Abstract
Osteosarcoma is the bone tumor that most commonly affects children, adolescents, and young adults. Before 1970, treatment primarily included surgical resection. However, the introduction of chemotherapy led to a dramatic improvement in prognosis for patients with localized osteosarcoma; long-term survival rates of less than 20% improved to 65% to 70% after the advent of multiagent chemotherapy regimens. Controversy concerning the ideal combination of chemotherapy agents ensued throughout the last quarter of the 20th century because of conflicting and often nonrandomized data. However, large cooperative group studies and international collaboration have demonstrated that the most effective regimens include the combination of high-dose methotrexate, doxorubicin, and cisplatin (MAP). The introduction of biologic agents such as muramyl tripeptide and the use of additional cytotoxic chemotherapy such as ifosfamide have not definitively improved the survival of patients with osteosarcoma. Collaborative efforts to increase understanding of the biology of osteosarcoma and the use of preclinical models to test novel agents will be critical to identify the path toward improving outcomes for patients. Once promising agents are identified, an international infrastructure exists for clinical trials. Herein, biologic, preclinical, and clinical trial efforts will be described along with future international collaborative strategies to improve outcomes for patients who develop this challenging tumor.
INTRODUCTION
Osteosarcoma is the primary malignant bone tumor that most commonly affects children, adolescents, and young adults. Osteosarcoma exhibits a predilection to occur in the metaphysis of long bones, and most commonly occurs in the distal femur (43%), proximal tibia (23%), or humerus (10%).1 The typical presentation includes onset of pain and swelling in the affected bone; a hallmark of the pain is that it is sufficiently intense to wake the patient from sleep. Occasionally, patients will present dramatically, with the onset of severe pain or other signs associated with a pathologic fracture. Approximately 15% to 20% of patients will have clinically detectable metastases at presentation. More than 85% of metastatic disease occurs in the lung, the most common site of metastasis, whereas bone is the second most common site of distant disease.1
The treatment for osteosarcoma was described in the medical literature as early as the mid-19th century. Since that time, treatment has advanced from amputation to complex limb-sparing surgeries and has incorporated multiagent chemotherapy. However, the pathway to current standard therapy, as well as the advancement of treatment to improve prognosis, has been something of a maze; there have been complicated turns and walls have been frequently hit that have slowed progress, particularly during the last 30 years. Fortunately, collaboration between cooperative groups across the United States and Europe has led to more unified treatment strategies and most recently included a trial that spanned continents, with the goal of influencing prognosis.
Osteosarcoma Treatment Strategies
The current management strategy for newly diagnosed osteosarcoma includes neoadjuvant chemotherapy followed by surgical removal of the primary tumor along with all clinically evident metastatic disease, plus the addition of adjuvant chemotherapy after surgery. This seemingly straightforward approach has developed out of sequential clinical trials that assessed the importance and type of chemotherapy and timing of surgery. Multiple clinical trials conducted throughout the 1970s and early 1980s demonstrated improved prognosis using single-agent and combination chemotherapy.2 However, in a retrospective analysis at the Mayo Clinic, an improvement in prognosis was described that was independent of adjuvant chemotherapy, with a 35% to 40% reported survival rate.3 This led investigators at the Mayo Clinic to conduct a randomized clinical trial through the late 1970s that failed to demonstrate a benefit from methotrexate and raised a general concern about the use of historic controls4 as a basis for claiming benefit or lack of benefit of a treatment. Therefore, the Multi-Institutional Osteosarcoma Study (MIOS) was developed and definitively demonstrated the superiority of chemotherapy plus surgery compared with surgery alone. MIOS was conducted from 1982 to 1984, and 113 patients were randomly assigned to receive chemotherapy after surgery versus surgery alone. The results of this trial demonstrated an 11% 6-year survival rate for those who received surgery alone compared with a 61% 6-year survival rate for the group that received surgery and adjuvant chemotherapy.5
During this same time period, investigators focused on efforts to increase the number of patients receiving limb-sparing surgeries. At Memorial Sloan Kettering Cancer Center (MSKCC), neoadjuvant chemotherapy was introduced in the T10 protocol in an effort to allow extra time for construction of prosthetic devices, which also had the theoretical advantage of treating presumed micrometastatic disease that was present at diagnosis.6 The outcome of this trial was similar to that of MIOS, with a 65% survival rate at 5 years. In addition, data regarding the association between prognosis and histologic necrosis began to emerge in single- and multi-institutional studies in the United States and across Europe,7–9 supporting this general strategy with respect to the timing of surgery.
