Abstract
Phase I trials are increasingly including dose-expansion cohorts after the maximum-tolerated dose (MTD) has been reached to better characterize the toxicity profile or identify early signs of efficacy within a specific disease population. This article provides guidelines on how to monitor safety and re-evaluate the MTD using data obtained from expansion cohorts of phase I protocols. We illustrate how to implement a sequential monitoring rule for safety using a completed phase I trial that included an expansion cohort. We compare the accuracy of the revised MTD with the MTD obtained before expansion and with the true MTD based on simulated trials. The percent of trials that led to a change in the MTD, how far the revised MTD was from the true MTD, and the toxicity rates associated with each level are reported. When toxicity outcomes from the expansion cohort are taken into account, there is a 50% chance that a new, higher MTD will be recommended. Significant improvement in the accuracy of the MTD is obtained 30% of the time (ie, revised MTD is exactly the true MTD), and moderate improvement is obtained 80% of the time when the revised MTD is within a level from true MTD. Failure to include toxicity outcomes from additional patients treated during the expansion phase may result in a less accurate estimate of the MTD. This article provides investigators of phase I protocols with methodological tools to monitor safety and/or efficacy for patients accrued during the expansion phase and to update or confirm the established MTD.
INTRODUCTION
Recent reports show that phase I trials are increasingly using dose-expansion cohorts (DECs) to better characterize the toxicity profiles of experimental agents or study disease-specific cohorts.1–4 The protocols typically consist of two phases: the dose-escalation phase, followed by the dose-expansion phase. During the dose-escalation phase, a dose-finding algorithm identifies the maximum-tolerated dose (MTD), and during the expansion phase, an additional number of patients (six, 10, or more) are treated at the MTD. We define the MTD as that reached by the dose-escalation algorithm and the recommended phase II dose (RP2D) as the dose level recommended for future trials based on review of additional data and safety considerations, which could include data obtained during the expansion phase. As more patients are treated, there is always a chance of observing more dose-limiting toxicities (DLTs). These additional DLTs that occur during the safety expansion phase therefore provide investigators with the opportunity to refine the estimate of the MTD established during the dose-escalation phase. In this article, we consider the toxicity responses, along with other efficacy end points, from the additional patients added at the MTD as part of an expansion cohort and illustrate how we can inform the selection of the RP2D in light of these data.
The broader aim of expansion cohorts varies from exploring pharmacokinetic (PK), pharmacodynamic (PD), efficacy, or biomarker-related end points while the primary aim remains to further evaluate the safety profile of an experimental agent and confirm the MTD. Often, DECs have different eligibility criteria than dose-escalation cohorts to study a specific targeted population within a certain disease type. Is it appropriate to combine all the safety data from a heterogeneous patient population, such as patients with all solid tumors, with the more homogeneous patient population treated during the expansion phase? If a combined analysis is performed, and it recommends a revised MTD, how many patients need to be treated at the new MTD? How can efficacy or other secondary end points be taken into consideration when selecting the RP2D? In this article, we outline design considerations for phase I trials with expansion cohorts from both a clinical perspective as well as a statistical standpoint so that a more efficient and accurate dose is selected for future trials.
MOTIVATION FOR RE-EVALUATING THE MTD
First, we report a recent literature review summarizing key points relevant to design considerations of phase I trials with expansion cohorts. We then illustrate through a clinical trial that re-evaluation of the MTD can suggest a new phase II dose different from the MTD established during the dose-escalation phase. We quantify through simulated trials how often the estimated MTD and RP2D agree and how often each level agrees with the true and unknown MTD.
Literature Review
We reviewed phase I trials with dose expansion published between 2009 and 2012. We used the search terms ((“phase I”[Title/Abstract]) AND “dose expansion”[Title/Abstract]) in the PubMed database. This search was not exhaustive; it is meant to illustrate the fact that there is no consensus regarding the design of phase I trials with DECs. We found that there can be more than one expansion cohort at more than one dose level, drug combination, or schedule; the expansion phase can involve nine to 100 patients, and it often narrows eligibility criteria to enroll patients with specific tumor types to obtain a preliminary efficacy assessment (Table 1). A majority of DECs were treated at a single dose, with the exception of one study of a multitarget inhibitor that expanded at two dose levels to further assess hepatotoxicity at the lower dose.7 A phase I study of bevacizumab with temsirolimus combined with liposomal doxorubicin also expanded at two dose levels, varying the dose of liposomal doxorubicin from 20 to 30 mg/m2 because of an increased rate of grade 2 or higher toxicities that required dose reductions beyond cycle one.10
Table 1.
