Abstract
We compared the bactericidal activity of recombinant sets of chimeric IgG monoclonal antibodies against two important outer membrane meningococcal vaccine antigens: PorA and factor H binding protein (FHbp). The sets contained human Fc portions from IgG1, IgG3, and two IgG3 mutants (IgG3m15 and IgGm17) with hinge regions of 15 and 17 amino acids encoded by hinge exons h2 and h1, respectively (human IgG3 has a hinge region of 62 amino acids encoded by hinge exons h1, h2, h3, and h4, while human IgG1 has a hinge region of only 15 amino acids encoded by one hinge exon) and mouse V regions. IgG1 showed higher bactericidal activity than IgG3 when directed against PorA (an abundant antigen), while IgG3 was more bactericidal than IgG1 when directed against FHbp (a sparsely and variably distributed antigen). On the other hand, the IgG3 hinge-truncated antibodies IgG3m15 and IgGm17 showed higher bactericidal activity than both IgG1 and IgG3 regardless of the target antigen. Thus, the Fc region of IgG3 antibodies appears to have an enhanced complement-activating function, independent of their long hinge region, compared to IgG1 antibodies. The greater activity of the truncated IgG3 hinge mutants indicates that the long hinge of IgG3 seems to downregulate through an unknown mechanism the inherent increased complement-activating capability of IgG3 Fc when the antibody binds to a sparse antigen.
INTRODUCTION
Immune protection against invasive meningococcal disease depends on recognition of bacterial surface antigens by antibodies, followed by activation of complement, leading to degradation of the bacteria by bacteriolysis, also named serum bactericidal activity (SBA). The class 1 outer membrane porin protein PorA is abundantly expressed by almost all meningococcal strains (1–3), and antigenic variation among PorA proteins is the basis of serosubtyping (1). PorA can induce bactericidal antibodies in humans and mice when they are immunized with meningococcal outer membrane vesicle (OMV) vaccines (4–8), and monoclonal antibodies (MAbs) against PorA can be protective in an infant rat model (9). Factor H binding protein (FHbp) is a lipoprotein that is sparsely distributed on the outer membrane of many meningococcal strains (10–12). It is an immune system-evading protein protecting the meningococci from complement-mediated lysis by binding the human complement-inhibiting protein factor H (FH) (13). Antibodies to FHbp elicit SBA and confer passive protection in infant rat meningococcal bacteremia models (14, 15). PorA is estimated to make up 25% of the outer membrane of meningococci, while FHbp is estimated to make up 1% (16).
Human IgG consists of four subclasses (isotypes), IgG1, IgG2, IgG3, and IgG4, which differ greatly in effector functions, such as interaction with FcR on immune cells and the capacity to activate complement (17–19). By using monoclonal hapten (4-hydroxy-3-nitrophenacetyl [NP/NIP])-specific antibodies of all four IgG isotypes, we have demonstrated that IgG1 and IgG3 are best in inducing complement-mediated cellular lysis and IgG1 performs better than IgG3 when the antigen concentration on the target cells is high, while IgG3 performs better than IgG1 when the antigen concentration on the target cells is low (20, 21). In separate studies, IgG3 antibodies also showed higher SBA than IgG1 antibodies when the target antigen was sparsely expressed (as in the case of FHbp) (22), but IgG1 antibodies were more bactericidal than IgG3 antibodies when the target antigen was highly expressed, such as for PorA (23).
Human IgG3 and IgG1 antibodies are structurally very different in the hinge region, while the Fc region shows more than 95% sequence homology (17). IgG3 has an extended hinge region of 62 amino acids (24) encoded by four exons (25), and IgG1 has a shorter hinge of 15 amino acids encoded by one exon (25). IgG3 molecules also have higher flexibility than IgG1, which might be beneficial when the antigen is sparsely distributed or poorly accessible (26). In the present study, we investigated the functional activity of recombinant IgG1 and IgG3 antibodies as well as hinge-truncated mutants of IgG3 when interacting with complement on the surface of live meningococci. As target molecules we chose the two outer membrane antigens FHbp and PorA, and as effector antibodies we employed parallel sets of monoclonal chimeric (murine-human) antibodies with well-defined specificity for FHbp and PorA, respectively. All antibodies were produced using the same cellular and molecular biology technologies, allowing direct comparison of the in vitro protective activity of the antibodies.
MATERIALS AND METHODS
Murine MAbs.
The murine anti-FHbp MAbs JAR3 and MAb502 have been described previously (27–29) JAR3 and MAb502 react with nonoverlapping epitopes involving glycine at position 121 and arginine at position 204 of the N-terminal and C-terminal domains of FHbp, respectively (29, 30). The murine PorA P1.16-specific MAb 151,F-9 has been described previously (23). Its specificity has been verified by testing against synthetic peptides (31), and it has been shown to have specificity identical to that of the P1.16 MAb MN12H2 (32).
Synthesis of the MAb502, JAR3, and 151,F-9 H- and L-chain genes.
Chimeric IgG1, IgG3, IgG3 mutant IgG3m15, and IgG3 mutant IgG3m17 of JAR3, MAb502, and 151,F-9 were constructed on the basis of the sequences of the V-region genes of the mouse anti-FHbp MAbs JAR3 and MAb502 (22) and the PorA P1.16-specific MAb 151,F-9 (33). The full-length human κ light (L) chain and human γ1, γ3, γ3m15, and γ3m17 heavy (H) chains were produced synthetically by GenScript. The restriction enzyme recognition sites Esp3I and EcoRI were inserted into the flanks of the synthesized genes for subsequent use in cloning of the genes into a modified pLNO vector (34). The genes were codon optimized for expression in human HEK293E cells.
Cloning of Ig genes.
Each of the H- and L-chain genes of MAb502, JAR3, and 151,F-9 was supplied in the pUC57 vector (GenScript, Piscataway, NJ). Vector pUC57 containing the synthesized gene was digested with the restriction enzymes Esp3I and EcoRI (Fermentas, Burlington, ON, Canada), and a DNA fragment corresponding to the size of H or L chain was isolated by agarose gel electrophoresis using a GelElute kit (Qiagen). The cloning vector was processed in the same way by digestion with restriction enzymes Esp3I and EcoRI and subsequent isolation of the digested vector by agarose gel electrophoresis. The digested genes were ligated into the linearized vector using T4 DNA ligase (New England BioLabs, Ipswich, MA) and then transformed into XL-10 Gold competent cells (Stratagene, La Jolla, CA). Transformed cells were selected on ampicillin-containing growth agar. Bacterial colonies were selected by growing bacteria for 14 h in ampicillin-containing liquid medium, and vector DNA was isolated using a plasmid Spin miniprep kit (Qiagen GmbH, Germany). Vector DNA was verified to contain the correct insert by restriction enzyme analysis.
Transient transfection of HEK293E cells for expression of Abs MAb502, JAR3, and 151,F-9.