However, concerns regarding the possibility that the presence of a large tumor could lead to resistant cells or an increase in micrometastatic disease during neoadjuvant chemotherapy led the Pediatric Oncology Group to conduct a randomized clinical trial comparing neoadjuvant to adjuvant chemotherapy.10 The results of this study revealed a similar outcome for the adjuvant and neoadjuvant groups. On the basis of these results, the use of preoperative chemotherapy has become the standard of care, given its advantages: it allows time for surgical planning, potentially facilitates tumor removal, and permits evaluation of response to therapy.
Initial trials assessing good- and poor-responder groups revealed that those with more than 90% necrosis after neoadjuvant chemotherapy had a greater than 90% 5-year event-free survival.11,12 However, for patients with poor histologic response, considered to be less than 90% necrosis, the 5-year survival was only 50% to 60%.7,11–13 These data have been critical in attempts to identify patients who may benefit from therapy modifications that aim to increase the number of good responders after neoadjuvant therapy. An initial attempt to alter therapy indicated a benefit for those who had poor necrosis.8 However, longer follow-up of these patients14 and additional studies by other investigators have not been able to reproduce that result.13 Intensification of neoadjuvant chemotherapy increased the number of good responders but did not alter the overall survival and diminished the value of prognostication on the basis of histologic response.15 In addition, the European Osteosarcoma Intergroup (EOI) evaluated dose compression of neoadjuvant chemotherapy with the use of granulocyte colony-stimulating factor so that chemotherapy could be given every 2 versus every 3 weeks as a means to enhance tumor response. Although 50% of patients in the dose-compression group achieved good necrosis versus 36% of those who received chemotherapy according to standard timing, there was no survival advantage with dose compression.16
Choice of Chemotherapy
A variety of chemotherapy regimens have been used to treat osteosarcoma since chemotherapy was introduced more than 40 years ago. The regimen used in the MIOS trial included the MAP combination.5 Some controversy had existed regarding the importance of methotrexate in treating osteosarcoma, although many of the initial concerns were raised in nonrandomized studies.17,18 In addition, although a randomized EOI study did not show an advantage with inclusion of methotrexate, the dose used may have been suboptimal, and the survival of the patients in the study overall was poor.19 More recently, methotrexate has been accepted as a standard agent in North America and Europe, and at this point, the use of methotrexate is a historical debate that is no longer relevant in these regions.20 Marti et al21 first reported a 33% response rate for ifosfamide in lung recurrence of osteosarcoma; other groups subsequently confirmed its activity as monotherapy.22–24 Since that time, a variety of cooperative groups in the United States and Europe have incorporated ifosfamide into multiagent regimens, either alone or in combination with etoposide.2,25 However, the lack of benefit and increased toxicity seen in recent trials, which will be discussed subsequently, leaves ifosfamide as a useful option in the relapsed or refractory setting.
Lack of Improvement in Outcome in Phase III Clinical Trials
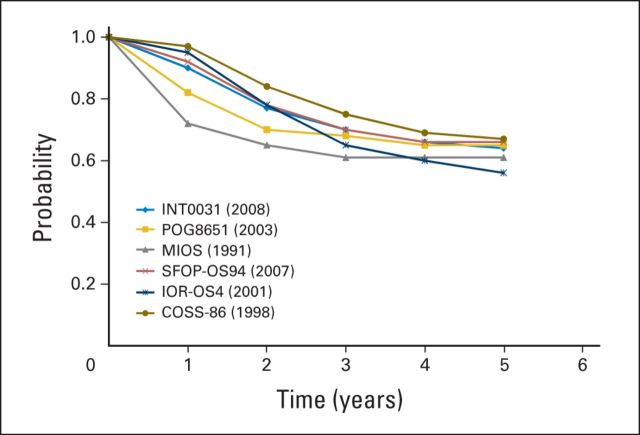
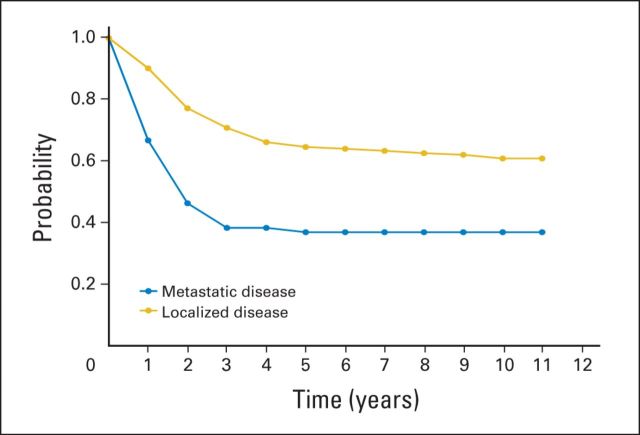
Unfortunately, since the mid-1980s, little progress has been made in improving the survival of patients diagnosed with osteosarcoma. This is readily observed in National Cancer Institute SEER program data, in which the 5-year event-free survival of patients age 15 to 19 years diagnosed with osteosarcoma was 60.6% between 1987 and 1990, 68.1% between 1991 and 1994, 61.7% between 1995 and 1998, and 66.4% between 1999 and 2002.26 In Europe, population-based data were similar for patients treated with osteosarcoma between 1988 and 1997: the 5-year survival rate for those age 15 to 19 years was 51%.27 These data can similarly be inferred by considering the major randomized