Abstract
Rare pediatric tumors account for approximately 10% of all childhood cancers, which in themselves are a rare entity. The diverse histologies and clinical behaviors of rare pediatric tumors pose challenges to the investigation of their biologic and clinical features. National and international cooperative groups such as the Rare Tumor Committee of the Children's Oncology Group, Rare Tumors in Pediatric Age Project, and European Cooperative Study Group for Pediatric Rare Tumors have developed several initiatives to advance knowledge about rare pediatric cancers. However, these programs have been only partially effective, necessitating the development of alternative mechanisms to study these challenging diseases. In this article, we review the current national and international collaborative strategies to study rare pediatric cancers and alternative methods under exploration to enhance those efforts, such as independent registries and disease-specific, National Cancer Institute–sponsored clinics.
INTRODUCTION
Pediatric cancer, in itself, is a rare disease; of the estimated 1.6 million annual cases of cancer in the United States, only 15,000 (< 1%) affect persons age < 20 years.1 Within pediatric cancer, there are groups of diseases that occur so infrequently that they are not captured by currently available registries or treatment protocols. The small numbers and diversity of these histologic subtypes pose challenges to the investigation of their biologic and clinical behavior. Therefore, children and adolescents with rare tumor histologies represent a vulnerable population that has not benefitted from the treatment success achieved in other childhood cancer populations.
DEFINING RARE CHILDHOOD CANCER
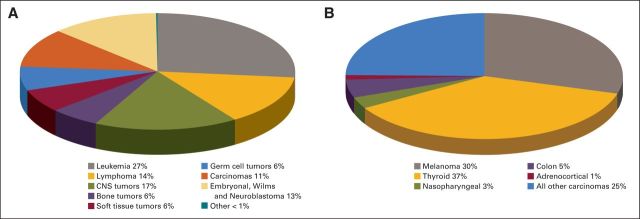
The definition of an infrequent or rare childhood cancer is complex and has been interpreted differently by various investigators. The European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT) defines a rare childhood cancer as one that has an incidence rate ≤ 2 per million per year, is not considered in clinical trials, or both.2 This definition emphasizes the concept that a rare childhood cancer should not be defined solely based on its incidence rate and that rare childhood cancers should be viewed as orphan diseases. For example, despite the fact that the incidence of melanoma in younger patients is 6 per million per year, the lack of registries and trials makes this a rare childhood cancer.2 Within the Children's Oncology Group (COG), the Rare Tumor Committee has adopted a qualitative definition of a rare cancer based on some common features that include low prevalence in younger patients, higher incidence in adults, and epithelial (rather than mesenchymal) tumor origin.3 Thus, the COG has opted to define rare childhood cancers as those classified as “other malignant epithelial neoplasms and melanomas” in the International Classification of Childhood Cancer subgroup 11 of the SEER database (Fig 1A).4 The histologic features of this subgroup include carcinomas, such as adrenocortical, thyroid, and nasopharyngeal carcinomas, as well as melanoma and other unspecified carcinomas. However, this definition does not include rare cancers seen almost exclusively in children (eg, pancreatoblastoma and pleuropulmonary blastoma [PPB]). The frequency of these histologies within subgroup 11 of the SEER database is shown in Figure 1B. Obviously, defining a rare cancer based solely on epidemiologic or pathologic traits is problematic, and a collaborative effort to better refine this concept is desperately needed.
Fig 1.
Annual incidence of (A) malignancies and (B) carcinomas and melanomas in those age < 20 years with proportion of specific histologies as coded according to SEER adolescent and young adult classification of International Classification of Diseases for Oncology (version 3), standardized to the 2000 US standard population.4
Rare Cancers Are Not That Rare
Despite their presumed relative rarity, as defined by the COG, rare childhood cancers collectively account for 11% of all cancers in those age < 20 years (Fig 1A). Furthermore, 75% of those cancers occur in patients who are age 15 to 19 years, a population that is underrepresented in National Cancer Institute (NCI) –sponsored clinical trials and in whom survival improvements have lagged behind those of younger age groups.5
Collaborative Efforts to Study Rare Childhood Cancers
In 2000, the Rare Tumors in Pediatric Age Project (TREP) was launched in Italy, and more recently, in 2008, European investigators formed the EXPeRT group, in which national working groups from Italy, France, Poland, the United Kingdom, and Germany came together to enhance collaborative clinical and biologic research in rare pediatric cancers.2,6 These groups have published several registry-based reports on various rare cancers, including PPB, pancreatoblastoma, thymic tumors, melanoma, and appendiceal neuroendocrine tumors. In addition, the TREP and EXPeRT groups have developed treatment and staging recommendations for selected rare cancers and have identified a group of experts who assist in consultations and clinical decisions.2,7–10 The EUROCARE (European Cancer Registry) project, a population-based cancer database that reports the survival rates from 74 population-based registries in 29 European countries, has also served as an important resource for investigators and offers opportunities for improved collaboration.11
The COG Rare Tumor Committee used preexisting resources (eg, COG registry) to explore the epidemiologic landscape of rare childhood cancers. On the basis of estimates from the SEER database, over a 6-year period (2002 to 2007), only 7% of the expected numbers of rare cancers, as defined by the COG, were registered.3 Since then, the COG has developed a more robust effort called the Children's Cancer Research Network (CCRN), which uses an informed authorization process to register all patients with childhood cancer who are age < 20 years and treated at COG institutions in the United States or Canada.12 Over a 2-year period, 42% of all pediatric oncology patients who were age < 20 years were registered; the rates of registration were highest for those age 5 to 9 years (57%) and lowest for those age 15 to 19 years (24%), a pattern that has been seen previously in COG and European studies.2,3 The registration rates were highest for leukemias, peripheral nervous system tumors, and renal tumors and lowest for germ cell tumors, CNS tumors, and retinoblastoma. For rare cancers, 1,862 registrations were recorded from 2008 to 2013, representing 5% of the total number of registrations during this time period. Table 1 compares the numbers of rare tumors registered with the expected numbers of selected rare tumors as estimated by the SEER database and demonstrates the large discrepancies between observed and expected cases, particularly among older patients with so-called adult-type tumors.