Examples of Phase I Trials Using DECs Published Between 2009 and 2012
| Trial | No. of Patients |
No. of Dose Levels | No. of DECs | End Point |
RP2D Same As MTD | Patient Eligibility Changed | Efficacy End Point for Expansion | Details of DECs | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Escalation | Dose Expansion | Total | Primary | Secondary | |||||||
| Phase I trial of pazopanib in patients with advanced cancer5 | 43 | 20 | 63 | 11 | 3 | Safety and PKs | Clinical activity, biomarker evaluation | MTD not reached; RP2D based on safety and secondary end points (PKs, PDs, CB); DEC at two doses in three schedules | No | Yes | Three schedules: 800 mg once per day; 300 mg twice per day; 400 mg twice per day |
| Phase I study of bosutinib, Src/ABL tyrosine kinase inhibitor, administered to patients with advanced solid tumors6 | 51 | 100 | 151 | 7 | 3 | Safety | Clinical activity, PKs, PDs | RP2D < MTD; DEC at single dose | Yes, specific tumor types | Yes | Three disease groups: colorectal, pancreatic, NSCLCs |
| Phase I study of AEE788, novel multitarget inhibitor of ErbB and VEGF receptor family tyrosine kinases, in patients with recurrent glioblastoma7 | 40 | 24 | 64 | 9 | 4 | Safety | Tolerability, PKs, efficacy | No MTD or RP2D; study was terminated early because of toxicity, PKs, and efficacy data; DEC at two dose levels | No | Yes | Four patient groups: EIACD, non-EIACD, surgical candidates, nonsurgical candidates |
| Phase I study of saracatinib (AZD0530) in combination with paclitaxel and/or carboplatin in patients with solid tumors8 | 96 | 20 | 116 | 20 (four drug combinations) | 2 | Safety | Tolerability, PKs, efficacy | Yes, RP2D is more than a single level; DEC at single dose | No | Yes | Four drug combinations: S + C + P once every 3 weeks; S + C once every three weeks (DEC); S + P once every 3 weeks; S + P once every week (DEC) |
| Phase I single-agent study of twice-per-week consecutive-day dosing of proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma9 | 37 | 11 | 48 | 9 | 2 | Safety, tolerability, PKs | PDs, antitumor activity | MTD not reached; RP2D is highest administered dose; DEC at single dose | No | Yes | Two DECs: carfilzomib ± dexamethasone |
| Phase I study of the antiangiogenic antibody bevacizumab and mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biologic activity10 | 39 | 97 | 136 | 8 | 2 | Safety, biologic activity | Yes; DEC at two doses | Yes, specific tumor types | Yes | Two drug combinations: B + T + LD 30 mg/m2 once every 3 weeks; B + T + LD 20 mg/m2 once every 3 weeks | |
| Pixantrone dimaleate in combination with fludarabine, dexamethasone, and rituximab in patients with relapsed or refractory indolent non-Hodgkin lymphoma: phase I study with DEC4 | 9 | 19 | 28 | 2 | 1 | Safety, preliminary efficacy | Yes; RP2D is highest administered dose | No | Yes | DEC at single dose | |
| Safety, PKs, and PDs of AMG102, fully human hepatocyte growth factor–neutralizing monoclonal antibody, in first-in-human study of patients with advanced solid tumors2 | 31 | 9 | 40 | 6 | 1 | Safety | Changes in vital signs, laboratory results, presence of antibodies, PKs, efficacy | MTD not reached; RP2D is up to highest administered dose | No | Yes | DEC at single dose |
| First-in-human study of conatumumab in adult patients with advanced solid tumors3 | 22 | 15 | 37 | 5 | 2 | Safety, tolerability, PKs | Changes in caspase-3 activity, efficacy | MTD not reached; RP2D is up to highest administered dose; DEC at single dose | Yes, specific tumor types | Yes | Two disease groups: colorectal and NSCLCs |
| Safety, tolerability, and PKs of anti–IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma11 | 24 | 43 | 67 | 4 | 2 | Safety, tolerability | PKs, PDs, CB | MTD not reached; RP2D is highest administered dose; DEC at single dose | Yes, specific tumor types | Yes | Two disease groups: ACC, solid tumors (not ACC) |
Abbreviations: ACC, adrenocortical carcinoma; B, bevacizumab; C, carboplatin; CB, clinical benefit; DEC, dose expansion cohort; EIACD, enzyme-inducing anticonvulsant drug; IGF-1R, insulin-like growth factor 1 receptor; LD, liposomal doxorubicin; MTD, maximum-tolerated dose; mTOR, mammalian target of rapamycin; NSCLC, non–small-cell lung cancer; P, paclitaxel; PD, pharmacodynamic; PK, pharmacokinetic; RP2D, recommended phase II dose; S, saracatinib; T, temsirolimus; VEGF, vascular endothelial growth factor.