Five million HEK293E cells were added to 25 ml Dulbecco modified Eagle medium (DMEM; Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) and 4 mM l-glutamine. Cell-containing medium was transferred to a standard medium cell culture flask (T75), and the cells were incubated for 18 h in humidified atmosphere with 5% CO2 at 37°C. A transfection mixture was prepared by adding 5 mg vector DNA (0.1 mg/ml) expressing the L chain (κ), 5 mg vector DNA (0.1 mg/ml) expressing the desired H chain (γ1, γ3, γ3m15, or γ3m17), and 375 μl RPMI 1640 into a test tube. The mixture was preheated to 80°C and cooled to 4°C. Polyethylenimine (PEI) Max (2 mg/ml; Polysciences, Warrington, PA) was heated simultaneously but cooled to room temperature (RT) to prevent precipitation. Sixty-five microliters PEI solution was added to the transfection mixture before the tube was left to incubate at RT for 8 min. After incubation, the reaction mixture was added to a 15-ml conical test tube with 3,375 μl DMEM supplemented with 10% FBS and 4 mM l-glutamine and gently mixed. Medium was removed from the cell culture flask with HEK293E adherent cells and replaced with the reaction mixture. The reaction mixture was allowed to cover the cells for 2 h before addition of 25 ml DMEM supplemented with 10% FBS and 4 mM l-glutamine. Transfected cells were allowed to grow for 2 to 5 days before the supernatant was harvested and tested for the production of IgG. The concentrations of human IgG1 and IgG3 in the samples were quantified by enzyme-linked immunosorbent assay (ELISA) with goat anti-human IgG Fc (Sigma-Aldrich) as the coating antibody (Ab) and alkaline phosphatase-conjugated goat anti-human IgG Fc-specific antibody (Sigma-Aldrich) as the detection Ab. The goat anti-human IgG Fc apparently has the same avidity for IgG1 and IgG3, since we obtained the same respective results by using anti-human κ-chain-specific Abs. Purified human myeloma plasma IgG1 and IgG3 (Sigma-Aldrich) were used as internal standards.
Flow cytometry.
Binding of the chimeric MAbs to the surface of live encapsulated bacteria was measured by flow cytometry as described previously (22). The test strain was wild-type (WT) strain H44/76 (H44/76-WT; with the phenotype B:15:P1.7,16; sequence type 32), which expresses the target FHbp identification (ID) 1 antigen as well as PorA P1.16 (23). In some experiments, we also used a mutant of H44/76 (H44/76-OE) in which FHbp was overexpressed (OE) (22). For the flow cytometry experiments to measure MAb binding, a fixed concentration of anti-FHbp MAb (4 μg/ml) or, as a negative control, 100 μg/ml of an irrelevant MAb (a monoclonal κ-chain antibody from a human myeloma [Sigma]) was incubated with ∼107 bacteria/ml. Bound antibody was detected using CF488-conjugated goat anti-human IgG (Biotium).
SPR analysis.
Surface plasmon resonance (SPR) technology was used to assess the binding properties of the MAbs (Biacore X-100 Plus instrument; Biacore AB, Uppsala, Sweden). A total of 2,700 response units (RU) of anti-human IgG MAb (human antibody capture kit) was coupled to a CM5 sensor chip using standard amine coupling chemistry. In single-cycle kinetics experiments, ∼1,200 RU of each human-mouse chimeric anti-FHbp MAb was captured on the anti-human IgG MAb. Various concentrations of soluble recombinant FHbp ID 1 antigen ranging from 2.5 nM to 40 nM were injected over the chip surface at a flow rate of 30 μl/min. An association step of 60 s was followed by a dissociation step of 180 s, and the final dissociation step was 600 s. Regeneration of the sensor chip surface was accomplished using 3 M MgCl2. Experiments were performed at 25°C. Kinetic data were analyzed using Biacore X100 Evaluation (version 2.0) software and a 1:1 binding model. All chemicals for the Biacore experiment were purchased from Sigma-Aldrich.
Mutant group B strain H44/76 with increased FHbp expression.
The shuttle vector pFP12, which has an origin of replication from a naturally occurring plasmid in Neisseria gonorrhoeae (35), was used to increase FHbp expression as described previously for Neisseria meningitidis group C strain RM1090 (18). A mutant of H44/76 in which the gene encoding FHbp had been inactivated (12) was transformed with pFP12-FHbp containing the full-length gene encoding the FHbp ID 1 antigen. The transformation was performed as previously described (36), and transformants were selected on GC agar plates containing 5 μg of chloramphenicol/ml. By flow cytometry, the mutant (designated H44/76-OE) bound ∼3-fold more anti-FHbp MAb than the parent wild-type strain (22).
Human complement sources.
The complement used to measure bactericidal activity was serum from a healthy adult with normal total hemolytic complement activity and no detectable serum bactericidal antibodies against the test strain. To eliminate nonbactericidal IgG antibodies that might augment or inhibit the activity of the test MAbs, the serum was depleted of IgG using a protein G column (HiTrap protein G; GE Life Sciences, Piscataway, NJ), which was performed as previously described (37). The IgG-depleted fraction had a >95% decrease in the IgG concentration and an ∼30% decrease in hemolytic complement activity, which resulted, in part, from dilution of the serum. To compensate for the lower CH50 activity (the CH50 measures the total hemolytic activity of a test sample and is the reciprocal of the dilution of serum complement needed to lyse 50% of a standardized suspension of sheep erythrocytes coated with antierythrocyte antibody), we added 12 μl of the IgG-depleted serum to the 40-μl (30%) bactericidal reaction mixture instead of 8 μl (20%) of nondepleted serum (see below).
Serum bactericidal assay.
Human complement-mediated bactericidal activity was measured as previously described (30, 37) using group B strain H44/76-WT or the H44/76-OE mutant described above with increased FHbp expression. In brief, the bacterial cells were grown in Mueller-Hinton broth to mid-log phase, harvested, and resuspended in buffer as described elsewhere (38). Immediately before the assay was performed, the MAbs were centrifuged for 2 h at 100,000 rpm to remove possible aggregates, since the aggregation of antibodies that occurs during development and storage may lead to the loss of biological activity (39). The 40-μl bactericidal reaction mixture contained 1 to 100 μg of MAb/ml, ca. 300 to 400 CFU of bacteria, and 30% IgG-depleted human complement (see above). The concentration resulting in 50% bactericidal activity (BC50) was defined by the MAb concentration that resulted in a 50% decrease in the number of CFU per milliliter after a 60-min incubation in the reaction mixture compared to the number of CFU per milliliter in the negative-control wells at 60 min.
C1q deposition on N. meningitidis.