Table 1.
Patient Cases Registered in CCRN COG Trial From 2008 to 2013 Compared With Expected Patient Cases During Same Time Period Based on SEER Estimates
| Diagnosis | Age Group (years) |
All |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 to 4 |
5 to 9 |
10 to 14 |
15 to 19 |
|||||||
| Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | Observed | Expected | |
| Adrenocortical carcinoma | 29 | 162 | 9 | 36 | 17 | 66 | 9 | 72 | 64 | 336 |
| Colon carcinoma | 13 | 84 | 13 | 186 | 37 | 786 | 47 | 3,396 | 110 | 4,452 |
| Melanoma (cutaneous) | 20 | 324 | 41 | 582 | 54 | 1,464 | 47 | 5,922 | 162 | 8,292 |
| Naospharyngeal carcinoma | 27 | 18 | 36 | 162 | 77 | 906 | 53 | 1,692 | 193 | 2,778 |
| Thyroid carcinoma | 0 | 66 | 14 | 444 | 44 | 2,202 | 47 | 7,854 | 105 | 10,566 |
Abbreviations: COG, Children's Oncology Group; CCRN, Children's Cancer Research Network.
In addition to poor registration rates, tissue samples of these cancers are scarce, and many investigators have labeled this problem as a biologic specimen repository gap.3 In a previous publication, we estimated that tissues for banking were submitted for only 11% of all cases of rare tumors in the COG registry.3 More recent estimates show that 9% of cases registered in the CCRN have rare tumor tissue for banking. These numbers highlight the urgent need to develop novel funding mechanisms to incentivize researchers to provide tissue samples for genomic analysis and the need for alternative methods of collaboration to address the clinical and biologic peculiarities of individual rare childhood cancers. Finally, the COG Rare Tumor Committee conducted two prospective trials in adrenocortical carcinoma and nasopharyngeal carcinoma; these efforts required collaboration with Brazilian investigators and took approximately 7 years to complete. These studies highlight the difficulty in performing single-arm trials, even within the context of a national collaborative group effort.3 In this review, we will describe three alternative mechanisms that have been implemented to study rare childhood cancers, including research initiatives through NCI-funded cooperative groups, independently funded registries, and the creation of specialized clinics for rare cancers.
COOPERATIVE GROUP STUDIES: HEPATOBLASTOMA
Primary liver cancers in children are rare, with approximately 100 to 150 new diagnoses each year in the United States; two thirds of those cases are hepatoblastomas.4 The rarity of hepatoblastoma precludes any single center from being able to diagnose and treat a sufficient number of patients to meaningfully evaluate new treatment approaches; thus, multi-institutional collaborations have been in place to study this disease since 1972.
In North America, the first two studies were launched by SWOG (SWOG-7495) and the Children's Cancer Study Group (CCSG-831) and, in 1976, demonstrated that hepatoblastoma is a chemosensitive tumor when treated with vincristine, cyclophosphamide, doxorubicin, and fluorouracil.56 The important role of cisplatin was identified in a single-institution study in which nine of 11 patients had objective responses when cisplatin was administered alone or with vincristine and fluorouracil.13 Later, investigators of the Pediatric Oncology Group pursued the combination of cisplatin, vincristine, and fluorouracil,14 and CCSG investigators evaluated the feasibility of combining cisplatin and continuously infused doxorubicin.15 These studies led to the development of an intergroup study (INT-0098) to compare these combinations in a setting that would provide sufficient statistical power to draw sound conclusions about the effectiveness of the treatment.16 Similar initiatives promptly followed, with the formation of the International Liver Tumors Strategy Group (SIOPEL), the liver tumor study group of the German Society for Pediatric Oncology and Hematology (GPOH), and the Japanese Study Group for Pediatric Liver Tumors (JPLT). These sustained multinational scientific collaborations have dramatically improved survival from < 20% in the prechemotherapy era with surgery alone to survival > 70% for patients without metastatic disease at diagnosis16–19 and approaching 100% in some subgroups.20 Table 2 highlights the most important findings from these four groups. The current management guidelines derived from these studies include the following: 1) surgery is the most effective modality to secure long-term survival; 2) cisplatin is the single most effective chemotherapeutic agent; and 3) cisplatin alone or in combination with other agents can facilitate successful complete surgical resection in most patients. The optimal timing of surgery (at diagnosis or delayed until after chemotherapy) and the optimal chemotherapy regimen for patients are still unknown.
Table 2.
