Introduction
Melanoma is the most aggressive of the cutaneous malignancies, projected to cause over 9,500 deaths in the United States in 2014.1 More effective immune-based treatments and molecularly targeted therapies have improved the overall outcome and have begun to play an expanding role in the treatment of patients with metastatic melanoma in the last few years.2–4 Ipilimumab (Yervoy; Bristol-Myers Squibb, New York, NY) is a fully humanized monoclonal antibody directed against the immune checkpoint cytotoxic T-lymphocyte antigen-4 (CTLA-4) that functionally removes a key modulator of the immune system. Tumor-specific cellular immunity is thereby enhanced by promotion of cytotoxic T cells and, possibly, reduction of intratumoral regulatory T cells.5 Ipilimumab improved overall survival for patients with melanoma compared with an experimental vaccine in previously treated patients and in combination with dacarbazine in the first-line setting.4,6 However, the immune activation caused by ipilimumab may also result in potentially severe autoimmune toxicity, most commonly involving the GI tract, liver, skin, and endocrine system.7 With increased clinical use, more rare adverse effects are emerging. To our knowledge, no cases of ipilimumab-induced myasthenia gravis have been reported in the medical literature to date. We describe two such cases below.
Case 1
A 69-year-old woman was initially diagnosed with a localized melanoma in 2011. She underwent wide local excision of a 3-mm deep, Clark level IV, nonulcerated melanoma (T3a) on her right lower extremity. Sentinel lymph node biopsy revealed a 0.25-mm deposit of melanoma in an ipsilateral inguinal lymph node (N1a, American Joint Committee on Cancer stage IIIA). She declined completion lymph node dissection or adjuvant therapy and was followed with close observation. In mid-2012, she developed several cutaneous melanoma nodules on her lower extremity; molecular testing did not reveal a BRAFV600 mutation. Positron emission tomography showed extensive hypermetabolic inguinal and popliteal adenopathy and subcutaneous nodules. The patient's history was significant for anxiety and hypothyroidism; medications included levothyroxine, aspirin, citalopram, and trazodone. She began receiving commercial ipilimumab at a dose of 3 mg/kg every 3 weeks for a maximum of four doses.
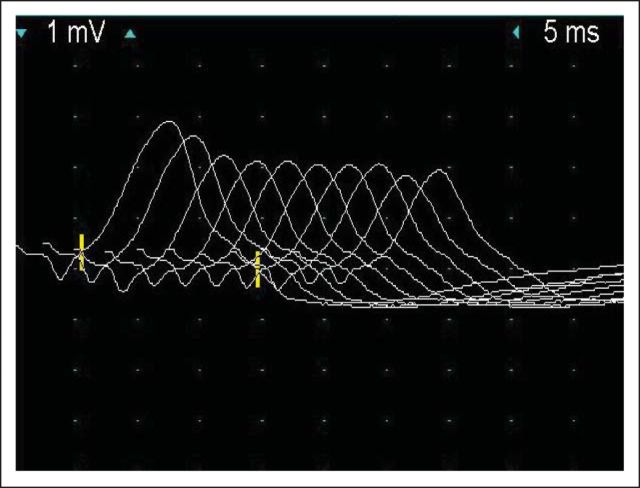
Shortly after her first dose, she developed a mild rash and pruritus with increased erythema at the site of her primary melanoma resection. After her second dose, she developed minimal blurring of vision and photosensitivity in her left eye. Physical examination at that time was unremarkable except for a mild macular rash; magnetic resonance imaging of the brain showed no intracranial metastatic disease, pituitary enlargement, or other abnormalities. Complete blood counts, electrolytes, and cortisol were normal; thyroid-stimulating hormone was elevated (7.5 μU/mL). Several days after her third dose of ipilimumab, she developed diplopia, ptosis, and dysphagia for solid foods. Her acetylcholine receptor (AChR) binding antibodies were 1.9 nmol/L (normal level, < 0.5 nmol/L). Additional examination demonstrated bilateral fatiguing ptosis, dysconjugate eye movements with decreased horizontal gaze, weakness of the orbicularis oculi and oris, and neck muscle weakness. Electromyography showed significant compound muscle action potential decrement at baseline on low-rate repetitive stimulation of the left spinal accessory-trapezius nerve-muscle pair, consistent with a postsynaptic neuromuscular junction disorder such as myasthenia gravis (Fig 1). Despite initiation of pyridostigmine 30 mg three times per day, she developed mild shortness of breath, worsening dysphagia, fatigable weakness, and inability to hold up her head. She was admitted to the hospital and received intravenous methylprednisolone 2 mg/kg and plasmapheresis and had gradual symptom improvement. Her corticosteroids were tapered after discharge and her symptoms continued to improve. Positron emission tomography scans performed 1 month and 3 months after her third dose of ipilimumab showed decreased size and [18F]fluorodeoxyglucose avidity in her lower extremity lymphadenopathy. Her strength has improved markedly; she complains only of mild fatigue and is receiving a current corticosteroid dose of prednisone 40 mg per day.