Flow cytometry was used to measure the deposition of human C1q on the surfaces of live bacteria of the H44/76-WT strain or the H44/76-OE mutant strain (38). In brief, bacteria were grown, harvested as described previously (12), and resuspended in Dulbecco phosphate-buffered saline (PBS) containing 0.1 g of CaCl2/liter and 0.1 g of MgCl2 · 6H2O/liter (Mediatech), pH 7.4, with 1% (wt/vol) bovine serum albumin (BSA; Equitech-Bio, Kerrville, TX) (D-PBS-BSA) to a density of ∼108 cells/ml. The bacteria were incubated with 40 μg/ml of human complement C1q (Complement Technology, Tyler, TX). Different concentrations of the chimeric human-mouse MAbs diluted in D-PBS-BSA were added. After 45 min of incubation at room temperature, bound human C1q was detected with 1:100 dilutions of fluorescein isothiocyanate-conjugated anti-human C1q (Meridian Life Science, Memphis, TN).
Antibody binding activity measured by ELISA.
Binding of the anti-FHbp MAbs to FHbp (amino acid sequence variant ID 1, as designated on the website http://pubmlst.org/neisseria/FHbp/) was measured by ELISA with recombinant FHbp on the plate, which was performed as previously described (40). For measurement of anti-PorA MAb titers, we used native outer membrane vesicles (nOMVs) from strain H44/76. The secondary detecting antibody was goat anti-human Fc-specific antibody conjugated with alkaline phosphatase (MyBioSource, San Diego, CA) diluted 1:2,000 in PBS-BSA and incubated for 1 h at room temperature.
RESULTS
Characterization of chimeric IgG antibodies.
The antibodies were isolated from the supernatant of transfected HEK293 cells using a protein G column as previously described (38). After purification and concentration, the protein preparations were analyzed by SDS-PAGE and size exclusion chromatography, showing the expected molecular weights (data not shown). The schematic structures of IgG1, IgG3, IgG3m15, and IgG3m17 are shown in Fig. 1.
FIG 1.
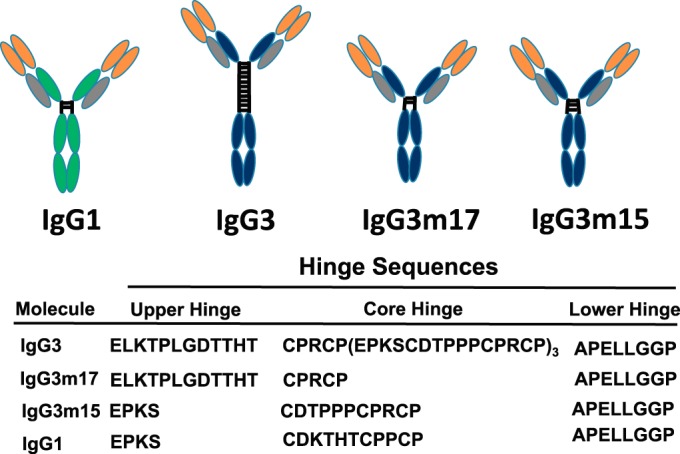
Schematic depiction of the structure of human IgG1, IgG3, and the IgG3m17 and IgG3m15 mutant antibodies. The length and amino acid sequence of the hinge region of the IgG3 variants are also shown. The functional hinge consists of the upper hinge, which is from the end of the CH1 domain to the N-terminal S-S bridge (cystine); the core hinge, which is from the N-terminal S-S bridge to the end of the beginning of the CH2 domain; and the lower hinge, which is the 8 N-terminal amino acids of the CH2 domain (47, 60). The genetic hinge consists of the upper hinge and the core hinge. Gray, the constant κ light chains; green, the constant IgG1 heavy chains; blue, the constant IgG3 heavy chains; orange, variable regions.
The PorA antigen has a higher level of expression on live H44/76 meningococci than FHbp.
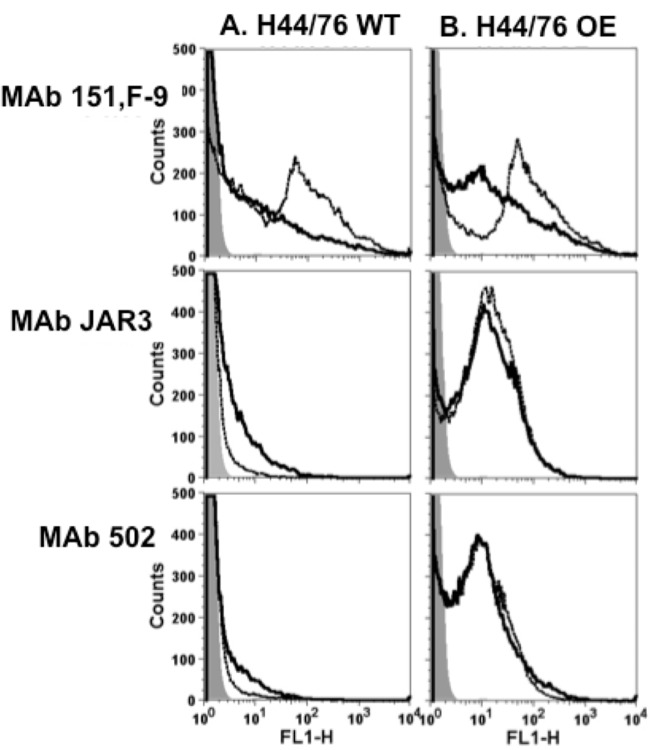
The level of PorA and FHbp expression by meningococcal strain H44/76-WT was analyzed by flow cytometry. The analysis showed an ∼10-fold higher level of expression of PorA than FHbp (Fig. 2). These results are consistent with previous estimates of the much lower level of expression of FHbp than PorA in native outer membrane vesicle preparations, as measured by SDS-PAGE and quantitative Western blotting (16, 41).
FIG 2.
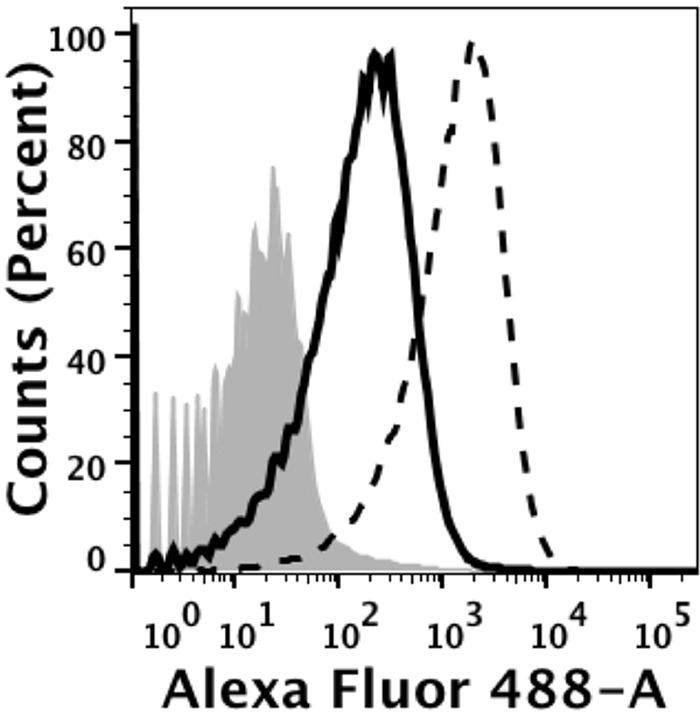
Binding of anti-FHbp MAb JAR3 and anti-P1.16 PorA MAb 151,F-9 (10 μg/ml each) to live bacteria of group B strain H44/76-WT, as measured by flow cytometry. Solid black line, anti-FHbp chimeric human-mouse JAR3 IgG1 MAb; dashed black line, anti-PorA 151,F-9 IgG1 MAb; gray filled area, bacteria only. The binding of the anti-PorA MAb is ∼10-fold greater than the binding of anti-FHbp MAb.