Cooperative Group Studies of Childhood Hepatoblastoma
| Study | No. of Patients | Treatment | Outcome | Conclusion/Comments |
|---|---|---|---|---|
| Single-Arm Cooperative Group Studies | ||||
| CCG/SWOG study 221 | 62 | V-CTX-DOX-FU | 12 of 27 patients with measurable disease who completed ≥ one 6-week course had ≥ PR; 5-year survival, approximately 38% | Hepatoblastoma is responsive to chemotherapy |
| CCG-823F15 | 33 | CDDP-DOX | 2-year survival, 67% | Preoperative chemotherapy can facilitate complete resection in substantial number of children; patients with unresectable or metastatic disease remain largely incurable |
| POG 869714 | 60 | CDDP-V-FU | 4-year DFS for stage III disease, 67% (SE, 10.8%) | |
| GPOH/HB 8957 | 72 | IFOS-CDDP-DOX | Long-term EFS, 75% | |
| SIOPEL-121 | 154 | CDDP-DOX | 5-year survival, 75% | |
| JPLT-158 | 134 | CDDP-pirarubicin | 3-year survival, 77.8% | |
| SIOPEL-259 | 67 standard-risk patients (PRETEXT I, II, III) | CDDP only | 3-year survival, 91% (± standard deviation, 7%); CDDP alone with surgery may be sufficient for standard-risk patients | |
| GPOH/HB 9417 | 69 | IFOS-CDDP-DOX | 3-year survival, 77% | |
| SIOPEL-422 | 62 | High-risk patients (PRETEXT IV; P+, V+, E+, metastasis, AFP < 100 ng/mL, or tumor rupture); CDDP-DOX-CBDCA | 3-year EFS, 76%; 3-year OS, 83% | Unique dose-dense CDDP regimen may improve survival in patients with unresectable or metastatic disease |
| Randomized Studies | ||||
| POG/CCG INT009816 | 173 | CDDP-V-FU v CDDP-DOX | 5-year survival, 69% v 72%; P = .88 | Combination chemotherapy without doxorubicin is less toxic but results in similar survival |
| SIOPEL 318 | 126 v 129 | CDDP only v CDDP-DOX | 3-year survival, 95% v 93% | DOX can be safely omitted from treatment of standard-risk patients |
Abbreviations: CBDCA, carboplatin; CCG, Children's Cancer Group; CDDP, cisplatin; COG, Children's Oncology Group; CTX, cyclophosphamide; DOX, doxorubicin; E+, extrahepatic abdominal disease; EFS, event-free survival; FU, fluorouracil; GPOH, German Society for Pediatric Oncology; HB, hepatoblastoma; IFOS, ifosfamide; JPLT, Japanese Study Group for Pediatric Liver Tumors; P+, portal vein involvement; POG, Pediatric Oncology Group; PRETEXT, pretreatment extent of disease; SIOPEL, International Childhood Liver Tumors Strategy Group; V, vincristine; V+, vascular invasion.
A major limitation to further progress is that there is no common risk-stratification system for hepatoblastoma. The COG currently uses a four-group stratification system: very low risk, patients with pure fetal histology and completely resected tumors at diagnosis20; low risk, patients with completely resected tumors at diagnosis but with histology that is not pure fetal; intermediate risk, patients with unresectable tumors localized to the liver; and high risk, patients with metastasis at diagnosis or those who present with α-fetoprotein (< 100 ng/mL). Once patients are risk stratified, their treatment is tailored to the very low–risk group, consisting of surgery alone (COG approach), or to the low- or intermediate-risk group, consisting of two to six cycles of chemotherapy. Despite the significant progress made in treating localized hepatoblastoma, the prognosis of patients presenting with metastatic disease has been uniformly poor; historically, the 5-year probability of event-free survival (EFS) was 21% to 28%.16,19 However, a recent report from SIOPEL was encouraging; 39 patients with metastasis at diagnosis had a 3-year EFS of 77% (95% CI, 63% to 90%).22 The results of this single-arm trial will need further validation.
We are only now beginning to investigate the biology of hepatoblastoma and hepatocellular carcinoma. In addition to identifying targets for new agents, such efforts may identify key biologic characteristics of the tumor that can be used to improve the risk-classification system. Efforts to obtain tissue samples have been hampered in the past not only by technical issues of tissue procurement but also by the lack of a protocol for banking and collection of clinical data to supplement the biologic materials, particularly when the patient is not enrolled onto a therapeutic trial. The COG started such an effort with the P9346 study.23 This effort will be subsumed in the upcoming COG Every Child protocol, which will support tissue banking and collection of clinical follow-up data for all rare pediatric cancers.
Despite the advances described here, several limitations to further improving the outcome of children with hepatoblastoma persist. First, the rarity of hepatoblastoma poses challenges to designing new clinical trials. In addition, regulatory hurdles across cooperative groups and the variety of staging systems used do not allow accurate comparisons of therapy and outcomes. The COG has historically staged patients by their histologic and surgical criteria for upfront surgery and uses this system to define risk categories. Thus, stage I is defined as complete gross total resection with clear margins; stage II, gross total resection with microscopic residual disease at the resection margins; stage III, gross total resection with tumor spill, nodal involvement, or gross residual intrahepatic disease; and stage IV, presence of metastatic disease with either complete or incomplete resection of the primary tumor.16 In contrast, SIOPEL has developed a staging system called PRETEXT (Pretreatment Extent of Disease), based on imaging acquired before therapy is initiated.24 The GPOH and JPLT have adapted this system, and the COG is now using PRETEXT to help define surgical resectability.