Simulated Results
In this section, we compare through simulated trials how often the MTD established by the dose-escalation algorithm alone agrees with the RP2D at the end of the study when all data from the two phases are combined. The likelihood of the RP2D being different from the MTD found during the dose-escalation phase depends on the number of DLTs observed during the expansion phase. That, in turn, depends on the underlying dose-toxicity curve, which in practice is unknown. In simulated trials, the underlying dose-toxicity curve is known, and thus, we are able to assess whether the estimated MTD before and after the expansion phase is indeed the actual/true MTD.
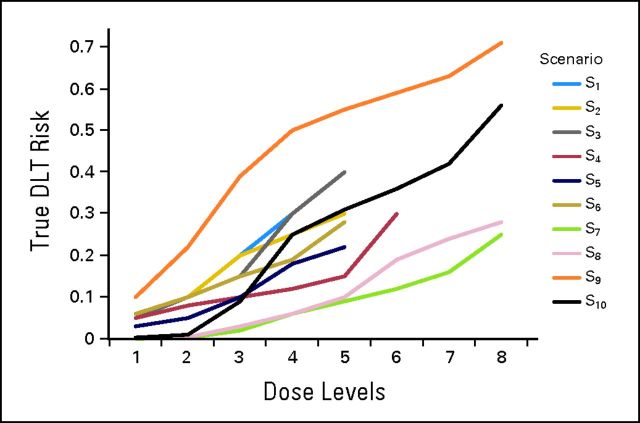
We simulated 1,000 trials testing five, six, or eight prespecified dose levels and varying the location of the true MTD based on some underlying hypothetical DLT rates. We evaluated 10 scenarios for the true dose-toxicity curve (Appendix Fig A1, online only), six of which were reported previously.12 All trials followed the 3 + 3 design12 (Appendix Table A1, online only), and once the MTD was found, an additional 10 patients (expansion cohort) were added at the MTD for a total of 16 patients treated at the MTD. Simulations with 10 (six plus four) patients at the MTD were also performed. The acceptable toxicity rate for the complete analysis varied from 25% to 30% (scenarios S1 to S4 target 30% DLT rate, whereas scenarios S5 to S10 target 25% DLT rate). To estimate a new MTD using the combined toxicity data from the dose-escalation cohort and DEC, a retrospective dose-toxicity curve was fitted for each simulated trial using methodology previously described.13 The approach described here can be used regardless of design choice, on the premise that this is a complete analysis of the combined observed toxicities.
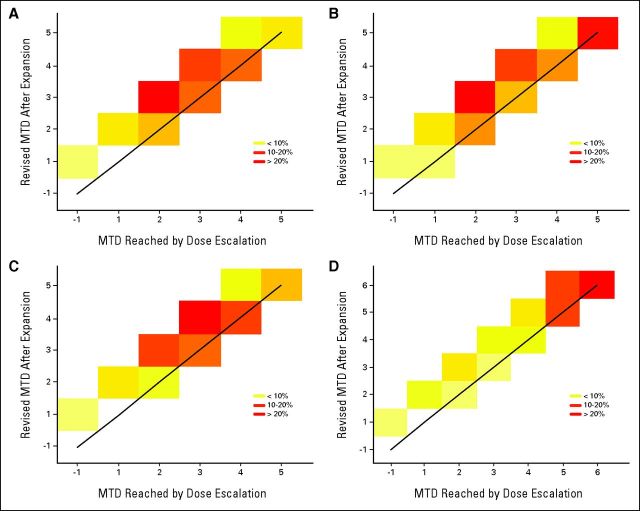
First, we compare how close the revised MTD or RP2D is to the MTD reached before the expansion phase. Figure 1 shows the percentage of trials that recommended each level based on the design and final analysis method for the first four scenarios. The x-axis shows the recommended level based on the escalation algorithm alone, whereas the y-axis shows the recommendation based on the final analysis, which includes data from the dose-escalation cohort and DEC. Figure 1 demonstrates that the complete analysis often recommends a higher dose compared with the MTD reached before the expansion cohort, as indicated by the trials falling above the diagonal line. Moreover, this higher dose in all four scenarios was closer to the true MTD than the level estimated using dose-escalation data alone. For example, in scenario S1, 47% of the trials recommend a higher dose toward level four, which is the true MTD, as opposed to 5% of the trials where recommendation is for level five.
Fig 1.
Percentage of 1,000 simulated trials that recommended each level based on dose-escalation phase alone (x-axis) versus revised maximum-tolerated dose (MTD) based on dose-escalation and -expansion cohorts (y-axis). Diagonal cells falling along solid black line indicate agreement between the two procedures. True MTDs are levels 4, 5, 4, and 6 (indicated in brackets) for scenarios (A) 1, (B) 2, (C) 3, and (D) 4, respectively.