Chimeric IgG1, IgG3, IgG3m15, and IgG3m17 MAbs show binding activity similar to that of their respective target PorA P1.16 and FHbp antigens.
The binding activity of MAbs to target antigens on bacteria is one of several factors that influence the effector functions of antibodies, such as complement-mediated serum bactericidal activity. We therefore tested IgG1, IgG3, IgG3m15, and IgG3m17 anti-FHbp MAbs for their binding activity to recombinant FHbp using surface plasmon resonance. Overall, JAR3 had a higher affinity (an ∼5- to 14-fold lower equilibrium dissociation constant [KD]) than MAb502 (Table 1). However, for each MAb, the respective functional affinities of both isotypes and the IgG3 hinge mutants were not significantly different from each other (Table 1). For the recombinant anti-PorA 151,F-9 MAbs, we lacked purified recombinant PorA that expressed the P1.16 epitope, which prevented us from measuring binding affinity by surface plasmon resonance. Instead, we tested the binding of IgG1, IgG3, IgG3m15, and IgG3m17 by ELISA using nOMVs from strain H44/76 that were adhered to the wells of the microtiter plate. The respective concentration-dependent binding was indistinguishable (Fig. 3).
TABLE 1.
IgG3m15 and IgG3m17 anti-FHbp MAbs show binding affinity similar to that of IgG3 and IgG1 anti-FHbp MAbs as measured by SPRa
| MAb | ka (1/Ms) | kd (1/s) | KD | χ2 |
|---|---|---|---|---|
| MAb502 IgG1 | 1.23 E+6 | 0.0063 | 5.12 E−9 | 1.97 |
| MAb502 IgG3 | 1.46 E+6 | 0.0065 | 4.57 E−9 | 3.98 |
| MAb502 IgGm15 | 1.05E+6 | 0.0061 | 5.75 E−9 | 3.06 |
| MAb502 IgG1m17 | 1.06 E+6 | 0.0065 | 6.10 E−9 | 3.77 |
| JAR3 IgG1 | 1.92 E+6 | 0.0007 | 0.36 E−9 | 5.60 |
| JAR3 IgG3 | 1.94 E+6 | 0.0016 | 0.84 E−9 | 0.15 |
| JAR3 IgG3m17 | 2.80 E+6 | 0.0021 | 0.76 E−9 | 2.56 |
The data shown are the mean values for 2 replicates performed in two independent experiments. KD, equilibrium dissociation constant; ka, association rate constant; kd, dissociation rate constant; χ2, quality of the fit of a 1:1 binding model to the data. The JAR3 IgG3m15 MAb construct was not expressed by the cell line, and the anti-PorA P1.16 MAbs were not tested because we lacked recombinant PorA that expressed the P1.16 epitope.
FIG 3.
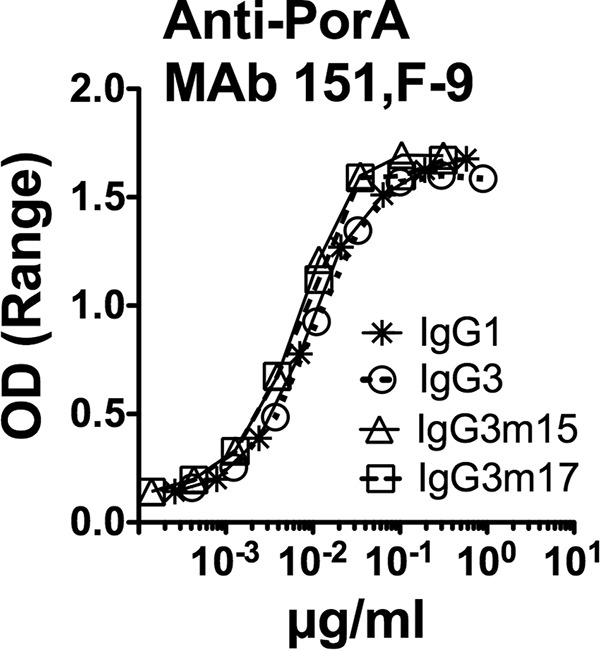
Concentration-depending binding of recombinant IgG1, IgG3, and IgG3m17 and IgG3m15 mutants of anti-P1.16 PorA MAb 151,F-9 to native OMV from serogroup B strain H44/76-WT, as measured by ELISA. The ranges of duplicate values for each data point are small and are not shown. OD, optical density.
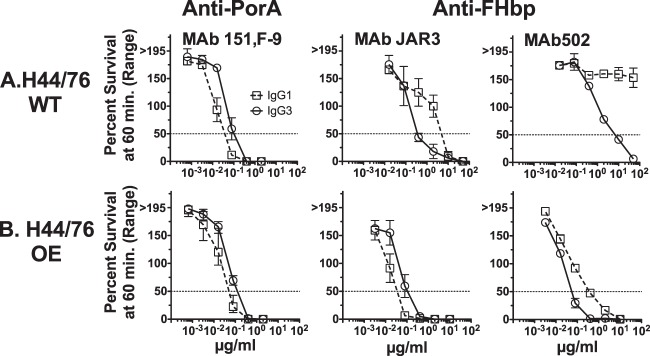
Chimeric IgG3 anti-FHbp has greater complement-mediated bactericidal activity than IgG1 MAbs, but the opposite is the case for anti-PorA Mabs.
Against the wild-type (WT) meningococcal group B strain H44/76, both the JAR3 and the MAb502 anti-FHbp MAbs with the IgG3 isotype had greater complement-mediated bactericidal activity than those with the respective IgG1 isotype (Fig. 4A). In contrast, for the anti-PorA MAb 151,F-9, it was the opposite: the IgG1 isotype had greater bactericidal activity than the IgG3 isotype.
FIG 4.