Moving forward, an international cooperative effort has been undertaken in which study databases from various international studies have been combined into the single Childhood Hepatic Tumor International Consortium (CHIC) database, allowing evaluation of variables such as extent of disease, α-fetoprotein values, tumor pathology and biology, extent of tumor necrosis, microscopic surgical margins, intravascular tumor invasion, and cytogenetics. Also, additional collaborative efforts are proceeding to develop an international pediatric liver tumor consensus pathologic classification.25 Third, the optimal timing of surgical resection, its role in managing metastatic disease, and whether negative microscopic surgical margins are important need to be addressed. Another surgical controversy centers on the indication for liver transplantation. To that end, SIOPEL has developed the Pediatric Liver Unresectable Tumor Observatory (PLUTO) registry to better define the role of liver transplantation in pediatric liver cancers, including hepatoblastoma.26
Finally, as summarized in Table 2, the optimal chemotherapeutic regimen is still undefined; thus, international cooperative groups are working to design the Pediatric Hepatoblastoma International Therapeutic Trial (PHITT), which will test novel drugs to treat metastatic disease, develop a uniform risk categorization and standard surgical approach, and further define the optimal chemotherapy regimen for pediatric patients with these challenging and rare diseases.
RARE TUMOR REGISTRIES FACILITATE CANCER RESEARCH: INTERNATIONAL PPB REGISTRY
PPB was recently described as a rare and aggressive malignancy of early childhood. The International PPB Registry (IPPBR) was established in 1988 in an effort to better understand the biology and clinical characteristics of this tumor. Since the inception of the registry, 408 children with centrally reviewed PPBs have been enrolled from six continents and 42 countries. A follow-up initiative, the IPPBR Web site,31 was launched in 2006 and has become a commonly cited reference for providers, an invaluable source of information for families, and an important portal through which to access new patients and their physicians.
The IPPBR collects medical and family history information and biologic samples for central review. Careful collection of clinical data, radiographic images, and pathologic specimens for central review has expanded our understanding of the natural history and biology of the disease. We now know that PPB starts as type I, a purely cystic neoplasm with 5-year overall survival (OS) of 91%, and can progress to type II, a mixed cystic and solid neoplasm with 5-year OS of 71%, or type III, a solid aggressive sarcomatous tumor with 5-year OS of only 53%.27 This association between a stepwise progression of PPB and decreasing cure rate led to a focus on early detection of the disease. Interestingly, not all type I PPBs are destined to progress to the more malignant type.29 Type Ir PPB seems to represent a spontaneously regressed type I PPB.31 The factors associated with spontaneous regression of this tumor are currently under investigation.
Since its first description, childhood PPB has been recognized as having a strong familial component that is suggestive of a familial cancer syndrome. In 2009, Hill et al30 performed linkage analysis on samples and histories obtained through the IPPBR to identify heterozygous germline DICER1 mutations in children with PPB. Since then, germline heterozygous loss-of-function DICER1 mutations have been found in the majority of children with PPB. The PPB tumor syndrome now includes many other tumors and entities, including lung cysts, ovarian stromal tumors, nodular thyroid hyperplasia, cystic nephroma, ciliary body medulloepthelioma, brain tumors such as pineoblastoma, ovarian sex cord stromal tumors, and cervical rhabdomyosarcoma.32–35 Many of the tumors have specific missense mutations in the DICER1 ribonuclease IIIb domain.38,39 Loss-of-function mutation in the germline DICER1 allele with a somatic second hit results in decreased processing of the 5p mature microRNA (miRNA) from its precursor miRNA, whereas processing of the 3p miRNA is unaffected, resulting in bias toward increased 3p miRNA. The loss of 5p miRNAs, such as the tumor-suppressing let-7 family miRNA, may lead to derepression of oncofetal genes and uncontrolled proliferation. Thus, the model of oncogenesis in this syndrome is not entirely the result of haploinsufficiency, as previously thought, and requires two hits without complete loss of function.36
The IPPBR-based procedures have also been used to develop treatment guidelines. Thus far, 62 patients with type II or III PPB have been uniformly treated using the guidelines, and preliminary results suggest that these patients have improved outcomes compared with those of historical controls.37 This has laid the groundwork for an upcoming treatment study to further reduce mortality in PPB, which is caused mostly by local recurrence and brain metastasis.27
To further study the association of DICER1 mutations and ovarian stromal tumors, a parallel registry, the International Ovarian and Testicular Stromal Tumor (OTST) Registry,38,39,43 was started in 2011. The OTST Registry has identified germline DICER1 mutations in > 50% of women with Sertoli-Leydig cell tumors.39 Identification of germline mutations in DICER1 can facilitate early detection of PPB in patients and their relatives, resulting in earlier diagnosis and increased likelihood of cure with decreased treatment-related adverse effects.40
SPECIALIZED CLINICS FOR STUDY OF RARE CANCERS: NATIONAL INSTITUTES OF HEALTH PEDIATRIC AND WILD-TYPE GIST CLINIC
