
| ≥ 65 | 27 | 52 | 33 | 45 | ||
| < 70 | 49 | 57 | 1.00 | 60 | 48 | .7929 |
| ≥ 70 | 10 | 60 | 19 | 42 | ||
| Prior rituximab | ||||||
| Yes | 35 | 54 | .5984 | 43 | 44 | .6553 |
| No | 24 | 63 | 36 | 50 | ||
| Prior FCR | ||||||
| Refractory to FCR† | 16 | 50 | .5585 | 16 | 44 | 1.00 |
| Other† | 43 | 60 | 63 | 48 | ||
| Prior FC | ||||||
| Refractory to FC‡ | 33 | 64 | .4264 | 46 | 50 | .6480 |
| Other‡ | 26 | 50 | 33 | 42 | ||
| Palpable lymph node size, cm | ||||||
| ≤ 5 | 43 | 56 | 1.00 | 39 | 54 | .1647 |
| > 5 | 11 | 55 | 36 | 36 | ||
| Disease stage | ||||||
| Rai stage I or II | 26 | 58 | 1.00 | 24 | 54 | .4654 |
| Rai stage III or IV | 32 | 56 | 55 | 44 | ||
| ECOG PS | ||||||
| 0-1 | 46 | 59 | 1.00 | 66 | 50 | .2384 |
| 2 | 12 | 58 | 13 | 31 | ||
| FISH cytogenetic abnormalities§ | ||||||
| 17p del | 17 | 41 | .1429 | 14 | 14 | .0073 |
| No 17p del | 40 | 65 | 62 | 55 | ||
| 11q del | 24 | 63 | .5963 | 22 | 64 | .0838 |
| No 11q del | 33 | 55 | 56 | 41 | ||
| 12q trisomy | 3 | 33 | .5669 | 8 | 38 | .7147 |
| No 12q trisomy | 54 | 59 | 70 | 49 | ||
Abbreviations: CLL, chronic lymphocytic leukemia; FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory; ORR, overall response rate; FCR, fludarabine plus cyclophosphamide and rituximab; FC, fludarabine plus cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; PS, performance status; FISH, fluorescent in situ hybridization.
Two-sided Fisher's exact test.
Patients considered refractory to FCR, with or without other drugs; other represents patients refractory to a fludarabine-based regimen other than that containing FCR.
Patients considered refractory to FC, with or without other drugs; other represents patients refractory to a fludarabine-based regimen other than that containing FC.
Categories adapted from Döhner hierarchical classification of FISH cytogenetic abnormalities.27
Measures of clinical improvement, based on components of the NCI-WG response criteria, are listed in Table 3. For patients who had baseline thrombocytopenia or anemia, improvements to normal values occurred by week 8 of treatment in approximately 50% of the patients (Appendix Figs A1A and A1B, online only). Furthermore, 45% of patients with decreased ECOG performance status at baseline (worse than 0) experienced an improvement during the treatment period.
Table 3.
Summary of Clinical Improvement for a Minimum Duration of 2 Months in Patients With Refractory CLL Treated With Ofatumumab
| Improvement in Clinical Parameters | FA-ref |
BF-ref |
||||
|---|---|---|---|---|---|---|
| No. of Patients With Abnormal Clinical Parameters at Baseline | Patients With Improvement From Baseline to Week 24 |
No. of Patients With Abnormal Clinical Parameters at Baseline | Patients With Improvement From Baseline to Week 24 |
|||
| No. | % | No. | % | |||
| Complete resolution of constitutional symptoms | 31 | 15 | 48 | 46 | 29 | 63 |
| Complete resolution of lymphadenopathy (nodes < 1 cm) | 55 | 9 | 16 | 74 | 8 | 11 |
| Complete resolution of splenomegaly | 30 | 14 | 47 | 46 | 16 | 35 |
| Complete resolution of hepatomegaly | 18 | 9 | 50 | 21 | 11 | 52 |
| Normalization of neutrophil count (from < 1.5 × 109/L to ≥ 1.5 × 109/L) | 19 | 1 | 5 | 17 | 5 | 29 |
| Improvement in hemoglobin level (from ≤ 11.0 g/dL to > 11.0 g/dL) | 26 | 8 | 31 | 42 | 11 | 26 |
| Improvement in platelet count (from ≤ 100 × 109/L to > 50% increase or > 100 × 109/L) | 29 | 12 | 41 | 44 | 17 | 39 |
Abbreviations: CLL, chronic lymphocytic leukemia; FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory.