Survival of N. meningitidis group B strain H44/76 after 60 min of incubation with IgG1 or IgG3 anti-FHbp or anti-P1.16 PorA chimeric MAbs and 20% human complement. (A) (Left) The IgG1 anti-PorA MAb has greater bactericidal activity than the respective IgG3 anti-PorA MAb against H44/76-WT. The reverse is true for the anti-FHbp MAbs, where IgG3 JAR3 has greater activity then the respective IgG1 JAR3 (middle) and IgG3 MAb502 has greater activity than the respective IgG1 MAb502 (right). (B) The IgG1 JAR3 has greater activity than the IgG3 JAR3 against the H44/76-OE (FHbp) mutant; however, IgG3 MAb502 still had more activity than IgG1 MAb502.
Effect of increased FHbp expression on IgG subclass anti-FHbp MAb complement activation and bactericidal activity.
One possible explanation for the opposite bactericidal activities of the two IgG isotypes directed at different target antigens could be the difference in the expression levels of the PorA antigen and the FHbp antigen on live group B meningococcal strain H44/76-WT (Fig. 2). We therefore investigated the complement-mediated bactericidal activity of the two IgG isotypes against a mutant strain that overexpressed FHbp (H44/76-OE). As previously reported, the mutant group B strain H44/76-OE bound ∼3-fold more anti-FHbp MAb, as determined by flow cytometry, than the parent wild-type strain (42). The mutant H44/76-OE strain was similarly tested by flow cytometry for the expression of the PorA P1.16 epitope employing the MAb 151,F-9 and showed equal levels of expression on H44/76-WT and H44/76-OE (data not shown).
The H44/76-WT and H44/76-OE mutant strains showed similar susceptibilities to human complement-mediated bactericidal activity elicited by chimeric anti-PorA P1.16 MAbs (compare the left panels of Fig. 4A and B). In contrast, the mutant OE strain was >10-fold more susceptible than the H44/76-WT strain to anti-FHbp bactericidal activity (compare Fig. 4A, which shows the activity of the JAR3 and MAb502 MAbs against the WT strain, with Fig. 4B, which shows the respective activity against the H44/76-OE mutant). Further, against the OE mutant, the JAR3 MAb with the IgG1 isotype was more bactericidal than the JAR3 MAb with the IgG3 isotype. This result paralleled the respective results with the IgG1 and IgG3 isotypes of anti-PorA P1.16 MAb 151,F-9 against the H44/76-WT strain.
Deposition of complement C1q on live meningococcal strain H44/76 as a marker for classical pathway (CP) activation.
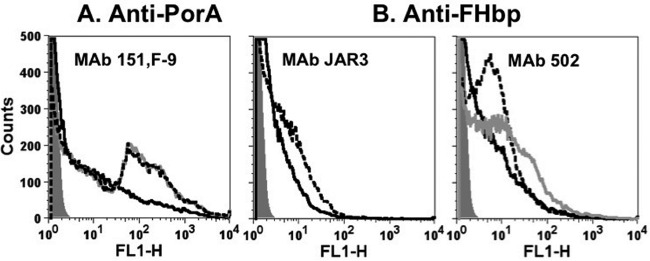
The deposition of complement factor C1q on the membrane of live strains H44/76 and H44/76-OE during complement activation induced by IgG1 and IgG3 MAbs 151,F-9, JAR3, and MAb502 was tested by flow cytometry. Overall, for the anti-FHbp MAbs, C1q deposition on the H44/76-OE strain was enhanced compared to that on H44/76-WT (Fig. 5), and this enhanced deposition paralleled the increase in anti-FHbp bactericidal activity against the OE mutant (Fig. 4B). With the OE mutant, similar C1q deposition was elicited by the respective IgG1 and IgG3 isotypes, while with the WT strain, IgG3 elicited more C1q than IgG1 for both the JAR3 and MAb502 MAbs. For the anti-PorA MAbs, the IgG1 subclass elicited more C1q deposition than the IgG3 subclass independently of FHbp expression.
FIG 5.
Effect of IgG1 and IgG3 MAbs on deposition of human C1q on live bacterial cells of group B strains H44/76-WT and mutant H44/76-OE, as measured by flow cytometry. The bacteria were incubated with human complement and 10 μg/ml of chimeric MAbs. Black dashed lines, IgG1; black solid lines, IgG3. An irrelevant human MAb (10 μg/ml) was used as a control. (A) H44/76-WT; (B) H44/76-OE.
Role of the extended hinge region of IgG3 in complement-mediated bactericidal activity against group B meningococcal strain H44/76.
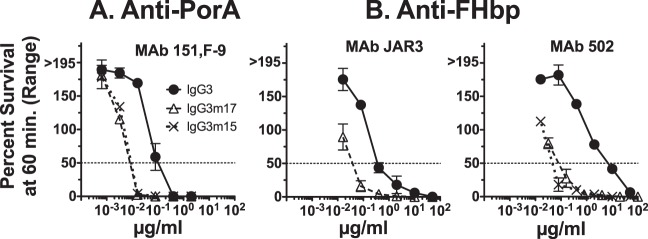
The human IgG3 isotype has an extended hinge region of 62 amino acids, whereas the human IgG1 isotype has an extended hinge region of 15 amino acids (Fig. 1) (24, 25). In order to study the role of the extended hinge region of IgG3, we therefore created two IgG3 hinge-truncated mutants, IgG3m15 and IgG3m17 (Fig. 1). The IgG3m15 mutant contained a 15-amino-acid hinge region encoded by one of the identical hinge exons h2, h3, or h4, while the IgG3m17 had a hinge encoded by hinge exon 1 (Fig. 1) (25). The complement-mediated bactericidal activity of both hinge-truncated IgG3 mutants was enhanced for both the FHbp and PorA specificities. We were able to create both IgG3m15 and IgG3m17 for the PorA MAb 151,F-9 and FHbp MAb502 (Fig. 6A and B, respectively) but only the IgG3m17 for the FHbp MAb JAR3 (Fig. 6B). We further tested by flow cytometry the deposition of C1q on live meningococcal strain H44/76-WT during complement activation and observed the same enhancement by the IgG3m15 and IgG3m17 mutants as that by the respective wild-type IgG3 (Fig. 7).
FIG 6.
Effect of IgG3 hinge truncation of anti-FHbp or anti-PorA P1.16 MAbs on survival of wild-type N. meningitidis group B strain H44/76 after 60 min of incubation with 20% human complement. Note that for JAR3, only WT IgG3 and IgG3m17 hinge-truncated MAbs were available.
FIG 7.
Deposition of human C1q on live bacterial cells of wild-type group B strain H44/76, as measured by flow cytometry. The bacteria were incubated with human complement and 10 μg/ml of chimeric WT IgG3 or the hinge-truncated mutants IgG3m17 and IgG3m15 MAbs. Black solid lines, WT IgG3; gray solid lines, IgG3m15; black dashed lines, IgG3 M17; gray filled histograms, bacteria without MAb, used as a negative control. Similar respective results were obtained in two independent experiments (data not shown).