phase III trials conducted during this interval of time, none of which have resulted in a change to the standard of care in North America (Fig 1). Lacking prognostic factors at the time of diagnosis other than the presence or absence of metastatic disease (Fig 2), numerous trials have been conducted in an attempt to augment chemotherapy in patients with an inferior histologic response, and in some cases, to reduce therapy in those with a favorable response. As mentioned previously, other than the initial MSKCC T10 study, none of the studies augmenting chemotherapy in poor responders has improved the outcome of patients with osteosarcoma. Similarly, reduction of therapy in favorable responders resulted in inferior survival outcomes. With the inability to change outcome after response, several studies have assessed the ability to improve the outcome of patients with osteosarcoma by intensifying preoperative chemotherapy to increase the proportion of patients with a favorable degree of necrosis. These studies, reported by the Rizzoli Institute, the EOI, and MSKCC, all succeeded in increasing the proportion of patients with a favorable histologic response but failed to improve patient survival. Other studies have explored the utility of specific chemotherapy agents or delivery of chemotherapy via intra-arterial routes,28,31 none of which has changed current treatment approaches. In addition, no clinical or biologic factors beyond the presence or absence of radiographically visible metastatic disease exist at the time of diagnosis with which to stratify patient therapy (Fig 2).
Fig 1.
Event-free survival by clinical trial and year published for patients with nonmetastatic osteosarcoma.5,10,25,28–30 COSS, Cooperative German-Austrian-Swiss Osteosarcoma Study Group; INT, Intergroup study; IOR, Istituto Ortopedico Rizzoli; MIOS, Multi-Institutional Osteosarcoma Study; POG, Periatric Oncology Group; SFOP, Société Française d'Oncologie Pédiatrique.
Fig 2.
Event-free survival for patients with metastatic and localized disease enrolled onto the Children's Cancer Group/Pediatric Oncology Group Intergroup study INT0133 clinical trial.
The most recently completed Children's Cancer Group/Pediatric Oncology Group trial INT-0133 was an evaluation in a 2 × 2 factorial design to determine whether the addition of ifosfamide and/or muramyl tripeptide–phosphatidyl ethanolamine to a standard high-dose methotrexate, cisplatin, and doxorubicin chemotherapy regimen improved outcomes for patients with osteosarcoma.29 Results of INT-0133 have been difficult to interpret because the original publication, reporting on event-free survival, suggested an interaction between the experimental strategies that precluded statistical analysis.11 However, additional follow-up and use of an overall survival end point suggested no evidence of such an interaction, and a significant improvement in overall survival was observed for patients receiving mifamurtide, regardless of chemotherapy regimen.29 This has created significant controversy, and although mifamurtide has been approved in Europe for the treatment of patients with osteosarcoma, its use there remains sporadic, and mifamurtide is unavailable in the United States. This study did clearly establish the lack of benefit of the addition of ifosfamide.
Collaborative Efforts to Improve Outcomes
Histologic response to preoperative chemotherapy was long identified as an important prognostic factor, with poor responders, usually defined as those with 10% or more of viable tumor remaining in the resected specimen, faring significantly worse than good responders. However, previous studies that modified treatment on the basis of histologic response were not randomized. It is estimated that any randomized trial focusing on either good or poor responders would require more than 600 to 700 patients each, bringing the total to well above 1,200 patients. Even the largest international groups would need more than a decade, and perhaps even several decades, to complete such trials individually. It therefore became obvious that any prospective randomized trial of response-guided treatment modifications would require large-scale, multisite, multinational, intergroup collaboration.