In responding patients, median time to response was 1.8 months for both the FA-ref and BF-ref groups. Approximately 80% of responses were observed within 2 months of initiating treatment. One patient in the BF-ref group had a delayed PR 9 months after initiating treatment. The median duration of response was 7.1 months (95% CI, 3.7 to 7.6 months) in the FA-ref group and 5.6 months (95% CI, 3.6 to 7.0 months) in the BF-ref group.
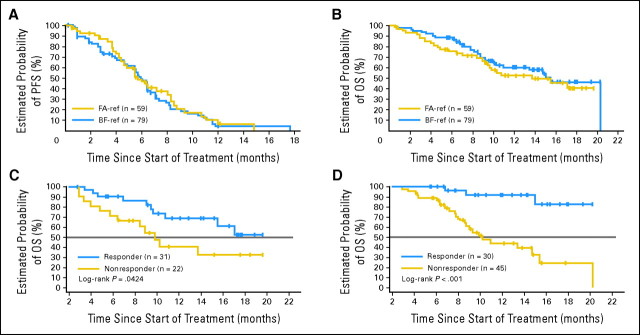
The median PFS time was 5.7 months (95% CI, 4.5 to 8.0 months) in the FA-ref group and 5.9 months (95% CI, 4.9 to 6.4 months) in the BF-ref group (Fig 1A). Median OS time was 13.7 months (95% CI, 9.4 months to not yet reached) in the FA-ref group and 15.4 months (95% CI, 10.2 to 20.2 months) in the BF-ref group (Fig 1B). On the basis of the landmark analysis at week 12, median OS time was significantly longer (by ≥ 10 months) among responding patients compared with nonresponders; the median OS time had not yet been reached for responders in both the FA-ref and BF-ref groups; whereas for nonresponders, the OS time was 9.8 months (P = .0424; Fig 1C) in the FA-ref group and 10.2 months (P < .0001; Fig 1D) in the BF-ref group.
Fig 1.
(A) Progression-free survival (PFS) by patient group. PFS was defined as time from baseline (week 0) to progression (assessed by independent end point review committee) or death. (B) Overall survival (OS) by patient group. OS was defined as time from baseline (week 0) to death. (C) OS according to response status in the fludarabine- and alemtuzumab-refractory (FA-ref) group. (D) OS according to response status in the bulky fludarabine-refractory (BF-ref) group. OS in panels C and D based on landmark analysis at week 12, which includes patients who were alive at the week 12 time point; P value derived from two-sided log-rank test.
Safety
Overall, infusion-related reactions were seen in 64% of patients in the FA-ref group and 61% of patients in the BF-ref group, nearly all of which were grade 1 or 2 (Fig 2). These reactions predominantly occurred during the first and second infusions and subsided during the course of treatment, decreasing from 38% of patients at the first infusion to 7% at the 12th infusion. The most common AEs (≥ 10% of patients) occurring during treatment (between the first ofatumumab infusion and up to 30 days after the last infusion) were infections (67%), cough (18%), diarrhea (16%), anemia (16%), fatigue (15%), fever (15%), neutropenia (15%), dyspnea (13%), nausea (11%), and rash (10%). Among these AEs, those judged by investigators to be related to ofatumumab treatment are listed in Table 4. One patient with FA-ref CLL had grade 4 thrombocytopenia during treatment. In the BF-ref group, during treatment, one patient each experienced grade 3 febrile neutropenia, thrombocytopenia, and hemolytic anemia, and one patient experienced grade 4 hemolytic anemia. No HAHAs were detected in any of the evaluable patients.
Fig 2.
Infusion-related reactions by infusion number.
Table 4.