DISCUSSION
Measurement of the SBA of antibodies elicited in response to vaccination is regarded as a reliable in vitro surrogate test for vaccine protection against meningococci (43) and has been used by regulatory agencies as a basis for licensing new vaccines (44, 45). SBA is the consequence of the antibody effector function and depends on the antigen specificity of the antibody, the isotype (class and subclass) of the antibody, the binding affinity of the antibody, and the glycosylation pattern of the antibody. The antibody functional activity derives from the antigen concentration and availability on the meningococcal cell surface as well as the intrinsic properties of the target antigen for the survival of the pathogen. Thus, specificity (antibody avidity and serum concentration) dominates the protective power of the antibodies formed during vaccination. The nature of the vaccine antigen and the formulation of the vaccine directly determine the isotype formed, which is usually a mixture of isotypes. Both vaccine antigens used in this study are proteins, which usually stimulate an IgG1 plus IgG3 response, where IgG1 normally dominates.
The fine specificity of anti-FHbp antibodies further determines whether they show SBA against meningococci. Thus, anti-FHbp antibodies that simultaneously inhibit FH binding to FHbp and activate complement deposition have greater SBA than antibodies that do not inhibit FH binding, as shown for monoclonal chimeric IgG1 antibodies (38) and recent studies of serum anti-FHbp antibodies elicited in human FH transgenic mice (46). The reason is probably that anti-FHbp complement activation via the classical pathway (CP) was insufficient to give SBA by itself, but the threshold was lowered when the antibody blocked FH binding, which permitted recruitment of the alternative pathway (AP) (38). Strikingly, when the parallel IgG3 isotype was tested, the non-FH-blocking antibody (MAb502) became bactericidal (22). The mechanism underlying the ability of MAb502 IgG3 to elicit an SBA against strain H44/76-WT while MAb502 IgG1 was completely negative (Fig. 3) (22) is not known. Apparently, the ability to deposit C4b on live bacteria (an indication of classical pathway complement activation) is not strikingly better for MAb502 IgG3 than for MAb502 IgG1 (22).
The mechanism behind the superior complement activation of IgG3 compared to that of IgG1 at a low antigen concentration on the target cell is poorly understood. One obvious reason might be because the hinge region of IgG3 (62 amino acids) is longer than that of the hinge region of IgG1 (15 amino acids) (Fig. 1). This feature naturally makes IgG3 more flexible than IgG1 (26) and supposedly gives IgG3 a better reach and adaptability, which might be particularly important at a low antigen concentration. In order to test this hypothesis in a clinical setting against meningococci and using the meningococcal outer membrane protein instead of an artificial, small hapten like NIP, we made two hinge-truncated mutants of IgG3 coding for the N-terminal hinge element (h1) or one of the succession of identical hinge elements (h2, h3, or h4), IgG3m17 and IgG3m15, respectively (Fig. 1). It is particularly important to compare IgG3m17 and IgG3m15, as they differ strikingly in the structure of the upper hinge (Fig. 1). The SBAs of both IgG3m17 and IgG3m15 were enhanced compared to the SBA of IgG3 for both chimeric anti-FHbp (both MAb502 and JAR3) and anti-P1.16 PorA antibodies (Fig. 6). We have previously tested the functional flexibility of IgG1, IgG3, and IgG3m15 (also called IgG3-15) by electron microscopy, analyzing the formation of ring dimer immune complexes (47). The enhanced formation of ring dimer immune complexes indicates flexibility, and IgG3m15 showed a low level of flexibility compared to WT IgG3 (47), which is probably a result of a short upper hinge of 4 amino acids of IgG3m15 (Fig. 1) (47). The length of the upper hinge seems to directly dictate the IgG flexibility, as also measured by fluorescence anisotropy (48). On the basis of the findings of our previous study, the IgG3m17 mutant is expected to have a high degree of flexibility, as also observed by fluorescence anisotropy (48), as it has an upper hinge region of 12 amino acids identical to that of IgG3 (Fig. 1). Thus, neither a long hinge giving a long spacer between Fab and Fc nor the high degree of flexibility of IgG3 is necessary for the superior SBA of IgG3 compared to that of IgG1 when the antigen concentration on the target cell is low, as is the case for the anti-FHbp antibodies. It is also unlikely the IgG3 has its C1q binding site partly shielded compared to the C1q binding sites of IgG3m15 and IgG3m17, since this site overlaps the binding site of classical FcγRs and IgG3 and IgG3m15 have the same antibody-mediated cellular cytotoxicity (ADCC) by engaging FcγRIIIa on NK cells (49).
Meningococci protect themselves from complement attack by expressing FHbp and, to a lesser extent, NspA (50) and PorB (51, 52), while human cells protect themselves by expressing three membrane-bound complement-regulatory proteins, CD59, CD55, and CD46. Recently, it has been shown that human IgG3 antibodies against epidermal growth factor receptor can efficiently perform complement-mediated lysis of tumor cells that express CD55 by activation of a C1q-dependent alternative pathway, while human IgG1 was inefficient (53). Similarly, we have earlier shown that chimeric IgG3 against an osteosarcoma antigen can elicit complement-mediated lysis of tumor cells better than IgG1 and that IgG3m15 elicited lysis even better than IgG3 (54).
The IgG effector function, such as complement activation, is highly dependent on glycosylation at the asparagine at position 297 in the Fc region (55). In the present study, we used the same recombinant technology, host cell transfection, and growth conditions to achieve nonbiased glycosylation of the recombinant antibodies, which otherwise can be altered (56). The differences in the functional activities of the IgG1, IgG3, IgG3m15 mutant, and IgG3m17 mutant MAbs against the meningococcal PorA and FHbp observed in our study therefore most likely result solely from the differences in the protein structures of the antibody molecules.
Human IgG1 and IgG3 share the amino acid residues that make up the epicenter of the complement activation site on the Fc part of the molecules (Asp270, Lys322, Pro329, and Pro331), but parallel mutations in other common surrounding and shared amino acids in IgG1 and IgG3 indicate that there are subtle differences in the complement activation site of human IgG1 and IgG3 (57). However, IgG1 is much more dependent on Asp270 than IgG3, and thus, the IgG3D270A mutant (which carries a mutation from aspartic acid to alanine at position 270) maintains most of the complement activation activity, while the IgG1D270A mutant is devoid of complement activation activity, and this difference is independent of the long hinge region of IgG3 (57).
Interestingly, IgG3 has attracted much attention in the field of vaccinology since a human IgG3 response correlates with protection (30% protection) in the only protective HIV vaccine trial published to date, thus pointing to a very important role of IgG3 in virus vaccine protection, at least for HIV (58). However, humans immunized with recombinant FHbp antigens primarily develop IgG1 antibody responses with only modest IgG3 (and IgG2) responses (D. M. Vu, unpublished data), whereas those immunized with OMV vaccines develop both serum IgG1 and IgG3, where IgG1 dominates but booster immunizations for IgG3 are often needed, as IgG3 fades faster than IgG1 (59).