GI stromal tumors (GISTs) are the most common mesenchymal tumors of the GI tract in adults and typically affect patients during their sixth decade of life.41 Histologically, GISTs are phenotypically related to the interstitial cells of Cajal, and immunohistochemical analysis has shown that > 95% of GISTs express KIT and DOG1.42 Approximately 85% of adult GISTs have oncogenic mutations of the KIT and PDGFR genes, which promote ligand-independent autophosphorylation and drive tumor formation.42 Targeted therapies using various tyrosine kinase inhibitors (ie, imatinib, sunitinib, and regorafenib) have dramatically improved the outcome of patients with high-risk, metastatic, or recurrent GIST.44
Approximately 10% to 15% of GISTs lack detectable mutations of KIT or PDGFR and are therefore called wild-type GISTs.49 A small subgroup of these GISTs arise in the small bowel of adult patients and either harbor an oncogenic BRAF V600E mutation or develop within the context of neurofibromatosis type 1. The remaining larger group of wild-type GISTs is now recognized as comprising succinate dehydrogenase (SDH) –deficient GISTs. These tumors are a component of two cancer syndromes: Carney's triad (GIST, pulmonary chondroma, and paraganglioma) and Carney Stratakis syndrome (paraganglioma and GIST); they most commonly affect female patients during the first two decades of life.50,51 Few reports have described the natural history of SDH-deficient GISTs, and those have been restricted to small institutional reviews. However, despite these limitations, pediatric SDH-deficient GISTs clearly have different clinical and biologic characteristics than adult KIT-mutant GISTs.46,49 These GISTs almost exclusively arise in the stomach and frequently present with GI bleeding and anemia. They are often multifocal, involve locoregional lymph nodes, have an indolent clinical course characterized by prolonged periods of disease stabilization followed by frequent recurrences that may span many years, and are unresponsive to the tyrosine kinase inhibitors used to treat KIT-mutant GISTs.46,49 Histologically, these GISTs have an epithelioid or mixed pattern; they express KIT and insulin-like growth factor receptor 1 (IGF1R) but not SDHB, and they have a relatively stable genome, with few or no copy number changes.48
Because of the unique characteristics and rarity of SDH-deficient GISTs (ie, SEER estimates of 0.01 per million per year in patients age < 20 years), investigators from various disciplines came together to develop a new paradigm to study its clinical and biologic characteristics. Under the auspices of the National Institutes of Health (NIH), the first NIH Pediatric and Wild-Type GIST Clinic55 was created in 2008. Since its inception, 126 patients have been evaluated in 11 consecutive clinics. These multidisciplinary clinics have brought together adult and pediatric oncologists, surgeons, geneticists, basic science investigators, psychologists, radiologists, nutritionists, and patient advocates to better define the natural history and biology of SDH-deficient GISTs. Local physicians refer patients with suspected wild-type GIST to the clinic for evaluation and treatment recommendations. Once informed consent has been obtained, clinical, radiographic, and pathologic analyses are completed; tumor and germline tissues are requested and collected when available. Additional laboratory, radiographic, and clinical measures are also obtained.
Review of the clinical, pathologic, and genomic material obtained through this clinic has confirmed previous observations that the population with wild-type GISTs consists of two major subgroups: a small subset of older patients (median age, 47 years) who have small bowel primaries and often carry BRAF or NF1 mutations and a large group of younger (median age, 20 years) mostly female patients (70%) with epithelioid or mixed-pattern tumors that arise exclusively in the stomach. More than 90% of these patients have metastatic disease at diagnosis that involves the liver and lymph nodes, and they present at a younger age (approximately 20 years). These patients do not respond well to the kinase inhibitors used to treat KIT-mutant GISTs. In a preliminary report from this clinic, 18 of 18 wild-type GISTs did not express SDHB (by immunohistochemical and Western blot analyses), and spectrophotometric analysis revealed reduced mitochondrial complex II activity.51 More importantly, four (12%) of 34 patients with no family history of paraganglioma harbored germline mutations of SDHB and SDHC.51 This report established conclusively that dysregulation of the SDH complex plays a central role in the pathogenesis of a subset of GISTs and that the term SDH-deficient GIST more adequately describes these tumors. In addition, because 10% of children with this disease may have a germline mutation of the SDH complex, this diagnosis should trigger genetic evaluation of their families. Subsequent analysis of larger numbers of patients with SDH-deficient GISTs revealed that ≥ 50% carry a germline mutation of the SDH complex, more often affecting SDHA. Most of these patients were female, with a median age at presentation of 23.5 years, and the primary site of their tumors was exclusively the stomach. The remaining group of SDH-deficient GISTs consists of younger patients (median age, 15.5 years) with exclusively primary gastric tumors and a slight female predominance. In this last group, SDH is inactivated by an alternative mechanism that is currently being investigated by members of the clinic.
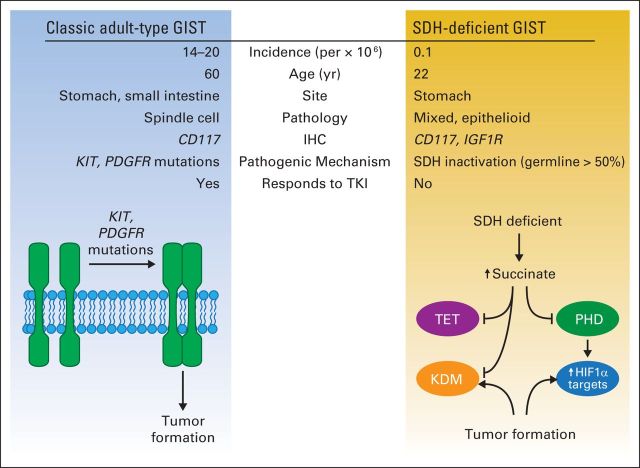
In an analysis of DNA-methylation profiles, investigators identified a global pattern of hypermethylation in the SDH-deficient tumors.52 This phenomenon resulted in the maintenance of C-phosphate-G demethylation because of inhibition of the dioxygenase-methylation pathway. Thus, the study identified SDH-deficient GIST as a tumor with a methyl-divergent pattern and identified succinate accumulation as a modifier of transcriptional regulation as a consequence of inhibition of α-ketoglutarate dioxygenases.52 Given the biologic differences leading to tumor formation between adult-type and SDH-deficient GISTs (Fig 2), alternative therapeutic trials incorporating angiogenesis inhibitors (vandetanib), IGF1R inhibitors (lisitinib), and demethylating agents are ongoing.
Fig 2.