After discussions during the 2001 SIOP meeting in Brisbane, Australia, four multinational groups from North America and Europe, each with a long history of successful trials, decided to join forces in the European and American Osteosarcoma Study Group (EURAMOS). These were the Children's Oncology Group (COG), the Cooperative Osteosarcoma Study Group, the EOI, and the Scandinavian Sarcoma Group. Taken together, they represent 17 countries with a population of close to 600,000,000 individuals. The objectives of the study included determining whether the addition of high-dose ifosfamide and etoposide to postoperative chemotherapy would improve outcomes for patients whose osteosarcomas responded poorly to standard preoperative chemotherapy, and determining whether maintenance treatment with pegylated interferon alfa-2b after completion of chemotherapy would further improve the prognosis of good responders.32
To simplify decision-making processes, the four participating groups nominated one principal investigator (PI) each. These four PIs acted as spokespersons for their respective constituencies, thereby mitigating potential heterogeneities arising from within the individual groups. Along with other individuals, the PIs participated in a Trial Management Group, which drafted the protocol and was responsible for day-to-day work. Several other intergroup bodies, such as a Coordinating Data Center and an Intergroup Safety Desk, were implemented and took on essential trial functions.
Although the groups came from different backgrounds and had used different chemotherapy backbones in their previous trials,2,33 agreeing on a three-drug standard of high-dose MAP proved one of the lesser challenges. Standards and procedures were defined in a joint uniform study protocol and a joint common appendix. In addition, group-specific appendices allowed additional aspects to be addressed that were relevant to individual groups. Using well-established infrastructures, with each of the four groups' data centers acting as regional hubs, proved beneficial and allowed for the required flexibility. It soon became obvious that separate national coordinators were also needed for obtaining ethical and regulatory approval and national funding and, if required, insurance.
The EURAMOS-1 trial fell during a time period when transatlantic collaboration in oncology was confronted with increasingly complex regulatory requirements.34 In Europe, for example, it coincided with the European Union (EU) Clinical Trials Directive 2001/20. This directive's original aim was to simplify and harmonize administrative procedures governing trials across Europe. This harmonization was impeded and diluted during translation of the directive into national legislation in the EU's many member states. Although the directive did impose greatly increased bureaucratic and financial burdens for clinical trials, it did not result in the harmonized regulatory framework from which academic trials would have benefited.35,36 Legal sponsorship was one topic that was affected by heterogeneous interpretation of the EU Trials Directive, with some competent national agencies insisting that there be only a single sponsor for all of Europe, a responsibility that was finally taken by the Medical Research Council in London, United Kingdom. Transatlantic collaboration added an additional layer of complexity, which was exemplified by having to follow US requirements to obtain Federalwide Assurance for the Protection of Human Subjects for European sites.
Obtaining funding for such a multinational endeavor proved an additional challenge. Although various national funding schemes were in place, none would cover all aspects of such an international project. In the end, European participation only became possible through successful grant applications within the Pan-European Clinical Trials EUROCORES (European Collaborative Research) of the European Science Foundation.
The first patient for EURAMOS-1 was recruited early in 2005, and international recruitment approached the expected rate by the end of that year. Altogether, 2,260 patients from 326 institutions were recruited by June 2011,37 and the primary outcome measures from both the good-responder and the poor-responder random assignments have now been reported. In the good-responder cohort, no significant difference was observed between those randomly assigned to continue standard chemotherapy and those randomly assigned to continue chemotherapy plus pegylated interferon alfa-2b.38 Analysis of the poor responders has revealed an increase in toxicity, with more patients receiving lower cumulative doses of methotrexate and unable to complete therapy, and a higher rate of second malignant neoplasms, with no difference in outcome for those randomly assigned to receive high-dose ifosfamide and etoposide.39 Therefore, although histologic response does provide prognostic information, this information should not be used to guide decisions about postoperative systemic treatment and may not have a role in future clinical trials. As an additional outcome, the EURAMOS trial serves as a model for international collaboration, and the resulting infrastructure can be used for future collaborative trials.37
Biologic Understanding and Its Impact on Clinical Trials Development
Tremendous efforts have been made to define the biology of osteosarcoma in the hopes of developing therapeutic leads from that information. The combination of osteosarcoma genome complexity40 with the low incidence of these tumors is an obstacle to thorough investigation of osteosarcoma genome biology. When considering the full spectrum of osteosarcoma subtypes, it is clear that there are significant biologic differences among subtypes. When this complexity is coupled with the lack of an obvious precursor lesion, it is difficult to define the initiating events in osteosarcoma formation. That said, alterations in p53 are invariable, and alterations in the Rb pathway are almost invariable. This information, along with the fact that patients with Li-Fraumeni syndrome with germline p53 abnormalities and patients with hereditary retinoblastoma with germ-line Rb pathway alterations are predisposed to osteosarcoma, implicates these pathways as central to osteosarcomas pathogenesis. When sequencing analyses are performed on osteosarcomas, regions of hypermutation called kataegis are frequently present.40 Although alterations in p53 and Rb result in genetic instability, it is not clear if all of the complexity is driven by these alterations. Notably, a large genome-wide association study identified two loci associated with osteosarcoma development, neither of which has a clear relationship to these pathways.41
Given the genome complexity of high-grade osteosarcomas, it is certain that these highly diverse structural and numeric aberrations exert diverse biologic effects. However, accumulating sufficient biologic data to overcome the complexity problem requires large studies. One such collaborative program, the National Cancer Institute's Therapeutically Applicable Research to Generate Effective Treatments program (TARGET), is currently investigating osteosarcoma.42 A multi-institutional project team is generating data from clinically annotated samples using sequencing and microarray platforms to detect mutations (exome, whole genome, single nucleotide polymorphism, copy number), gene expression (mRNA, miRNA), and epigenetic modifications (DNA methylation). The goal of this study is to use integrated genomic analysis of a sufficiently large sample set to enable dissection of the heterogeneity present among high-grade osteosarcomas with respect to mutation, gene expression, and epigenetic signatures. Although existing information suggests that mutations in drug targets will occur at a low frequency, currently available data do not provide sufficient sample numbers to derive clinical correlations that impact clinical trial design or motivate new laboratory and translational investigations of osteosarcoma biology. It is hoped that TARGET and other large genomics studies will provide a rich, publicly available data resource with significant impact on the future of osteosarcoma research.