As for the truncated hinge region of IgG3, the bactericidal activity of IgG3m15 and IgG3m17 mutants of MAb JAR3, MAb502 against FHbp, and MAb 151,F-9 against PorA P1.16 were equally enhanced compared to that of their parallel wild-type IgG3 antibodies (Fig. 6 and 7). However, the intrinsic higher molecular flexibility of IgG3 than the IgG3m15 mutant (26, 47) appears not to have been beneficial for the complement-mediated bactericidal activity IgG3 compared with that of the IgG3m15 mutant observed in this study. When an antigen is abundant on the cell surface, as for PorA, more epitopes are available and closer to each other. Thus, the extended hinge region of IgG3 could negatively affect complement activation due to steric hindrance, while the shorter hinge region of IgG1 could favor engagement of C1q. However, this is not the case for the engagement of FcγR on neutrophils, where IgG1 is less active than IgG3 in inducing the phagocytosis of meningococci when directed against PorA (23). Perhaps the long hinge region of IgG3 combined with the high level of PorA expression makes the deposition of the membrane attack complex (MAC) more distant from the outer membrane and thus mediates less SBA.
In summary, we compared the SBA of chimeric monoclonal human antibodies (IgG1, IgG3, IgG3m15, and IgG3m17) against two different meningococcal outer membrane proteins, FHbp (a monomeric antigen with generally low expression levels in meningococci) and PorA (a trimeric antigen with high expression levels). Against PorA, IgG1 performed better than IgG3, while against FHbp, IgG3 performed better than IgG1. For the hinge-truncated mutants, both IgG3m15, with a hinge region of 15 amino acids, and IgG3m17, with a hinge of 17 amino acids, performed better than IgG3, with a hinge region of 62 amino acids, irrespective of antibody specificity. Thus, the high SBA of IgG3 at a low antigen concentration on target cells does not need a long hinge region or a high degree of flexibility of the antibody to maintain its enhanced complement-mediated bactericidal activity against meningococci. The long hinge region of IgG3 seems, rather, to dampen the inherited high complement activation capability of IgG3 Fc through an unknown mechanism.
ACKNOWLEDGMENTS
The work was supported by grants R01 AI046464 (to D.M.G.), R01 AI099125 (to P.T.B.), and R01 AI114701 (to D.M.G. and P.T.B.) from the National Institute of Allergy and Infectious Diseases, NIH. The work was performed in a facility funded by Research Facilities Improvement Program grant C06 RR016226 from the National Center for Research Resources, NIH.
REFERENCES
- 1.Frasch CE, Zollinger WD, Poolman JT. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis 7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 2.Wang JF, Caugant DA, Li X, Hu X, Poolman JT, Crowe BA, Achtman M. 1992. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People's Republic of China. Infect Immun 60:5267–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JF, Caugant DA, Morelli G, Koumare B, Achtman M. 1993. Antigenic and epidemiologic properties of the ET-37 complex of Neisseria meningitidis. J Infect Dis 167:1320–1329. doi: 10.1093/infdis/167.6.1320. [DOI] [PubMed] [Google Scholar]
- 4.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001–1008. doi: 10.1016/0264-410X(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 5.Milagres LG, Gorla MC, Sacchi CT, Rodrigues MM. 1998. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect Immun 66:4755–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenqvist E, Hoiby EA, Wedege E, Bryn K, Kolberg J, Klem A, Ronnild E, Bjune G, Nokleby H. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun 63:4642–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman JT, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527. [DOI] [PubMed] [Google Scholar]
- 8.van der Ley P, Heckels JE, Virji M, Hoogerhout P, Poolman JT. 1991. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun 59:2963–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saukkonen K, Leinonen M, Abdillahi H, Poolman JT. 1989. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine 7:325–328. doi: 10.1016/0264-410X(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 10.Pajon R, Beernink PT, Harrison LH, Granoff DM. 2010. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 28:2122–2129. doi: 10.1016/j.vaccine.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. 2011. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl Trop Dis 5:e1302. doi: 10.1371/journal.pntd.0001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsch JA, Ram S, Koeberling O, Granoff DM. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis 197:1053–1061. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 13.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vu DM, Pajon R, Reason DC, Granoff DM. 2012. A broadly cross-reactive monoclonal antibody against an epitope on the N-terminus of meningococcal fHbp. Sci Rep 2:341. doi: 10.1038/srep00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsch JA, Rossi R, Comanducci M, Granoff DM. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol 172:5606–5615. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 16.Pajon R, Fergus AM, Granoff DM. 2013. Mutant native outer membrane vesicles combined with a serogroup A polysaccharide conjugate vaccine for prevention of meningococcal epidemics in Africa. PLoS One 8:e66536. doi: 10.1371/journal.pone.0066536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton DR, Gregory L, Jefferis R. 1986. Aspects of the molecular structure of IgG subclasses. Monogr Allergy 19:7–35. [PubMed] [Google Scholar]
- 18.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. 1987. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidarsson G, Dekkers G, Rispens T. 2014. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelsen TE, Garred P, Aase A. 1991. Human IgG subclass pattern of inducing complement-mediated cytolysis depends on antigen concentration and to a lesser extent on epitope patchiness, antibody affinity and complement concentration. Eur J Immunol 21:11–16. doi: 10.1002/eji.1830210103. [DOI] [PubMed] [Google Scholar]
- 21.Garred P, Michaelsen TE, Aase A. 1989. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand J Immunol 30:379–382. doi: 10.1111/j.1365-3083.1989.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 22.Giuntini S, Reason DC, Granoff DM. 2012. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein. Infect Immun 80:187–194. doi: 10.1128/IAI.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaelsen TE, Ihle O, Beckstrom KJ, Herstad TK, Kolberg J, Hoiby EA, Aase A. 2003. Construction and functional activities of chimeric mouse-human immunoglobulin G and immunoglobulin M antibodies against the Neisseria meningitidis PorA P1.7 and P1.16 epitopes. Infect Immun 71:5714–5723. doi: 10.1128/IAI.71.10.5714-5723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelsen TE, Frangione B, Franklin EC. 1977. Primary structure of the “hinge” region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J Biol Chem 252:883–889. [PubMed] [Google Scholar]
- 25.Huck S, Fort P, Crawford DH, Lefranc MP, Lefranc G. 1986. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C gamma genes. Nucleic Acids Res 14:1779–1789. doi: 10.1093/nar/14.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux KH, Strelets L, Michaelsen TE. 1997. Flexibility of human IgG subclasses. J Immunol 159:3372–3382. [PubMed] [Google Scholar]