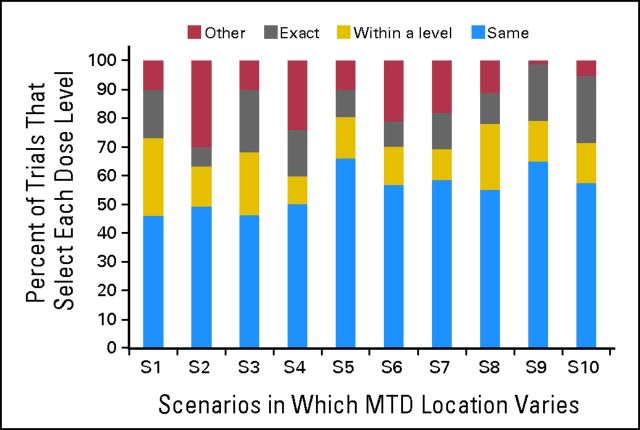
Figure 2 summarizes the changes in the MTD recommendation in relation to the true MTD. There is an approximately 50% chance of changing the MTD based on the new data obtained from the expansion cohort. When the recommendation is to change the MTD, on average, 30% of the recommended levels will point exactly to the true MTD, whereas approximately 50% will point to a level within a range ± one level from the true MTD. In our examples, the acceptable toxicity rate varied from 25% to 30%, so the levels around the MTD that were considered within an acceptable toxicity range were levels at which the corresponding toxicity rate was 15% to 33%. If the actual MTD is not the last level, there is an 80% chance that the refined estimate of the MTD will be a dose level within an acceptable toxicity range. Thirteen percent of simulated trials had a DLT rate between 20% and 30% at the MTD after expansion, with the majority of trials (60%) having a DLT rate of less than 15%.
Fig 2.
Percent of 1,000 simulated trials in which dose recommended by combined analysis using dose-escalation and -expansion cohorts was same as level recommended by dose-escalation alone, regardless if that is true maximum-tolerated dose (MTD). Within a level is defined as dose ± one level from true MTD; exact is defined as true MTD; other includes dose recommendations that are within two levels from true MTD.
RE-EVALUATING THE MTD
In this section, we outline four methods for how reassessment of the MTD using complete data can be carried out. The first method combines all the toxicity data, before and after expansion, into a final retrospective analysis. No changes in the design or other considerations are taken into account during the enrollment of patients onto the expansion cohort. The second approach allows for re-evaluation of the MTD as more data are obtained from the expansion cohort and incorporates stopping rules for safety. The third approach allows re-evaluation of the MTD when safety and efficacy are taken into consideration simultaneously before recommending a new level. Finally, issues pertaining to patient heterogeneity are discussed in the context of dose-finding algorithms, such as when it is appropriate to have separate MTDs for separate groups.
Option One: Using Safety Data Alone—Retrospective Assessment
A final, complete analysis including the toxicity responses from the additional patients could confirm or revise the DLT rate at the MTD with a higher precision and accuracy as follows. We use the completed phase I trial of intravenous aflibercept in combination with docetaxel (at a fixed dose) in patients with advanced solid tumors14 for illustration. The trial included a dose-escalation phase using a 3 + 3 design followed by a DEC. A total of 54 patients were accrued: 34 patients during the dose-escalation phase, and 20 patients during the expansion phase. Initially, 10 patients were treated as part of the dose-escalation phase at the MTD of 6 mg/kg. The recommended MTD was 6 mg/kg based on two DLTs: one event of hypertension at dose 7 mg/kg, and one patient who had progressive increase in blood pressure treated at dose 9 mg/kg. Table 2 shows observed DLT rates at the six levels included during the dose-escalation phase. The recommended dose based on reanalysis of data from the 34 patients, without including the expansion cohort, is 7 mg/kg, assuming an acceptable toxicity rate of 25% (details listed in Appendix Table A2, online only). However, the expansion phase of the protocol treated 20 more patients at 6 mg/kg, because that was the MTD based on the adverse events seen at higher doses.
Table 2.
DLTs and Suggested MTDs for Illustrative Trial of IV Aflibercept in Combination With Docetaxel
| DLT Rate | Actual Dose (mg/kg) |
|||||
|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 7 | 9 | |
| Observed | 1 of 7 | 0 of 3 | 0 of 6 | 0 of 10 (MTD) | 1 of 5 | 1 of 3 |
| Predicted | 0.01 | 0.04 | 0.09 | 0.16 | 0.25 (revised MTD) | 0.35 |
NOTE. Data adapted.14
Abbreviations: DLT, dose-limiting toxicity; IV, intravenous; MTD, maximum-tolerated dose.