Current and Future Clinical Trials
Before investing significant resources to study a novel agent, substantial consideration of many factors should be considered. As described by Khanna et al, 43 rigorous preclinical data, including animal modeling and an understanding of mechanism of action, among other factors, will be critical when considering agents for clinical investigation. With this as the background, numerous preclinical studies have been performed or initiated to look for the various described levels of evidence, including expression of the target on osteosarcoma tumor samples, evidence of efficacy in preclinical mouse models, and evidence of efficacy in dogs that develop osteosarcoma spontaneously. Indeed, the study of ch14.18 that will be described subsequently was preceded by an analysis of GD2 expression in osteosarcoma tumor tissue. The Pediatric Preclinical Testing Program (PPTP), another example of advances through collaboration, is a National Cancer Institute–funded initiative to screen new drugs though patient-derived xenograft tumors grown in severe combined–immunodeficiency mice. Osteosarcoma is included in those screening efforts to identify eribulin and glembatumumab as agents with potential efficacy. Transgenic mouse models with alterations in receptor activator of nuclear factor κB ligand (RANKL) that develop osteosarcoma suggest the potential efficacy of denosumab. A comparative oncology effort will screen other agents in canines with osteosarcoma, including rapamycin, which, if efficacious, may be of interest for further development in human patients with osteosarcoma.
Following the aforementioned guidelines, COG has begun to develop clinical trials to evaluate a variety of agents in patients with osteosarcoma.44 At present, few new agents are recognized to have efficacy in the treatment of osteosarcoma. This is likely in part because of the relative resistance of osteosarcoma to chemotherapy, but may be related in part to the use of radiographic response as the primary end point in osteosarcoma phase II trials. Because of extensive calcified matrix, osteosarcoma that is responsive to therapy often does not shrink radiographically. Thus, the objective in subsequent studies will be to identify agents that demonstrate an improvement in the time to progression for patients with relapsed and refractory disease when compared with the consistent rapid progression seen in previous phase II studies of the mostly ineffective agents listed in Table 1. It should be noted that some recent trials, including a study of sorafenib reported by the Italian Sarcoma Group, seem to suggest activity of the experimental agent when using prolonged stabilization of disease as an end point, which is effectively a progression-free survival end point.54
Table 1.
Selected Phase II Trials in Osteosarcoma
| Study | No. of Patients With Osteosarcoma | Agent | Response | Years Study Open |
|---|---|---|---|---|
| A0971345 | 10 | Topotecan | No responses | 1995-1998 |
| ADVL012246 | 12 | Imatinib | No responses | 2002-2004 |
| ADVL042147 | 13 | Oxaliplatin | No responses | 2004-2006 |
| ADVL052448 | 11 | Ixabepilone | No responses | 2006-2007 |
| CCG-096249 | 23 | Docetaxel | 1 PR, 1 CR, 2 NE, 19 with no response | 1997-2001 |
| EORTC phase II50 | 15 | Iproplatin | 1 SD, 14 with no response | 1997-1998 |
| P976151 | 10 | Irinotecan | 9 with no response, 1 NE | 1999-2005 |
| P996352 | 16 | Rebeccamycin | No responses | 2000-2003 |
| Phase II ridaforolimus53 | NR | Ridaforolimus | 2 PR (total No. of patients NR) | 2004-2005 |
Abbreviations: ADVL, Children's Oncology Group Developmental Therapeutic committee; CCG, Children's Cancer Group; CR, complete response; EORTC, European Organisation for the Research and Treatment of Cancer; NE, not evaluable; NR, not reported; PR, partial response; SD, stable disease.