- 27.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor H-binding protein. Infect Immun 76:4232–4240. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuliani MM, Santini L, Brunelli B, Biolchi A, Arico B, Di MF, Cartocci E, Comanducci M, Masignani V, Lozzi L, Savino S, Scarselli M, Rappuoli R, Pizza M. 2005. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect Immun 73:1151–1160. doi: 10.1128/IAI.73.2.1151-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarselli M, Cantini F, Santini L, Veggi D, Dragonetti S, Donati C, Savino S, Giuliani MM, Comanducci M, Di Marcello F, Romagnoli G, Pizza M, Banci L, Rappuoli R. 2009. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J Mol Biol 386:97–108. doi: 10.1016/j.jmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Beernink PT, LoPasso C, Angiolillo A, Felici F, Granoff D. 2009. A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol Immunol 46:1647–1653. doi: 10.1016/j.molimm.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Jarvis GA, Achtman M, Rosenqvist E, Michaelsen TE, Aase A, Griffiss JM. 2000. Functional activities and immunoglobulin variable regions of human and murine monoclonal antibodies specific for the P1.7 PorA protein loop of Neisseria meningitidis. Infect Immun 68:1871–1878. doi: 10.1128/IAI.68.4.1871-1878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Elsen J, Vandeputte-Rutten L, Kroon J, Gros P. 1999. Bactericidal antibody recognition of meningococcal PorA by induced fit. Comparison of liganded and unliganded Fab structures. J Biol Chem 274:1495–1501. [DOI] [PubMed] [Google Scholar]
- 33.Ihle O, Beckstrom KJ, Michaelsen TE. 2003. Cloning, sequencing and expression of immunoglobulin variable regions of murine monoclonal antibodies specific for the P1.7 and P1.16 PorA protein loops of Neisseria meningitidis. Scand J Immunol 57:453–462. doi: 10.1046/j.1365-3083.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- 34.Norderhaug L, Olafsen T, Michaelsen TE, Sandlie I. 1997. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J Immunol Methods 204:77–87. doi: 10.1016/S0022-1759(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 35.Pagotto FJ, Salimnia H, Totten PA, Dillon JR. 2000. Stable shuttle vectors for Neisseria gonorrhoeae, Haemophilus spp. and other bacteria based on a single origin of replication Gene 244:13–19. [DOI] [PubMed] [Google Scholar]
- 36.Koeberling O, Giuntini S, Seubert A, Granoff DM. 2009. Meningococcal outer membrane vesicle vaccines derived from mutant strains engineered to express factor H binding proteins from antigenic variant groups 1 and 2. Clin Vaccine Immunol 16:156–162. doi: 10.1128/CVI.00403-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 79:3751–3759. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hari SB, Lau H, Razinkov VI, Chen S, Latypov RF. 2010. Acid-induced aggregation of human monoclonal IgG1 and IgG2: molecular mechanism and the effect of solution composition. Biochemistry 49:9328–9338. doi: 10.1021/bi100841u. [DOI] [PubMed] [Google Scholar]
- 40.Beernink PT, Shaughnessy J, Ram S, Granoff DM. 2010. Impaired immunogenicity of a meningococcal factor H-binding protein vaccine engineered to eliminate factor H binding. Clin Vaccine Immunol 17:1074–1078. doi: 10.1128/CVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zollinger WD, Donets MA, Schmiel DH, Pinto VB, Labrie JE III, Moran EE, Brandt BL, Ionin B, Marques R, Wu M, Chen P, Stoddard MB, Keiser PB. 2010. Design and evaluation in mice of a broadly protective meningococcal group B native outer membrane vesicle vaccine. Vaccine 28:5057–5067. doi: 10.1016/j.vaccine.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Carulli G, Ottaviano V, Cannizzo E, Giuntini S, Manetti C, Ciancia EM, Azzara A. 2012. Multiparameter immunophenotyping by flow cytometry as a diagnostic tool in multiple myeloma and monoclonal gammopathy of undetermined significance. Clin Ter 163:387–392. [PubMed] [Google Scholar]
- 43.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrow R, Andrews N, Goldblatt D, Miller E. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 69:1568–1573. doi: 10.1128/IAI.69.3.1568-1573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frasch CE, Borrow R, Donnelly J. 2009. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 27(Suppl 2):B112–B116. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 46.Costa I, Pajon R, Granoff DM. 2014. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5:e01625-14. doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux KH, Strelets L, Brekke OH, Sandlie I, Michaelsen TE. 1998. Comparisons of the ability of human IgG3 hinge mutants, IgM, IgE, and IgA2, to form small immune complexes: a role for flexibility and geometry. J Immunol 161:4083–4090. [PubMed] [Google Scholar]
- 48.Tan LK, Shopes RJ, Oi VT, Morrison SL. 1990. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci U S A 87:162–166. doi: 10.1073/pnas.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michaelsen TE, Aase A, Norderhaug L, Sandlie I. 1992. Antibody dependent cell-mediated cytotoxicity induced by chimeric mouse-human IgG subclasses and IgG3 antibodies with altered hinge region. Mol Immunol 29:319–326. doi: 10.1016/0161-5890(92)90018-S. [DOI] [PubMed] [Google Scholar]
- 50.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog 6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giuntini S, Pajon R, Ram S, Granoff DM. 2015. Binding of complement factor H to PorB3 and NspA enhances resistance of Neisseria meningitidis to anti-factor H binding protein bactericidal activity. Infect Immun 83:1536–1545. doi: 10.1128/IAI.02984-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. 2013. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 4:e00339-13. doi: 10.1128/mBio.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosner T, Lohse S, Peipp M, Valerius T, Derer S. 2014. Epidermal growth factor receptor targeting IgG3 triggers complement-mediated lysis of decay-accelerating factor expressing tumor cells through the alternative pathway amplification loop. J Immunol 193:1485–1495. doi: 10.4049/jimmunol.1400329. [DOI] [PubMed] [Google Scholar]
- 54.Olafsen T, Munthe Lund CK, Bruland OS, Sandlie I, Michaelsen TE. 1999. Complement-mediated lysis of cultured osteosarcoma cell lines using chimeric mouse/human TP-1 IgG1 and IgG3 antibodies. Cancer Immunol Immunother 48:411–418. doi: 10.1007/s002620050594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jefferis R. 2007. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther 7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 56.Vestrheim AC, Moen A, Egge-Jacobsen W, Bratlie DB, Michaelsen TE. 2013. Different glycosylation pattern of human IgG1 and IgG3 antibodies isolated from transiently as well as permanently transfected cell lines. Scand J Immunol 77:419–428. doi: 10.1111/sji.12046. [DOI] [PubMed] [Google Scholar]
- 57.Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. 2009. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol 70:553–564. doi: 10.1111/j.1365-3083.2009.02338.x. [DOI] [PubMed] [Google Scholar]
- 58.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naess LM, Aarvak T, Aase A, Oftung F, Hoiby EA, Sandin R, Michaelsen TE. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754–764. doi: 10.1016/S0264-410X(98)00259-X. [DOI] [PubMed] [Google Scholar]
- 60.Beale D, Feinstein A. 1976. Structure and function of the constant regions of immunoglobulins. Q Rev Biophys 9:135–180. doi: 10.1017/S0033583500002390. [DOI] [PubMed] [Google Scholar]