Fig 1.
Case 2
A 73-year-old woman developed a focal skin lesion in her right heel in 2008. After initial biopsy, she underwent a wide local excision and sentinel lymph node biopsy of the right groin. Pathology revealed an ulcerated, Clark level IV, 4.1-mm-deep acrolentiginous melanoma with a mitotic index of 8.5/mm2 (T4b). One of three sentinel lymph nodes was involved by melanoma; completion right inguinal lymph node dissection was performed, and all remaining lymph nodes were negative for metastases (N1a, American Joint Committee on Cancer stage IIIB). She was free of systemic metastases and subsequently declined adjuvant therapy. One year after surgery, she developed in-transit metastases and underwent a radical resection of two upper thigh nodules and isolated limb perfusion of the right extremity. Two months later, she underwent re-excision of recurrent in-transit metastases on her right lower limb after a second isolated limb perfusion procedure. In mid-2009, restaging scans demonstrated multiple new, hypermetabolic cutaneous and subcutaneous nodules in the right lower extremity, new iliac and inguinal lymphadenopathy, and hepatic metastasis. She was initiated on a phase I clinical trial with ipilimumab.
After two doses of ipilimumab 10 mg/kg, she developed shortness of breath and proximal limb muscle weakness. A pulmonary and cardiac work-up was performed and was notable for a Sniff test demonstrating decreased diaphragmatic excursion. Thyroid-stimulating hormone, free thyroxine, adrenocorticotrophic hormone, and cortisol levels were normal. Serum serologies were significant for elevated AChR binding antibodies of 13.6 nmol/L (normal level, < 0.5 nmol/L) and modulating antibodies of 26% (normal level, < 21%). On the basis of these findings, a diagnosis of myasthenia gravis secondary to ipilimumab was made. She began high-dose corticosteroids and pyridostigmine with gradual improvement in her muscle strength over the next 8 weeks. Ipilimumab was discontinued permanently. Restaging scans demonstrated initial regression of disease; however, it ultimately progressed despite multiple therapies including carboplatin and paclitaxel, local radiotherapy, and a phase II clinical trial. Because of significant progression of disease burden and functional decline, she was transitioned to hospice care and died.
Discussion
In this report, we describe two patients with myasthenia gravis complicating ipilimumab therapy. To our knowledge, these are the first patients with ipilimumab-associated myasthenia gravis to be reported in the medical literature. Other severe neurologic disorders following ipilimumab were observed previously, including acute inflammatory demyelination polyneuropathy and an ascending motor paralysis with common features of both myasthenia gravis and acute inflammatory demyelination polyneuropathy.7–9 More recently, an experimental immune checkpoint inhibitor blocking programmed cell death ligand 1 was reported to cause myasthenia gravis in a phase I trial.10
Myasthenia gravis is an autoimmune disorder affecting the neuromuscular junction and is characterized by fatigable weakness variably involving ocular, bulbar, respiratory, and limb muscles. Autoantibodies to the acetylcholine receptor (AChR) or muscle-specific tyrosine kinase are believed to be responsible for the clinical features. T lymphocytes also seem to play a role in the pathogenesis of myasthenia gravis by binding to the AChR and stimulating antibody production.11 AChR antibodies are present in approximately 85% of patients with generalized myasthenia and 50% of those with ocular myasthenia, and are highly specific for the diagnosis.12,13 Acetylcholine esterase inhibitors (pyridostigmine) are generally used as first-line therapy to potentiate the action of acetylcholine in the neuromuscular junction and to alleviate symptoms.14 Most patients require additional immunomodulatory therapy to suppress autoantibody production and T-cell activation, including plasmapheresis, intravenous immune globulin, and corticosteroids.15,16 Although symptoms may worsen transiently 4 to 10 days after initiation of corticosteroids in some patients, we ultimately elected to treat our patients with prednisone under close observation, given the corticosteroid-responsive nature of most ipilimumab-induced adverse events.14
Many medications, such as aminoglycosides and corticosteroids, can aggravate myasthenia, but only a few, such as D-penicillamine, interferon alfa, and perhaps the statins, can induce it.17–19 In our patients, ipilimumab possibly triggered the onset of myasthenia gravis by exuberant T-cell activation. Preclinical and observational studies have long concerned investigators that myasthenia gravis could develop during anti–CTLA-4 therapy. An anti–CTLA-4 antibody worsened myasthenia gravis in an experimental murine model.20 Furthermore, patients with myasthenia gravis have lower T-cell membrane expression of CTLA-4 compared with healthy controls.21 Particular CTLA-4 single nucleotide polymorphisms or alternative splicing lead to this decreased expression.22–24
Our patients had clinical presentations and confirmatory testing that were consistent with myasthenia gravis. The first patient developed predominantly ocular and bulbar symptoms, whereas the second patient presented with shortness of breath and limb muscle weakness. Other initial diagnostic considerations included hypophysitis, uveitis, and intracranial metastases in the first patient, and cardiopulmonary dysfunction or myopathy in the second patient. However, the development of typical physical examination findings, markedly positive AChR antibody laboratory testing, and electromyography results confirmed myasthenia gravis. Our patients experienced marked clinical improvement after initiation of pyridostigmine and corticosteroids and in one case required plasmapheresis.