A phase II study of eribulin, a novel microtubule inhibitor, is now open through COG and available to adult-based cooperative groups using the Clinical Trials Support Unit through the National Cancer Institute. Eribulin has gained interest on the basis of data reported by the PPTP demonstrating complete responses in three of six osteosarcoma xenografts.55 This agent has been used successfully in patients with multiply relapsed breast cancer56 and has demonstrated activity in patients with soft tissue sarcoma.57 This phase II evaluation is currently enrolling patients and that process is expected to be complete in 2015.
Denosumab, a fully human monoclonal antibody against RANKL, has been demonstrated to have efficacy with advanced giant-cell tumor of bone. Interest in using denosumab for patients with osteosarcoma has since arisen on the basis of the aforementioned transgenic model. Further support is derived from transgenic mouse models with manipulations in the parathyroid hormone receptor, which affect RANK signaling. In addition, RANK signaling has been shown to be important in osteosarcoma cell growth and motility.58 A phase II clinical trial is expected to open in 2015 to assess the efficacy of denosumab in patients with refractory or relapsed osteosarcoma.
Glembatumumab vedotin is a novel antibody-auristatin conjugate that targets cells expressing the transmembrane glycoprotein GPNMB (glycoprotein nonmelanoma protein B, osteoactivin) and is present with a high level of activity in osteosarcoma cell lines. Preclinical testing in the PPTP demonstrated a statistically significant improvement in osteosarcoma xenograft event-free survival distribution compared with control; three of six xenografts demonstrated a maintained clinical response.59 On the basis of these data, a phase II clinical trial is being developed by COG to assess efficacy in patients with relapsed osteosarcoma.
A fourth trial in development through COG includes the use of an anti-GD2 (disialoganglioside) antibody.44 Therapy directed against GD2 has been typically used for patients with stage IV neuroblastoma, for which the anti-GD2 antibody ch14.18 has been shown to improve event-free survival when given in the setting of minimal residual disease. Because more than 95% of osteosarcoma tumors express GD2, this is a rational target for patients with osteosarcoma.60 Expected to open in 2015, this planned trial will include patients younger than age 30 years with relapsed osteosarcoma who have had a complete tumor resection.
In conclusion, the treatment of osteosarcoma has evolved greatly during the last 40 years. In early times, the treatment involved ablative surgery that was associated with a morbid outcome and with only a small probability of long-term survival. The recognition and understanding of the presence of micrometastatic disease, along with the advent of chemotherapy and complex surgical procedures, have led to tremendous progress in curing a greater proportion of patients while achieving an improved quality of life. However, the great progress that was seen in the 1970s and early 1980s has since stalled. There is now an increased focus on understanding the complex biology of osteosarcoma and identifying novel agents that have the best chance of success when efficacy is assessed. These efforts have been enhanced by collaborative initiatives such as the PPTP, TARGET, and EURAMOS. Using a new prioritization schema43 to identify agents that are believed to have the strongest rationale for efficacy testing will hopefully streamline resources and allow for more efficient progress in advancing treatment. In addition, continued international collaboration through the EURAMOS group will be critical to the future design of trials so that rapid and efficient accrual can occur while eliminating redundancy and consolidating efforts to improve prognosis for this rare disease. The currently open and future clinical trials will assess these new therapies and will increase hope that we will successfully navigate through the maze and see the increase in survival that has been so elusive in the past.