Option Two: Prospectively Guiding Dose Expansion Using Safety
Alternatively, instead of treating all patients at the MTD during the expansion phase, investigators might guide the dose expansion prospectively using a dose-finding algorithm. The recommendation will be to stay at the MTD as long as the estimated DLT rate is sufficiently safe, based on some threshold of acceptable toxicity. If a high number of DLTs is observed, de-escalation will be recommended. Alternatively, if a low rate of DLTs is observed in the DEC, the dose recommendation will be higher. Table 3 summarizes how a sequential safety monitoring rule can be implemented using hypothetical DLT outcomes for the 20 patients. The recommendation is dose 7 mg/kg as long as we observe ≤ eight DLTs in the expansion cohort (eight of 30 [26.6%]). However, if we observe ≥ nine DLTs among 30 patients, the predicted DLT rate increases for dose 7 mg/kg and, the recommendation would be to stay at dose 6 mg/kg. This example illustrates that the dose recommendation can be lower or higher than the pre-expansion dose, because model-based methods are adaptive to new data.
Table 3.
Hypothetical No. of DLTs of 20 Patients Enrolled in DEC at Dose 6 mg/kg
| No. of DLTs (of 20) | MTD Based on Final Analysis (mg/kg) | Predicted DLT Rates |
|
|---|---|---|---|
| 6 mg/kg | 7 mg/kg | ||
| 2 | 7 | 0.15 | 0.24 |
| 4 | 7 | 0.16 | 0.25 |
| 6 | 7 | 0.17 | 0.26 |
| 8 | 7 | 0.20 | 0.29 |
| 10 | 6 | 0.22 | 0.32 |
| 12 | 6 | 0.24 | 0.33 |
NOTE. MTD recommendation includes zero DLTs observed among 10 patients during escalation phase as well as all patients treated at other levels. This sequential analysis included all 30 patients treated at dose 6 mg/kg plus 24 patients treated at other levels.
Abbreviations: DEC, dose-expansion cohort; DLT, dose-limiting toxicity; MTD, maximum-tolerated dose.
Option Three: Prospectively Guiding Dose Expansion Using Safety and Efficacy
Thus far, we have presented methods that either treat all patients in the expansion cohort at the same level or allow re-evaluation of the MTD depending on the number of DLTs. Both assessments are based on safety alone, and the decision to continue treating patients at the revised MTD until some level of confidence is achieved can be made using statistical considerations (like a CI estimation around the DLT rate15) or using a practical rule (eg, until six patients are treated at the revised MTD16). CIs tend to be wide in the context of phase I trials, so treating a minimum of six patients at the revised MTD is a useful rule of thumb. The third option is to take into account efficacy during the expansion cohort when evaluating the MTD. This approach can be used when the primary aim of the DEC is to simultaneously assess safety and efficacy. In this case, efficacy measures can be used to guide the dose assignment in the expansion cohort. We can use previous patients' outcomes, both in terms of toxicity and efficacy, to inform the dose selection for the next patients to be treated. This can be done using sequential model-based dose-finding algorithms that aim to recommend a dose optimal in both safety and efficacy.17,18 On the basis of some prespecified promising and low efficacy rates (eg, 30% v 5%), the algorithm points to a level that is safe and simultaneously tests if this level has an efficacy rate of, for example, ≥ 30%. If a safe and efficacious level does not exist, then at the end of the study, we still have a recommended MTD based on safety, but the test indicates that the efficacy rate is likely close to the 5% rate under the null hypothesis.
Option Four: Experimenting at More Than a Single Level
Another option, which is rarely employed, is to allow patients being treated during the expansion phase to be randomly assigned to two levels near or at the MTD.19 The hypothesis is that regardless of the design being used in the dose-escalation phase, during the expansion phase, we are experimenting in the neighborhood of a target dose with an acceptable rate of toxicity, often near the true MTD. Random assignment allows us to refine our initial estimate of the MTD by continuing experimentation in a narrower dose range at and around the initial estimate of the MTD. This will further enable us to address the question of whether a higher level is promising in terms of efficacy, but that level might not be acceptable because of the rate and types of serious adverse events. The algorithm that sequentially assigns patients to one level ± the MTD can take into account the estimated DLT rates such that overdose control can be maintained via safety constraints. Of course, the number of patients treated at levels above the MTD depends on the steepness of the dose-toxicity and dose-efficacy curves and safety constraints, which in turn can depend on the particular disease setting, investigational drug, and previous experience with this drug (eg, type, severity, resolution of adverse events). Finally, if the safety criterion points to a lower level, it is possible that the number of patients treated at the higher level will not be sufficient, and thus, the efficacy result may not be conclusive at the end of the study. This is an equally important finding, because investigators might want to experiment more at that level depending on the types of adverse events, or they might accept the inconclusive result given the safety concerns. These tools are meant to guide the process along with clinical judgment so that any final decisions can be based on a review of all accumulated data. These algorithms are flexible through the choice of model parameters, which in turn control the operating characteristics, and should be evaluated in the particular trial before being used.