Related Adverse Events With Ofatumumab Therapy
| Adverse Events* | FA-ref (n = 59) |
BF-ref (n = 79) |
||||||
|---|---|---|---|---|---|---|---|---|
| All Grades |
Grade 3 or 4 |
All Grades |
Grade 3 or 4 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Infection | 12 | 20 | 7 | 12 | 17 | 22 | 6 | 8 |
| Neutropenia | 11 | 19 | 8 | 14 | 6 | 8 | 5 | 6 |
| Fatigue | 3 | 5 | 0 | 0 | 7 | 9 | 0 | 0 |
| Cough | 5 | 8 | 0 | 0 | 4 | 5 | 0 | 0 |
| Anemia | 2 | 3 | 0 | 0 | 7 | 9 | 4 | 5 |
| Diarrhea | 5 | 8 | 0 | 0 | 3 | 4 | 0 | 0 |
| Dyspnea | 5 | 8 | 1 | 2 | 3 | 4 | 0 | 0 |
| Nausea | 3 | 5 | 0 | 0 | 5 | 6 | 0 | 0 |
| Rash | 5 | 8 | 0 | 0 | 2 | 3 | 0 | 0 |
| Fever | 5 | 8 | 1 | 2 | 1 | 1 | 0 | 0 |
Abbreviations: FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory.
Adverse events judged by investigators to be related to ofatumumab among the most common adverse events that occurred in ≥ 10% of patients from the first infusion of ofatumumab to within 30 days of the last infusion.
During treatment, 189 infectious events were reported among 92 patients; 139 of these events (74%) were grade 1 or 2 in severity. Among 37 grade 3 or 4 infections, pneumonia (14 events) and other respiratory tract infections (six events) were the most common. Thirteen infections with onset during treatment led to death, including sepsis (n = 6), pneumonia (n = 5), Fusarium infection (n = 1), and progressive multifocal leukoencephalopathy (PML; n = 1). Among these grade 5 infections, eight infections (FA-ref, n = 5; BF-ref, n = 3) led to death within 30 days of the last ofatumumab infusion (Table 5). Other causes of death within 30 days of last infusion are listed in Table 5.
Table 5.
Summary of Death With Ofatumumab Therapy
| Death* | FA-ref (n = 59) |
BF-ref (n = 79) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Total deaths | 6 | 10 | 5 | 6 |
| Cause of death | ||||
| Sepsis/septic shock | 3 | 5 | 1 | 1 |
| Pneumonia | 2 | 3 | 2 | 3 |
| CLL Richter's transformation | 1 | 2 | 0 | 0 |
| Myocardial infarction | 0 | 0 | 1 | 1 |
| Disease progression | 0 | 0 | 1 | 1 |
Abbreviations: FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory; CLL, chronic lymphocytic leukemia.
Occurring within 30 days of the last ofatumumab infusion.
Early deaths (≤ week 8) occurred in four patients (7%) in the FA-ref group and two patients (3%) in the BF-ref group. Four of these deaths are listed in Table 5, including one death each as a result of pneumonia and sepsis in the FA-ref group and one death each as a result of sepsis and myocardial infarction in the BF-ref group. Two other early deaths were a result of Fusarium infection (mentioned earlier) and bronchopneumonia in the FA-ref group.
DISCUSSION
Outcomes for patients with FA-ref or BF-ref CLL are poor with available salvage regimens, including intensive chemoimmunotherapy, with low response rates (23% ORR), short time to treatment failure (median, 2 to 3 months), and short survival (median, 9 months)7; new treatment options are needed for these patients. Comparisons with available historical data are limited; however, an ORR of 47% to 58% and a median PFS time of approximately 6 months with ofatumumab, as assessed by an IRC, clearly demonstrate clinical activity and are significant given outcomes reported with current salvage therapies.7 Furthermore, the activity with single-agent ofatumumab is remarkable, given the ORR of 0% in 14 patients with FA-ref or BF-ref CLL treated with other types of mAb therapy in the retrospective report.7 The median OS time with ofatumumab treatment was 14 to 15 months, and a significant survival benefit was observed in responding patients in both patient groups. The landmark method was used to minimize the survival bias in responders, which would otherwise occur when using a direct comparison of survival among all responders versus nonresponders.
In this study, treatment with ofatumumab was associated with considerable relief of disease-related constitutional symptoms and improvements in performance status, even among patients who did not qualify as responders strictly based on NCI-WG criteria. Complete resolution of splenomegaly and hepatomegaly and/or substantial reduction in lymphadenopathy were observed in a large proportion of patients (Table 3), and patients with thrombocytopenia or anemia at baseline experienced improvements in hematologic parameters.