Footnotes
Supported by the Deutsche Forschungsgemeinschaft (DFG Reference Nos. BI 1045/1-1 and BI 1045/1-2; S.S.B.), Deutsche Krebshilfe (DKH Reference No. 50-2723-Bi2; S.S.B.), and the European Union's Seventh Framework Programme (Grant No. FP7/2007-2013; S.S.B.) under project European Network for Cancer Research in Children and Adolescents, Grant Agreement HEALTH-F2-2011-261474.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Michael S. Isakoff, Stefan S. Bielack, Richard Gorlick
Collection and assembly of data: Michael S. Isakoff, Paul Meltzer, Richard Gorlick
Data analysis and interpretation: Michael S. Isakoff, Richard Gorlick
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Osteosarcoma: Current Treatment and a Collaborative Pathway to Success
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Michael S. Isakoff
No relationship to disclose
Stefan S. Bielack
Consulting or Advisory Role: Celgene, Bayer, Chugai Pharma, Clinigen
Research Funding: Novartis (Inst), Janssen-Cilag (Inst)
Paul Meltzer
Research Funding: AstraZeneca, Ariad
Patents, Royalties, Other Intellectual Property: Monoclonal antibodies to NCOA3
Richard Gorlick
Stock or Other Ownership: Oncolytics Biotech
Consulting or Advisory Role: Oncolytics Biotech
REFERENCES
- 1.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Gorlick R, Bielack S, Teot LA, et al. Osteosarcoma: Biology, diagnosis, treatment, and remaining challenges. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology (ed 6) Philadelphia, PA: Lippincott Williams and Wilkins; 2010. pp. 1015–1044. [Google Scholar]
- 3.Taylor WF, Ivins JC, Pritchard DJ, et al. Trends and variability in survival among patients with osteosarcoma: A 7-year update. Mayo Clin Proc. 1985;60:91–104. doi: 10.1016/s0025-6196(12)60293-6. [DOI] [PubMed] [Google Scholar]
- 4.Edmonson JH, Green SJ, Ivins JC, et al. A controlled pilot study of high-dose methotrexate as postsurgical adjuvant treatment for primary osteosarcoma. J Clin Oncol. 1984;2:152–156. doi: 10.1200/JCO.1984.2.3.152. [DOI] [PubMed] [Google Scholar]
- 5.Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity: Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991:8–14. [PubMed] [Google Scholar]
- 6.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Bacci G, Avella M, Capanna R, et al. Neoadjuvant chemotherapy in the treatment of osteosarcoma of the extremities: Preliminary results in 131 cases treated preoperatively with methotrexate and cisdiamminoplatinum. Ital J Orthop Traumatol. 1988;14:23–39. [PubMed] [Google Scholar]
- 8.Rosen G, Marcove RC, Caparros B, et al. Primary osteogenic sarcoma: The rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Winkler K, Beron G, Kotz R, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: Results of a Cooperative German/Austrian study. J Clin Oncol. 1984;2:617–624. doi: 10.1200/JCO.1984.2.6.617. [DOI] [PubMed] [Google Scholar]
- 10.Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 11.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: A report from the Children's Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: Results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 14.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 16.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 17.Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: Prognostic factors and impact on survival. J Clin Oncol. 2006;24:1161–1168. doi: 10.1200/JCO.2005.03.5352. [DOI] [PubMed] [Google Scholar]
- 18.Tunn PU, Reichardt P. Chemotherapy for osteosarcoma without high-dose methotrexate: A 12-year follow-up on 53 patients. Onkologie. 2007;30:228–232. doi: 10.1159/000100776. [DOI] [PubMed] [Google Scholar]
- 19.Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: The first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579–1591. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe N, Gorlick R. High-dose methotrexate in osteosarcoma: Let the questions surcease—Time for final acceptance. J Clin Oncol. 2008;26:4365–4366. doi: 10.1200/JCO.2007.14.7793. [DOI] [PubMed] [Google Scholar]
- 21.Marti C, Kroner T, Remagen W, et al. High-dose ifosfamide in advanced osteosarcoma. Cancer Treat Rep. 1985;69:115–117. [PubMed] [Google Scholar]
- 22.Miser JS, Kinsella TJ, Triche TJ, et al. Ifosfamide with mesna uroprotection and etoposide: An effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5:1191–1198. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 23.Pratt CB, Douglass EC, Etcubanas EL, et al. Ifosfamide in pediatric malignant solid tumors. Cancer Chemother Pharmacol. 1989;24(suppl 1):S24–S27. doi: 10.1007/BF00253234. [DOI] [PubMed] [Google Scholar]
- 24.Schwartzman E, Scopinaro M, Angueyra N. Phase II study of ifosfamide as a single drug for relapsed paediatric patients. Cancer Chemother Pharmacol. 1989;24(suppl 1):S11–S12. doi: 10.1007/BF00253230. [DOI] [PubMed] [Google Scholar]
- 25.Le Deley MC, Guinebretière JM, Gentet JC, et al. SFOP OS94: A randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007;43:752–761. doi: 10.1016/j.ejca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiller CA, Bielack SS, Jundt G, et al. Bone tumours in European children and adolescents, 1978-1997: Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–2135. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–899. doi: 10.1023/a:1008391103132. [DOI] [PubMed] [Google Scholar]
- 29.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 30.Bacci G, Briccoli A, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: Long-term results of the Rizzoli's 4th protocol. Eur J Cancer. 2001;37:2030–2039. doi: 10.1016/s0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 31.Bacci G, Ferrari S, Tienghi A, et al. A comparison of methods of loco-regional chemotherapy combined with systemic chemotherapy as neo-adjuvant treatment of osteosarcoma of the extremity. Eur J Surg Oncol. 2001;27:98–104. doi: 10.1053/ejso.2000.1056. [DOI] [PubMed] [Google Scholar]
- 32.Marina N, Bielack S, Whelan J, et al. International collaboration is feasible in trials for rare conditions: The EURAMOS experience. Cancer Treat Res. 2009;152:339–353. doi: 10.1007/978-1-4419-0284-9_18. [DOI] [PubMed] [Google Scholar]
- 33.Bielack SS, Machatschek JN, Flege S, et al. Delaying surgery with chemotherapy for osteosarcoma of the extremities. Expert Opin Pharmacother. 2004;5:1243–1256. doi: 10.1517/14656566.5.6.1243. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins DS. Transatlantic collaboration in pediatric oncology: Now is the time. J Pediatr Hematol Oncol. 2004;26:783–784. [PubMed] [Google Scholar]
- 35.Cannell E. Clinical Trials Directive slows registration of paediatric studies. Lancet Oncol. 2007;8:10. doi: 10.1016/s1470-2045(06)70991-3. [DOI] [PubMed] [Google Scholar]
- 36.Carrle D, Dantonello T, Bielack SS. Pan-European sarcoma trials: Moving forward in a climate of increasing economic and regulatory pressure. Sarcoma 2007. http://dx.doi.org/10.1155/2007/76405.