Option Five: Taking Into Account Different Eligibility Criteria
Because the aim of expansion cohorts varies from exploring PK, efficacy, or biomarker-related end points, DECs often have different eligibility criteria to study a specific targeted population within a certain disease type. The underlying assumption is that the same toxicity profile and MTD apply to different populations. Given that investigators frequently base the RP2D on safety data from heterogeneous patient populations, it is often clinically appropriate to combine safety data from the dose-escalation cohort and DEC. In cases where a trial enrolls patients with solid tumors or a heterogeneous group during dose escalation but focuses on a particular disease type or a more homogenous group for the DEC, there are dose-finding algorithms that can estimate the MTD for each group.20 Such designs can indicate whether there is a single MTD for all groups or separate MTDs for each group. The basic idea is that there is a dose-toxicity model that is updated based on previous patients' responses, and there is a covariate effect measuring the difference in levels between the groups. The data might support no difference in the two groups or a shift in the MTD by one or two levels.
DISCUSSION
The assumption that we have reached the MTD during the dose-escalation phase and that thus patients in the expansion cohort are receiving the right dose is not always true. We argue that DECs in phase I studies need to be sequentially monitored for safety, and experimentation should be adaptively changed if supported by the results. The limited number of patients involved in dose-escalation trials, combined with patient heterogeneity in terms of prior therapies, disease setting, and concurrent illnesses, makes it impossible to know whether the MTD reached is indeed the true MTD. Moreover, the size of expansion cohorts varies, with recent phase I studies enrolling 100 to 200 patients when aiming to target five disease sites, for example, with 20 patients in each DEC.6,21 Our previous simulations have shown that on average, 20 to 35 patients are sufficient to estimate safety and efficacy in DECs with a homogeneous group.18 The precise estimate of how many patients are needed depends on the objective of the study, the number of levels in the dose-escalation phase, and whether multiple drugs, schedules, or heterogeneous disease groups are involved. In more complex settings, it also depends on the amount of information borrowed between treatment schedules, drug combinations, and patient groups. We suggest that the DEC size should be at least 50% of the pre-expansion sample size, or a minimum of 12 to 15 patients, to obtain some meaningful preliminary evidence for efficacy.
Trial duration of these studies depends on target accrual, accrual rate, and cohort size. The idea that model-based designs take longer to complete is not true.12 As phase I trials increasingly add expansion cohorts, they can no longer be viewed as small, short trials. Because the commitment in terms of both time and resources is increasing, it is imperative that we improve our estimation of the MTD by using all available information, including secondary and/or exploratory end points when available, such as PK/PD end points or lower-grade toxicities. For example, using subdose-limiting toxicities to guide dose escalation can get us to the neighborhood of the MTD faster, especially when we experiment with a large number of levels.22–24 The design we discussed using efficacy to guide dose expansion can be used with any other binary end point, such as plasma concentration, tumor absorption, biomarker expression, or PK/PD end points. If instead of efficacy we use biomarker expression, for example, this methodology can be coupled with the paradigm of targeted therapies by allowing dose expansion to focus experimentation in a targeted population in terms of both patient selection for future trials as well as selection of an appropriate dose level.
The approaches we have discussed here mainly address the simplest cases, where the toxicity end point is measured by presence or absence of DLT, and the efficacy end point is binary, such as responders versus nonresponders. We have not taken into account the effect of cumulative toxicities observed in a longitudinal setting from subsequent cycles of treatment, and we consider these to be important issues that require further research. Model-based designs can be extended to take into account correlated data such as information obtained from intrapatient dose de-escalations and intermediary or lower-grade toxicities. Some of these more complex situations and necessary design modifications have been discussed previously.19,20 Finally, the concepts discussed here can be used in the context of multiple-drug combinations, as long as there is an expectation of higher toxicity with higher dose levels. In cases where higher dose levels do not always correlate with higher toxicity, available designs can be modified to address issues specific to DECs.25
One reviewer pointed out that use of model-based methods depends on the willingness of investigators to recommend a new, higher phase II dose in the absence of toxicity information from additional patients being treated at the revised level. In this article, we provided various options for guiding DECs mainly grouped into two options. The first option is to treat all patients at the pre-expansion level (ie, initial MTD), and then use all the updated information to decide whether the combined data point toward a new dose. If the expansion cohort proves the current MTD as completely nontoxic, it is up to the investigators to decide whether they want to treat more patients at a higher dose for potential higher-efficacy activity. The second option is to allow patients in DECs to be treated at more than a single level, as described in detail in Options Two to Four in this article. Adaptive designs concentrate experimentation near the MTD, in which case toxicity information will be available from nearby levels, not just a single level. Clinical experience cannot be replaced by model recommendations. If, for example, we have observed zero DLTs of six and two DLTs of three patients at dose levels four and five, respectively, and zero DLTs in 10 patients treated in the DEC at level four, no method can find the dose associated with 33% of the DLT rate in this scenario, unless we allow interpolation (insertion) of intermediary levels or additional experimentation to further confirm the 67% rate at level five. If clinicians do not consider exposing more patients to the level at which two of three DLTs were observed as acceptable, and because the level at which zero of 16 patients experienced DLTs is likely to be inactive, the option to experiment at intermediate dose levels is a viable option. The decision to add intermediate dose levels can be based on subdose-limiting toxicities, PK data, or other data obtained beyond cycle one. Given preclinical or clinical data from other trials or previous formulations of the drug, and evaluation of the type and severity of DLTs, investigators might accept a higher toxicity rate. Different factors, including whether DLTs are reversible and not alarming, onset time, attribution, agent type (cytotoxic v molecular), and other considerations, can affect this decision. For example, in the drug combination study reported by Moroney et al,10 because of an increased rate of grade 2 toxicity that required dose reductions beyond cycle one, an intermediate dose level was added that was not part of the dose expansion but informed the choice of RP2D.