Classic adult-type GI stromal tumor (GIST; left) affects older patients, is CD117 positive by immunohistochemistry (IHC), and has KIT and PDGFR oncogenic mutations, resulting in ligand-independent kinase activation that contribute to tumor formation. These tumors are responsive to tyrosine kinase inhibitors (TKIs) such as imatinib. Succinate dehydrogenase (SDH) –deficient GIST (right) affects younger patients, is characterized by CD117 positivity by IHC, and IGF1R overexpression. These tumors are not responsive to TKIs and are characterized by loss of function of SDH complex; germline mutations of this complex are seen in > 50% of patients. Resultant succinate accumulation downregulates prolyl hydroxylase (PHD), leading to decreased proteasomal degradation and increased levels of hypoxia-inducible factor 1 (HIF1α). In addition, succinate accumulation leads to inhibition of 2-oxoglutarate–dependent dioxygenases, including ten-eleven translocation (TET) family of DNA-modifying enzymes and JmjC domain–containing histone lysine demethylases (KDM), resulting in dysregulated transcription and gene expression with consequent tumor formation.
DISCUSSION
Controlled clinical trials, both randomized and nonrandomized studies, have been instrumental for improving cures for pediatric cancers; however, progress in rare pediatric cancers has lagged. The reasons for these discrepancies in clinical outcome are multiple and include small numbers of patients with rare cancers, limited dedicated resources for protocol enrollment of rare cancers, and few available trials for these patients. As detailed in this article, several different approaches to the study of rare cancers exist. Collaborative group trials have been successful in studying and treating selected rare cancers such as hepatoblastoma, but efforts to study other rare cancers such as adrenocortical carcinoma have required the participation of international collaborators.3 Partnerships with adult groups (eg, to study melanoma) have been largely unsuccessful, likely in part because of infrastructural and resource constraints. However, continued collaboration with adults is crucial, because the incidence of adolescent melanoma continues to rise, and novel targeted and immune therapies are not widely available for these patients.3,53,54
It is clear that advances in treatment success will come through developing partnerships to increase the referral base for trials of rare pediatric tumors. In addition, understanding tumor biology will be necessary to identify tumor vulnerabilities and develop promising new therapies that maximize patient outcome while minimizing treatment toxicity. As noted in our review, advances leading to the understanding of the biologic bases of the disease are only possible when well-annotated biologic samples are available and are linked to patient outcome data. Advances made by investigators of the PPB registry and the GIST NIH clinic, for example, were only made possible through collaborative efforts that involved alternative strategies and funding mechanisms to study these diseases. The COG Every Child Project represents a new approach to support clinical and biologic research for rare tumors and will allow identification of patients, submission of biologic specimens, and provide status updates for children seen at a COG institution. We believe that this new research portal, along with true international scientific collaboration, provides an unparalleled opportunity to advance the understanding and treatment of rare pediatric cancers. Available resources and ongoing research initiatives focusing on rare pediatric tumors are summarized in Table 3.
Table 3.
Resources for Pediatric Rare Cancers
| Name | Web Site | Content |
|---|---|---|
| Children's Oncology Group | http://www.childrensoncologygroup.org | NCI-supported clinical trials group comprising > 8,000 experts from > 200 children's hospitals |
| Cancer.Net | http://www.cancer.net/coping-and-emotions/managing-emotions/finding-support-and-information/finding-information-and-support-resources-rare-cancers | Oncologist-approved information from ASCO |
| NCI PDQ | http://www.cancer.gov/cancertopics/pdq | PDQ is NCI comprehensive cancer database, with summaries on wide range of cancer topics, registry of > 8,000 open and > 19,000 closed cancer clinical trials from around world, and directory of professionals who provide genetic services; PDQ also contains NCI Dictionary of Cancer Terms, with definitions for > 6,800 cancer and medical terms, and NCI Drug Dictionary, with information on > 2,300 agents used in treatment of cancer or cancer-related conditions |
| American Cancer Society | http://www.cancer.org | Informational tools for various types of cancer from American Cancer Society |
| International Pleuropulmonary Blastoma Registry | http://www.ppbregistry.org | Registry and resource for physicians, families, and patients affected by pleuropulmonary blastoma |
| NORD | http://www.rarediseases.org | Provides advocacy and information for people affected with rare diseases |
| Rare Cancer Alliance | http://www.rare-cancer.org | Provides information and support for adult and pediatric patients with rare cancers |
| NCI | http://www.cancer.gov | Provides information on rare cancers as well as list of open clinical trials for various pediatric and adult cancers |
| ACOR | http://www.acor.org | Provides information and support for patients with cancer and those who care for them through creation and maintenance of cancer-related Internet mailing lists and Web-based resources |
| Orphanet | http://www.orpha.net | Internet portal that provides information on rare diseases and orphan drugs with aim of improving diagnosis and treatment of patients with rare diseases |
| Italian TREP Project | http://www.trepproject.org | TREP group brings together physicians from Italian Pediatric Surgery and Pediatric Oncology Centers, creating network of experts who are dedicated to improving knowledge and cure of patients affected by rare pediatric tumors |
| US SEER | http://www.seer.cancer.gov | NCI SEER program provides information on cancer in US population |
| German Childhood Cancer Registry | http://www.kinderkrebsregister.de | German Cancer Childhood Registry was founded in 1980; registers approximately 1,800 childhood cancer cases from Germany each year |
| IPACTR | http://www.stjude.org/ipactr | Provides resource and registry for patients with pediatric adrenocortical tumors |
| Pediatric and Wild-Type GIST Clinic | http://www.pediatricgist.cancer.gov | Collaborative effort to better understand biology and clinical characteristics of pediatric and wild-type GIST; Web site offers registration form for consideration for referral to clinic |
Abbreviations: ACOR, Association of Cancer Online Resources; ASCO, American Society of Clinical Oncology; GIST, GI stromal tumor; IPACTR, International Pediatric Adrenocortical Tumor Registry; NCI, National Cancer Institute; NORD, National Organization for Rare Disorders; PDQ, Physician Data Query; TREP, Tumori Rari in Età Pediatrica [Rare Tumors in Pediatric Age].