- 37.Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marina N, Smeland S, Bielack SS, et al. MAPIE vs MAP as postoperative chemotherapy in patients with a poor response to preoperative chemotherapy for newly-diagnosed osteosarcoma: Results from EURAMOS-1. Connective Tissue Oncology Society meeting; October 15-18, 2014; Berlin, Germany. (abstr 032) [Google Scholar]
- 40.Chen X, Bahrami A, Pappo A, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savage SA, Mirabello L, Wang Z, et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet. 2013;45:799–803. doi: 10.1038/ng.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Cancer Institute Office of Cancer Genomics. TARGET: Therapeutically Applicable Research to Generate Effective Treatments. https://ocg.cancer.gov/programs/target.
- 43.Khanna C, Fan TM, Gorlick R, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20:4200–4209. doi: 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorlick R, Janeway K, Lessnick S, et al. Children's Oncology Group's 2013 blueprint for research: Bone tumors. Pediatr Blood Cancer. 2013;60:1009–1015. doi: 10.1002/pbc.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seibel NL, Krailo M, Chen Z, et al. Upfront window trial of topotecan in previously untreated children and adolescents with poor prognosis metastatic osteosarcoma: Children's Cancer Group (CCG) 7943. Cancer. 2007;109:1646–1653. doi: 10.1002/cncr.22553. [DOI] [PubMed] [Google Scholar]
- 46.Bond M, Bernstein ML, Pappo A, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: A Children's Oncology Group study. Pediatr Blood Cancer. 2008;50:254–258. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 47.Beaty O, 3rd, Berg S, Blaney S, et al. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: A Children's Oncology Group study. Pediatr Blood Cancer. 2010;55:440–445. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs S, Fox E, Krailo M, et al. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: A report from the Children's Oncology Group. Clin Cancer Res. 2010;16:750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwerdling T, Krailo M, Monteleone P, et al. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: A report from the Children's Oncology Group. Cancer. 2006;106:1821–1828. doi: 10.1002/cncr.21779. [DOI] [PubMed] [Google Scholar]
- 50.Pawinski A, Crowther D, Keizer HJ, et al. The EORTC Phase II study of iproplatin in advanced osteogenic sarcoma. Eur J Cancer. 1999;35:163–164. doi: 10.1016/s0959-8049(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 51.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 52.Langevin AM, Bernstein M, Kuhn JG, et al. A phase II trial of rebeccamycin analogue (NSC #655649) in children with solid tumors: A Children's Oncology Group study. Pediatr Blood Cancer. 2008;50:577–580. doi: 10.1002/pbc.21274. [DOI] [PubMed] [Google Scholar]
- 53.Chawla SP, Staddon AP, Baker LH, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30:78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 54.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: An Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 55.Kolb EA, Gorlick R, Reynolds CP, et al. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer. 2013;60:1325–1332. doi: 10.1002/pbc.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortes J, O'Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 57.Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: A phase 2 study in four independent histological subtypes. Lancet Oncol. 2011;12:1045–1052. doi: 10.1016/S1470-2045(11)70230-3. [DOI] [PubMed] [Google Scholar]
- 58.Beristain AG, Narala SR, Di Grappa MA, et al. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci. 2012;125:943–955. doi: 10.1242/jcs.094029. [DOI] [PubMed] [Google Scholar]
- 59.Kolb EA, Gorlick R, Billups CA, et al. Initial testing (stage 1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61:1816–1821. doi: 10.1002/pbc.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roth M, Linkowski M, Tarim J, et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120:548–554. doi: 10.1002/cncr.28461. [DOI] [PMC free article] [PubMed] [Google Scholar]