In this article, we have shown that a final analysis that takes into account additional data can recommend a different dose level on average 50% of the time, and in no scenario studied have we found a reduction in accuracy. The majority of simulated trials that led to a revised RP2D recommended a higher level after the expansion compared with the level reached using the dose-escalation data alone. These data suggest that on average, failing to analyze the expansion cohort, in conjunction with the pre-expansion cohorts, is not exposing patients to increased risk, in that the recommended dose can still be considered a safe dose. However, in a large number of cases, this leads us to miss the fact that a level higher than that currently recommended by the dose-escalation cohorts alone could still be considered safe while simultaneously offering the potential of a greater treatment effect. Our results agree with previous findings of retrospective analyses of 3 + 3 trials,26–29 and other authors have also suggested monitoring of DECs with some sequential safety rules.30–32 There can be unique trials in which the RP2D is lower than the MTD,8,14 given auxiliary data observed in the DEC and based on recommendations of the data safety monitoring board. We view the methods discussed here as tools to provide the board with the necessary information to confirm or refine the RP2D in light of the complete data. These tools are not meant to be definitive. Investigators can incorporate them into phase I protocols without any practical changes in the way phase I trials are carried out, or they can allow more experimentation in expansion cohorts to further evaluate safety simultaneously with efficacy. Failure to monitor safety during the expansion phase raises ethical concerns, because investigators may continue treating patients at a dose that would otherwise be deemed invalid if all relevant data were used.
Appendix
Table A1.
Dose-Escalation Algorithm for 3 + 3 Method
| No. of Patients With DLTs | Action |
|---|---|
| 0 of 3 | Escalate to next-higher dose level and enroll up to three patients |
| 1 of 3 | Enroll up to three more patients at same dose level |
| 1 of 6 | Escalate to next-higher dose level and enroll up to three patients |
| ≥ 2 of 3 in first 3 or 6 | De-escalate* to lower dose level, and enroll total of six patients; if one of six has DLT, declare this lower level to be MTD |
Abbreviations: DLT, dose-limiting toxicity; MTD, maximum-tolerated dose.
If de-escalation occurs at dose level −1, additional enrollment will follow rules in first three rows only for dose level −1.
Table A2.
Final Analysis*
| DLT Rate | Actual Dose (mg/kg) |
|||||
|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 7 | 9 | |
| Initial (a priori) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 |
| Weights via simulations | 0.17 | 0.09 | 0.10 | 0.12 | 0.23 | 0.29 |
| Predicted | 0.01 | 0.04 | 0.09 | 0.16 | 0.25 (revised MTD) | 0.35 |
Abbreviations: DLT, dose-limiting toxicity; MTD, maximum-tolerated dose.
Using equation six from 2005 report by O'Quigley et al.13 We estimate dose-toxicity parameter to be estimated α = 2.0286 and the MTD to be dose 7 mg/kg for acceptable rate of 25%.
Fig A1.