Acknowledgment
We thank Angela McArthur for editorial assistance and the patients, families, and referring physicians who collaborated with the International Pleuropulmonary Blastoma Registry, Children's Oncology Group, and National Institutes of Health Wild-Type GIST Clinic.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Alberto S. Pappo, Mark D. Krailo
Provision of study materials or patients: Andrea Ferrari
Collection and assembly of data: Wayne L. Furman, Kris A. Schultz, Lee Helman, Mark D. Krailo
Data analysis and interpretation: Alberto S. Pappo, Lee Helman, Mark D. Krailo
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Rare Tumors in Children: Progress Through Collaboration
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Alberto S. Pappo
Consulting or Advisory Role: ZIOPHARM Oncology
Wayne L. Furman
No relationship to disclose
Kris A. Schultz
Research Funding: CSL Behring, Novo Nordisk
Andrea Ferrari
No relationship to disclose
Lee Helman
Employment: MedImmune (I)
Stock or Other Ownership: AstraZeneca (I)
Mark D. Krailo
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Bisogno G, Ferrari A, Bien E, et al. Rare cancers in children: The EXPeRT initiative—A report from the European Cooperative Study Group on Pediatric Rare Tumors. Klin Padiatr. 2012;224:416–420. doi: 10.1055/s-0032-1327608. [DOI] [PubMed] [Google Scholar]
- 3.Pappo AS, Krailo M, Chen Z, et al. Infrequent tumor initiative of the Children's Oncology Group: Initial lessons learned and their impact on future plans. J Clin Oncol. 2010;28:5011–5016. doi: 10.1200/JCO.2010.31.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LAG, Smith MA, Gurney JG, et al. Bethesda, MD: National Cancer Institute; 1999. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. NIH publication No. 99-4649. [Google Scholar]
- 5.Shaw PH, Hayes-Lattin B, Johnson R, et al. Improving enrollment in clinical trials for adolescents with cancer. Pediatrics. 2014;133(suppl 3):S109–S113. doi: 10.1542/peds.2014-0122F. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari A, Bisogno G, De Salvo GL, et al. The challenge of very rare tumours in childhood: The Italian TREP project. Eur J Cancer. 2007;43:654–659. doi: 10.1016/j.ejca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Virgone C, Cecchetto G, Alaggio R, et al. Appendiceal neuroendocrine tumours in childhood: Italian TREP project. J Pediatr Gastroenterol Nutr. 2014;58:333–338. doi: 10.1097/MPG.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 8.Casanova M, Bisogno G, Gandola L, et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: The Italian Rare Tumors in Pediatric Age (TREP) project. Cancer. 2012;118:2718–2725. doi: 10.1002/cncr.26528. [DOI] [PubMed] [Google Scholar]
- 9.Carretto E, Inserra A, Ferrari A, et al. Epithelial thymic tumours in paediatric age: A report from the TREP project. Orphanet J Rare Dis. 2011;6:28. doi: 10.1186/1750-1172-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dall'igna P, Cecchetto G, Bisogno G, et al. Pancreatic tumors in children and adolescents: The Italian TREP project experience. Pediatr Blood Cancer. 2010;54:675–680. doi: 10.1002/pbc.22385. [DOI] [PubMed] [Google Scholar]
- 11.Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999-2007: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 12.Musselman JR, Spector LG, Krailo MD, et al. The Children's Oncology Group Childhood Cancer Res Network (CCRN): Case catchment in the United States. Cancer. 2014;120:3007–3015. doi: 10.1002/cncr.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglass EC, Green AA, Wrenn E, et al. Effective cisplatin (DDP) based chemotherapy in the treatment of hepatoblastoma. Med Pediatr Oncol. 1985;13:187–190. doi: 10.1002/mpo.2950130405. [DOI] [PubMed] [Google Scholar]
- 14.Douglass EC, Reynolds M, Finegold M, et al. Cisplatin, vincristine, and fluorouracil therapy for hepatoblastoma: A Pediatric Oncology Group study. J Clin Oncol. 1993;11:96–99. doi: 10.1200/JCO.1993.11.1.96. [DOI] [PubMed] [Google Scholar]
- 15.Ortega JA, Krailo MD, Haas JE, et al. Effective treatment of unresectable or metastatic hepatoblastoma with cisplatin and continuous infusion doxorubicin chemotherapy: A report from the Children's Cancer Study Group. J Clin Oncol. 1991;9:2167–2176. doi: 10.1200/JCO.1991.9.12.2167. [DOI] [PubMed] [Google Scholar]
- 16.Ortega JA, Douglass EC, Feusner JH, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–2675. doi: 10.1200/JCO.2000.18.14.2665. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs J, Rydzynski J, von Schweinitz D, et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: A report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer. 2002;95:172–182. doi: 10.1002/cncr.10632. [DOI] [PubMed] [Google Scholar]
- 18.Perilongo G, Maibach R, Shafford E, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–1670. doi: 10.1056/NEJMoa0810613. [DOI] [PubMed] [Google Scholar]
- 19.Hishiki T, Matsunaga T, Sasaki F, et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: Report from the JPLT. Pediatr Surg Int. 2011;27:1–8. doi: 10.1007/s00383-010-2708-0. [DOI] [PubMed] [Google Scholar]
- 20.Malogolowkin MH, Katzenstein HM, Meyers RL, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: A report from the Children's Oncology Group. J Clin Oncol. 2011;29:3301–3306. doi: 10.1200/JCO.2010.29.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard J, Brown J, Shafford E, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach—Results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 22.Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol. 2013;14:834–842. doi: 10.1016/S1470-2045(13)70272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlinson GE, Douglass EC, Pollock BH, et al. Cytogenetic evaluation of a large series of hepatoblastomas: Numerical abnormalities with recurring aberrations involving 1q12-q21. Genes Chromosomes Cancer. 2005;44:177–184. doi: 10.1002/gcc.20227. [DOI] [PubMed] [Google Scholar]