Response to ofatumumab was consistent across various subgroups based on pretreatment characteristics, except for 17p deletion, which was associated with lower ORR in the BF-ref group. This study was not powered to identify subgroup differences; however, it is encouraging to appreciate responses in patients who may be considered higher risk, such as those with advanced disease stage, age ≥ 70 years, 11q deletion, poor performance status, or large palpable lymph nodes (> 5 cm). The dose of corticosteroid premedication used in this study has not been reported to have efficacy in refractory patients with CLL and was not likely to significantly affect the ORR.
With median response duration of 6 to 7 months, some patients experienced relapse soon after completing treatment. One possible explanation for this is the proliferative nature of disease in these refractory patients. Because all but one responder achieved PR, responders had residual disease that progressed after completion of ofatumumab treatment. The median number of malignant B cells in peripheral blood decreased rapidly with ofatumumab and remained depleted during the course of treatment (Appendix Fig A2, online only). The gradual disappearance of the tumor bulk during continued therapy was followed by a gradual return of the malignant clone after discontinuation of treatment (data not shown). Thus, loss of response did not seem to be a result of resistance to ofatumumab during active treatment; detailed pharmacokinetic and pharmacodynamic analyses may provide further insights.
Ofatumumab was well tolerated, there were no unexpected toxicities, and no formation of HAHAs was detected. The most common AEs were infusion reactions and infections, which were primarily grade 1 or 2 events; infusion reactions were common during the first two doses, as expected with this type of therapy, but largely subsided with subsequent infusions. The incidence of grade 3 or 4 infections was at an expected level, considering prior treatment, extent of disease, and immunosuppression among these patients.28 One case of PML (resulting in death 63 days after last dose of ofatumumab) occurred in a patient with FA-ref disease who had received eight prior treatments and had a low CD4 count at baseline (data not shown). PML has been reported in patients with B-cell malignancies treated with rituximab-containing regimens.29–31 The incidence of major infections (as previously defined by Tam et al7) in our patients with FA-ref and BF-ref CLL (32% and 23%, respectively; data not shown) compared favorably with that of similar patients treated with various other salvage regimens (60% and 45%, respectively)7; the incidence of early death in our patients with FA-ref and BF-ref CLL (7% and 3%, respectively) was also lower than that reported in the historical data (16% and 10%, respectively).7 Although median neutrophil counts in our patient population decreased during the first 4 to 8 weeks of treatment, they remained greater than 1.5 × 109/L and were stable during the course of treatment (Appendix Fig A3A, online only). Median platelet and hemoglobin values rapidly improved during the study for patients who stayed on the study (Appendix Figs A3B and A3C, online only), including patients with baseline thrombocytopenia and anemia, as a result of continued treatment and responses. These outcomes in hematologic parameters are notable considering the extent of disease in this patient population and the lack of blood count limits for trial enrollment.
Ofatumumab demonstrates significant activity and a favorable safety profile, providing meaningful clinical improvements in poor-risk patients with heavily pretreated FA-ref and BF-ref CLL. Results are especially encouraging for a single-agent mAb used in such heavily pretreated patients as in this salvage setting. Importantly, similar response rates were seen irrespective of prior exposure to rituximab-containing treatments and irrespective of refractoriness to fludarabine combined with cyclophosphamide and rituximab, a standard regimen in earlier lines of CLL therapy. Phase III trials are needed to confirm therapeutic efficacy in patients with CLL. Further investigation of ofatumumab is warranted in earlier disease settings.
Supplementary Material
Acknowledgment
We thank the patients and the following investigators in the Hx-CD20-406 Study Group for their participation in the study: Czech Republic – D. Belada (Hradec Kralova); Denmark – O. Gadeberg (Vejle), C. Geisler (Copenhagen); France – J. P. Casuuto (Nice), B. Coiffier (Lyon), P. Feugier (Vandoeuvre les Nancy), J. F. Rossi (Montpellier); Germany – C. Beck (Monchengladbach-Rheydt), G. Hess (Mainz), M. Kneba (Kiel), M. Pfreundschuh (Homburg/Saar), J. Schetelig (Dresden), N. Schmitz (Hamburg); Italy – R. Foa (Rome), M. Montillo (Milan); Poland – J. Holowiecki (Katowice), J. Kloczko (Bialystok); Spain – J. Delgado (Barcelona); Sweden – A. Aleskog (Uppsala); United Kingdom – G. A. Follows (Cambridge), J. G. Gribben (London); United States – K. Foon (Pittsburgh, PA), K. Hymes (New York, NY), B. Link (Iowa City, IA), D. Mulford (Rochester, NY), and K. Rai (New Hyde Park, NY).