Clinicians treating patients with ipilimumab should have a high index of concern for immune-related adverse events. Weakness, fatigue, dyspnea, and visual disturbances may herald a variety of endocrine, ophthalmologic, cardiopulmonary, or neurologic disorders. Diagnostic evaluation of these symptoms should be pursued in an expedited and comprehensive fashion. In the appropriate clinical setting, myasthenia gravis should be considered as a complication of ipilimumab therapy. Additionally, providers should screen for a history of myasthenia gravis or other neurologic problems before initiating ipilimumab.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ragini R. Kudchadkar, Bristol-Myers Squibb (C); Jeffrey A. Sosman, Genentech (C), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Ragini R. Kudchadkar, Bristol-Myers Squibb Research Funding: Jeffrey A. Sosman, Bristol-Myers Squibb, Novartis Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 7.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 8.Gaudy-Marqueste C, Monestier S, Franques J, et al. A severe case of ipilimumab-induced guillain-barré syndrome revealed by an occlusive enteric neuropathy: A differential diagnosis for ipilimumab-induced colitis. J Immunother. 2013;36:77–78. doi: 10.1097/CJI.0b013e31827807dd. [DOI] [PubMed] [Google Scholar]
- 9.Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barré syndrome in a melanoma patient. Ann Oncol. 2011;22:991–993. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi Q, Pirskanen R, Lefvert AK. Human muscle acetylcholine receptor reactive T and B lymphocytes in the peripheral blood of patients with myasthenia gravis. J Neuroimmunol. 1993;42:215–222. doi: 10.1016/0165-5728(93)90013-o. [DOI] [PubMed] [Google Scholar]
- 12.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Viegas S, Leite MI, et al. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalized myasthenia gravis. Arch Neurol. 2012;69:994–1001. doi: 10.1001/archneurol.2012.437. [DOI] [PubMed] [Google Scholar]
- 14.Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol. 2010;17:893–902. doi: 10.1111/j.1468-1331.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- 15.Gajdos P, Chevret S, Toyka K. Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev. 2002:CD002275. doi: 10.1002/14651858.CD002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15:893–908. doi: 10.1111/j.1468-1331.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- 17.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 18.Congeni JP, Kirkpatrick RB. Pegylated interferon induced myasthenia crisis: A case report. J Clin Neuromuscul Dis. 2013;14:123–125. doi: 10.1097/CND.0b013e318285257f. [DOI] [PubMed] [Google Scholar]
- 19.Purvin V, Kawasaki A, Smith KH, et al. Statin-associated myasthenia gravis: Report of 4 cases and review of the literature. Medicine (Baltimore) 2006;85:82–85. doi: 10.1097/01.md.0000209337.59874.aa. [DOI] [PubMed] [Google Scholar]
- 20.Wang HB, Shi FD, Li H, et al. Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J Immunol. 2001;166:6430–6436. doi: 10.4049/jimmunol.166.10.6430. [DOI] [PubMed] [Google Scholar]
- 21.Wang XB, Kakoulidou M, Giscombe R, et al. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: Effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130:224–232. doi: 10.1016/s0165-5728(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Mestre M, Sánchez K, Balbás O, et al. Influence of CTLA-4 gene polymorphism in autoimmune and infectious diseases. Hum Immunol. 2009;70:532–535. doi: 10.1016/j.humimm.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Wang XB, Pirskanen R, Giscombe R, et al. Two SNPs in the promoter region of the CTLA-4 gene affect binding of transcription factors and are associated with human myasthenia gravis. J Intern Med. 2008;263:61–69. doi: 10.1111/j.1365-2796.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Gu M, Kakoulidou M, Giscombe R, et al. Identification of CTLA-4 isoforms produced by alternative splicing and their association with myasthenia gravis. Clin Immunol. 2008;128:374–381. doi: 10.1016/j.clim.2008.05.006. [DOI] [PubMed] [Google Scholar]