- 24.Roebuck DJ, Aronson D, Clapuyt P, et al. PRETEXT: A revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. quiz 249-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Terrada D, Alaggio R, de Dávila MT, et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014;27:472–491. doi: 10.1038/modpathol.2013.80. [DOI] [PubMed] [Google Scholar]
- 26.Otte JB, Meyers R. PLUTO first report. Pediatr Transplant. 2010;14:830–835. doi: 10.1111/j.1399-3046.2010.01395.x. [DOI] [PubMed] [Google Scholar]
- 27.Messinger YH, Stewart DR, Priest JR, et al. Pleuropulmonary blastoma: A report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121:276–285. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill DA, Jarzembowski JA, Priest JR, et al. Type I pleuropulmonary blastoma: Pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282–295. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 29.Priest JR, Williams GM, Hill DA, et al. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44:14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 30.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Pleuropulmonary Blastoma Registry. http://www.PPBregistry.org.
- 32.Doros L, Schultz KA, Stewart DR, et al. DICER1-related disorders. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews. Seattle, WA: University of Washington; 2014. [Google Scholar]
- 33.Slade I, Bacchelli C, Davies H, et al. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48:273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 34.Doros L, Yang J, Dehner L, et al. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer. 2012;59:558–560. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doros LA, Rossi CT, Yang J, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol. 2014;27:1267–1280. doi: 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugh TJ, Yu W, Yang J, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. 2014;33:5295–5302. doi: 10.1038/onc.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doros LA, SK, Harris A, Williams GM, et al. IVADo treatment of type II and type III pleuropulmonary balstoma (PPB): A report form the International PPB Registry. J Clin Oncol. 2014;32(suppl 15s):659s. abstr 10060. [Google Scholar]
- 38.Schultz KA, Pacheco MC, Yang J, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: A report from the International Pleuropulmonary Blastoma Registry. Gynecol Oncol. 2011;122:246–250. doi: 10.1016/j.ygyno.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz KAP, Harris A, Doros LA, et al. Clinical and genetic aspects of ovarian stromal tumors: A report from the International Ovarian and Testicular Stromal Tumor Registry. J Clin Oncol. 2014;32(suppl 15s):355s. abstr 5520. [Google Scholar]
- 40.Schultz KA, Harris A, Williams GM, et al. Judicious DICER1 testing and surveillance imaging facilitates early diagnosis and cure of pleuropulmonary blastoma. Pediatr Blood Cancer. 2014;61:1695–1697. doi: 10.1002/pbc.25092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: Origin and molecular oncology. Nature reviews. Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 43.International Ovarian and Testicular Stromal Tumor Registry. http://www.OTSTregistry.org.
- 44.von Mehren M, Randall RL, Benjamin RS, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014;12:853–862. doi: 10.6004/jnccn.2014.0080. [DOI] [PubMed] [Google Scholar]
- 45.Pantaleo MA, Nannini M, Corless CL, et al. Quadruple wild-type (WT) GIST: Defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med. 2015;4:101–103. doi: 10.1002/cam4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs): A review. Int J Biochem Cell Biol. 2014;53:514–519. doi: 10.1016/j.biocel.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 48.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23:15–34. vii. doi: 10.1016/j.hoc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: A clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35:1712–1721. doi: 10.1097/PAS.0b013e3182260752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killian JK, Kim SY, Miettinen M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong JR, Harris JK, Rodriguez-Galindo C, et al. Incidence of childhood and adolescent melanoma in the United States: 1973-2009. Pediatrics. 2013;131:846–854. doi: 10.1542/peds.2012-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: Targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 55.National Cancer Institute. NIH Pediatric and Wild-Type GIST Clinic. http://www.pediatricgist.cancer.gov/Source/NIHGISTClinic/NIHGistClinic.aspx.
- 56.Evans AE, Land VJ, Newton WA, et al. Combination chemotherapy (vincristine, adriamycin, cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer. 1982;50:821–826. doi: 10.1002/1097-0142(19820901)50:5<821::aid-cncr2820500502>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 57.von Schweinitz D, Byrd DJ, Hecker H, et al. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma: Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur J Cancer. 1997;33:1243–1249. doi: 10.1016/s0959-8049(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki F, Matsunaga T, Iwafuchi M, et al. Outcome of hepatoblastoma treated with the JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J Pediatr Surg. 2002;37:851–856. doi: 10.1053/jpsu.2002.32886. [DOI] [PubMed] [Google Scholar]
- 59.Perilongo G, Shafford E, Maibach R, et al. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology: SIOPEL 2. Eur J Cancer. 2004;40:411–421. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]