Hypothetical true dose-limiting toxicity (DLT) risk per patient for each scenario (ie, true dose-toxicity curves). Acceptable rate of 25% or 30% was used to vary location of true MTD. Scenarios S1 to S4 target 30% DLT rate. Scenarios S5 to S10 target 25% DLT rate and were studied previously.12
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Alexia Iasonos
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Annunziata CM, Kohn EC, LoRusso P, et al. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest New Drugs. 2013;31:77–84. doi: 10.1007/s10637-012-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon MS, Sweeney CS, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Kurzrock R, Hong DS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 4.Srokowski TP, Liebmann J, Modiano MR, et al. Pixantrone dimaleate in combination with fludarabine, dexamethasone, and rituximab in patients with relapsed or refractory indolent non-Hodgkin lymphoma: Phase 1 study with a dose-expansion cohort. Cancer. 2011;117:5067–5073. doi: 10.1002/cncr.26121. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 6.Daud AI, Krishnamurthi SS, Saleh MN, et al. Phase I study of bosutinib, a src/abl tyrosine kinase inhibitor, administered to patients with advanced solid tumors. Clin Cancer Res. 2012;18:1092–1100. doi: 10.1158/1078-0432.CCR-11-2378. [DOI] [PubMed] [Google Scholar]
- 7.Reardon DA, Conrad CA, Cloughesy T, et al. Phase I study of AEE788, a novel multitarget inhibitor of ErbB- and VEGF-receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother Pharmacol. 2012;69:1507–1518. doi: 10.1007/s00280-012-1854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye S, Aamdal S, Jones R, et al. Phase I study of saracatinib (AZD0530) in combination with paclitaxel and/or carboplatin in patients with solid tumours. Br J Cancer. 2012;106:1728–1734. doi: 10.1038/bjc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsina M, Trudel S, Furman RR, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18:4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 10.Moroney J, Fu S, Moulder S, et al. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: Tolerance and biological activity. Clin Cancer Res. 2012;18:5796–5805. doi: 10.1158/1078-0432.CCR-12-1158. [DOI] [PubMed] [Google Scholar]
- 11.Haluska P, Worden F, Olmos D, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65:765–73. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iasonos A, Wilton AS, Riedel ER, et al. A comprehensive comparison of the continual reassessment method to the standard 3+3 dose escalation scheme in phase I dose-finding studies. Clin Trials. 2008;5:465–477. doi: 10.1177/1740774508096474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Quigley J. Retrospective analysis of sequential dose-finding designs. Biometrics. 2005;61:749–756. doi: 10.1111/j.1541-0420.2005.00353.x. [DOI] [PubMed] [Google Scholar]
- 14.Isambert N, Freyer G, Zanetta S, et al. Phase I dose-escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2012;18:1743–1750. doi: 10.1158/1078-0432.CCR-11-1918. [DOI] [PubMed] [Google Scholar]
- 15.O'Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 16.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova A. A new dose finding design for bivariate outcomes. Biometrics. 2003;59:1001–1007. doi: 10.1111/j.0006-341x.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Quigley J, Hughes MD, Fenton T. Dose-finding design for HIV studies. Biometrics. 2001;57:1018–1029. doi: 10.1111/j.0006-341x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Quigley J, Conaway M. Continual reassessment and related dose finding designs. Stat Sci. 2010;25:202–216. doi: 10.1214/10-STS332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Quigley J, Conaway M. Extended model-based designs for more complex dose-finding studies. Stat Med. 2011;30:2062–2069. doi: 10.1002/sim.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iasonos A, Zohar S, O'Quigley J. Incorporating lower grade toxicity information into dose finding designs. Clin Trials. 2011;8:370–379. doi: 10.1177/1740774511410732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SM, Cheng B, Cheung YK. Continual reassessment method with multiple toxicity constraints. Biostatistics. 2011;12:386–398. doi: 10.1093/biostatistics/kxq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Meter EM, Garrett-Mayer E, Bandyopadhyay D. Dose-finding clinical trial design for ordinal toxicity grades using the continuation ratio model: An extension of the continual reassessment method. Clin Trials. 2012;9:303–313. doi: 10.1177/1740774512443593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wages NA, Conaway MR, O'Quigley J. Continual reassessment method for partial ordering. Biometrics. 2011;67:1555–1563. doi: 10.1111/j.1541-0420.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iasonos A, Ostrovnaya I. Estimating the dose-toxicity curve in completed phase I studies. Stat Med. 2011;30:2117–2129. doi: 10.1002/sim.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Quigley J, Zohar S. Experimental designs for phase I and phase I/II dose-finding studies. Br J Cancer. 2006;94:609–613. doi: 10.1038/sj.bjc.6602969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, Liu J, Binkowitz B, et al. A model-based approach in the estimation of the maximum tolerated dose in phase I cancer clinical trials. Stat Med. 2006;25:2027–2042. doi: 10.1002/sim.2334. [DOI] [PubMed] [Google Scholar]
- 29.Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an up-and-down design. Stat Med. 2003;22:535–543. doi: 10.1002/sim.1351. [DOI] [PubMed] [Google Scholar]
- 30.Berry SM, Spinelli W, Littman GS, et al. A Bayesian dose-finding trial with adaptive dose expansion to flexibly assess efficacy and safety of an investigational drug. Clin Trials. 2010;7:121–135. doi: 10.1177/1740774510361541. [DOI] [PubMed] [Google Scholar]
- 31.Gönen M. A Bayesian evaluation of enrolling additional patients at the maximum tolerated dose in phase I trials. Contemp Clin Trials. 2005;26:131–140. doi: 10.1016/j.cct.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Hamber P, Ratain MJ, Lessafre E, et al. Dose escalation models for combination phase I trials. Eur J Cancer. 2010;46:2870–2878. doi: 10.1016/j.ejca.2010.07.002. [DOI] [PubMed] [Google Scholar]