We also thank the Independent Endpoint Review Committee: T. Lin, Columbus, OH; M. Keating, Houston, TX; N. Kay, Rochester, MN; A. M. Dalseg, Herlev, Denmark; and L. M. Pedersen, Odense, Denmark. The authors would also like to thank Michael Arning, MD, PhD (GlaxoSmithKline, Collegeville, PA), and Karin Havenith (Genmab, Utrecht, the Netherlands) for their contributions to the analysis and interpretation of study data and critical review of this article. Editorial support for this publication was provided by Andrew Owen, MSc, Medicus International.
Supported by Genmab (Copenhagen, Denmark) and GlaxoSmithKline (Collegeville, PA). Presented in part at the 50th Annual Meeting of the American Society of Hematology, December 6-9, 2008, San Francisco, CA; the 13th Annual International Congress on Hematologic Malignancies: Focus on Leukemias, Lymphomas, and Myelomas, February 11-15, 2009, Whistler, British Columbia, Canada; the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; and the 14th Congress of the European Haematology Association, June 4-7, 2009, Berlin, Germany.
Appendix
Fig A1.

(A) Median hemoglobin levels over time in patients with baseline anemia. (B) Median platelet count over time in patients with baseline thrombocytopenia. FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory.
Fig A2.
Median CD45+CD5+CD19+ cells in peripheral blood over time. FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory.
Fig A3.

(A) Median neutrophil count over time in all patients. (B) Median hemoglobin levels over time in all patients. (C) Median platelet count over time in all patients. FA-ref, fludarabine and alemtuzumab refractory; BF-ref, bulky fludarabine refractory.
Footnotes
Written on behalf of the Hx-CD20-406 Study Investigators.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: CT# NCT00349349.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Geoffrey Chan, GlaxoSmithKline (C); Randy Davis, GlaxoSmithKline (C); Nedjad Losic, Genmab (C); Joris Wilms, Genmab (C); Charlotte A. Russell, Genmab (C) Consultant or Advisory Role: William G. Wierda, GlaxoSmithKline (C), Genentech (C), Celgene (C); Thomas J. Kipps, GlaxoSmithKline (C), Genmab (C); Jiří Mayer, Fresenius (C), GlaxoSmithKline (C), Roche (C); Stephan Stilgenbauer, GlaxoSmithKline (C), Genmab (C); Tadeusz Robak, GlaxoSmithKline (C); Richard R. Furman, GlaxoSmithKline (C); Peter Hillmen, GlaxoSmithKline (C); Marek Trneny, GlaxoSmithKline (C); Swami Padmanabhan, GlaxoSmithKline (C) Stock Ownership: Geoffrey Chan, GlaxoSmithKline; Randy Davis, GlaxoSmithKline; Nedjad Losic, Genmab; Joris Wilms, Genmab; Charlotte A. Russell, Genmab Honoraria: Stephan Stilgenbauer, GlaxoSmithKline, Genmab; Tadeusz Robak, GlaxoSmithKline; Richard R. Furman, GlaxoSmithKline; Swami Padmanabhan, GlaxoSmithKline; Anders Österborg, GlaxoSmithKline Research Funding: William G. Wierda, GlaxoSmithKline, Genmab, Abbott; Thomas J. Kipps, Abbott, GlaxoSmithKline, Genmab, Genentech, sanofi-aventis, Celgene, Cephalon, Memgen; Jiří Mayer, BMS, Roche; Stephan Stilgenbauer, GlaxoSmithKline, Genmab; Tadeusz Robak, GlaxoSmithKline; Anders Österborg, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: William G. Wierda, Thomas J. Kipps, Geoffrey Chan, Nedjad Losic, Joris Wilms, Anders Österborg
Administrative support: Geoffrey Chan
Provision of study materials or patients: William G. Wierda, Thomas J. Kipps, Jiří Mayer, Stephan Stilgenbauer, Cathy D. Williams, Andrzej Hellmann, Tadeusz Robak, Richard R. Furman, Peter Hillmen, Marek Trneny, Martin J.S. Dyer, Swami Padmanabhan, Magdalena Piotrowska, Tomas Kozak, Anders Österborg
Collection and assembly of data: William G. Wierda, Thomas J. Kipps, Tadeusz Robak, Martin J.S. Dyer, Swami Padmanabhan, Tomas Kozak, Anders Österborg
Data analysis and interpretation: William G. Wierda, Thomas J. Kipps, Stephan Stilgenbauer, Swami Padmanabhan, Geoffrey Chan, Randy Davis, Nedjad Losic, Joris Wilms, Charlotte A. Russell, Anders Österborg
Manuscript writing: William G. Wierda, Thomas J. Kipps, Stephan Stilgenbauer, Richard R. Furman, Swami Padmanabhan, Geoffrey Chan, Nedjad Losic, Joris Wilms, Charlotte A. Russell, Anders Österborg
Final approval of manuscript: William G. Wierda, Thomas J. Kipps, Jiří Mayer, Stephan Stilgenbauer, Cathy D. Williams, Andrzej Hellmann, Tadeusz Robak, Richard R. Furman, Peter Hillmen, Marek Trneny, Martin J.S. Dyer, Swami Padmanabhan, Magdalena Piotrowska, Tomas Kozak, Geoffrey Chan, Randy Davis, Nedjad Losic, Joris Wilms, Charlotte A. Russell, Anders Österborg
REFERENCES
- 1.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 3.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 4.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 5.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 6.Keating MJ, O'Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- 7.Tam CS, O'Brien S, Lerner S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma. 2007;48:1931–1939. doi: 10.1080/10428190701573257. [DOI] [PubMed] [Google Scholar]
- 8.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 9.Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Rai KR, Freter CE, Mercier RJ, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20:3891–3897. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 11.Fiegl M, Falkner A, Hopfinger G, et al. Routine clinical use of alemtuzumab in patients with heavily pretreated B-cell chronic lymphocytic leukemia: A nation-wide retrospective study in Austria. Cancer. 2006;107:2408–2416. doi: 10.1002/cncr.22263. [DOI] [PubMed] [Google Scholar]
- 12.Lundin J, Osterborg A, Brittinger G, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: A phase II multicenter study—European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin's Lymphoma. J Clin Oncol. 1998;16:3257–3263. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 13.Osterborg A, Dyer MJ, Bunjes D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia: European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15:1567–1574. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 14.Robak T, Moiseev S, Dmoszynska A, et al. Rituximab, fludarabine, and cyclophosphamide (R-FC) prolongs progression free survival in relapsed or refractory chronic lymphocytic leukemia (CLL) compared with FC alone: Final results from the international randomized phase III REACH trial. Blood. 2008;112:LBA-1. abstr. [Google Scholar]
- 15.Hallek M, Fingerle-Rowson G, Fink A, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL) Blood. 2008;112:325. abstr. [Google Scholar]
- 16.Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–1331. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 17.Itälä M, Geisler CH, Kimby E, et al. Standard-dose anti-CD20 antibody rituximab has efficacy in chronic lymphocytic leukaemia: Results from a Nordic multicentre study. Eur J Haematol. 2002;69:129–134. doi: 10.1034/j.1600-0609.2002.02786.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 19.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 20.Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 21.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 22.Pawluczkowycz A, Beurskens F, Beum P, et al. Binding of submaximal C1q promotes complement dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): Considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 23.Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: A phase 1-2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 24.Coiffier B, Tilly H, Pedersen L, et al. Significant correlation between survival endpoints and exposure to ofatumumab (HuMax-CD20) in chronic lymphocytic leukemia. Blood. 2006;108:2842. abstr. [Google Scholar]
- 25.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 26.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 27.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 28.Perkins JG, Flynn JM, Howard RS, et al. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: Implications for clinical trials in this patient population. Cancer. 2002;94:2033–2039. [PubMed] [Google Scholar]
- 29.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: A report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopfinger G, Plessl A, Grisold W, et al. Progressive multifocal leukoencephalopathy after rituximab in a patient with relapsed follicular lymphoma and low IgG levels and a low CD4+ lymphocyte count. Leuk Lymphoma. 2008;49:2367–2369. doi: 10.1080/10428190802404048. [DOI] [PubMed] [Google Scholar]
- 31.Reddy N, Abel TW, Jagasia M, et al. Progressive multifocal leukoencephalopathy in a patient with follicular lymphoma treated with multiple courses of rituximab. Leuk Lymphoma. 2009;50:460–462. doi: 10.1080/10428190802695827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.