Abstract
Purpose
Long-term clinical outcome data have established imatinib 400 mg/d as standard front-line treatment for newly diagnosed patients with chronic myeloid leukemia (CML).
Patients and Methods
The Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) trial is a multicenter study of imatinib 400 mg twice a day as initial therapy in 115 patients (70% Sokal low risk) with newly diagnosed CML in chronic phase who were observed for both molecular and cytogenetic responses for up to 18 months. Eighty-three patients (72%) completed the study, 10 patients (9%) discontinued the study because of adverse events, and six patients (5%) discontinued because of unsatisfactory therapeutic effect.
Results
Polymerase chain reaction analysis demonstrated rapid kinetics of major molecular response (MMR), with 48% of patients achieving MMR by 6 months, 54% by 12 months, and 63% by 18 months. Corresponding complete molecular response rates were 39%, 44%, and 55%, respectively. Median dose-intensity was 98%. Overall, 79% of patients who received at least 90% dose-intensity achieved MMR. The most frequent adverse events included myelosuppression, rash, fatigue, and musculoskeletal symptoms.
Conclusion
This study suggests that imatinib 400 mg twice a day results in more rapid reduction in tumor burden than imatinib 400 mg/d with minimal added toxicity.
INTRODUCTION
Imatinib 400 mg/d is standard front-line therapy for the treatment of chronic myeloid leukemia (CML) based on the phase III International Randomized Study of Interferon and STI571 (IRIS) trial.1,2 Five-year follow-up of the IRIS trial3 demonstrated that achievement of complete cytogenetic response (CCyR) and major molecular response (MMR) within the first 12 months of imatinib therapy is a prognostic indicator for better long-term outcome.3 Given the apparent benefit of early CCyR and MMR in terms of long-term outcome, efforts have been made to determine whether higher initial doses of imatinib might result in more robust early responses and improved outcomes.
Prior evidence suggests that higher doses of imatinib may result in improved outcomes compared with standard doses. A starting dose of 600 mg/d resulted in improved response rates, time to progression, and overall survival in patients with CML in accelerated or blast phase (BP).4,5 In a study of patients with CML in chronic phase (CML-CP), dose escalation to imatinib 800 mg/d induced responses in some patients resistant to imatinib 400 mg/d.6 High-dose imatinib (400 mg twice a day) was first evaluated as a starting dose in patients with CML-CP who had experienced treatment failure after receiving interferon alfa; it induced CCyR in most patients, with high rates of molecular remission.7 High-dose imatinib was later demonstrated to yield high response rates in patients with newly diagnosed CML-CP,8 with higher rates of complete molecular response (CMR) compared with responses seen in earlier studies with imatinib 400 mg/d. High-dose imatinib was generally well tolerated in these single-institution studies. However, the efficacy of high-dose imatinib across academic and community settings and the ability to deliver higher-dose imatinib for sustained periods of time with acceptable toxicity remained to be elucidated. Based on results from earlier studies, we implemented the Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) trial, a multicenter study of imatinib 400 mg twice a day as initial therapy in patients with newly diagnosed Philadelphia chromosome–positive or Philadelphia chromosome–negative BCR-ABL–positive early CML-CP. Here we report the findings of the RIGHT trial.
PATIENTS AND METHODS
Trial Design and Study Objectives
The RIGHT trial was a single-arm, phase II, open-label multicenter study in nine academic and 26 community sites. The primary objective was to determine the rate of MMR at 6, 12, and 18 months, defined as a ≥ 3-log reduction in BCR-ABL transcripts relative to a standardized baseline. Secondary end points included rate of and time to hematologic response, cytogenetic response, CMR (defined as undetectable BCR-ABL transcript with a sensitivity of ≥ 4.5 logs), safety, and correlation between clinical outcomes and delivered dose-intensity compared with historical data from the imatinib 400-mg/d arm of the IRIS study. Patients who had received any treatment for CML for more than 1 month, with the exception of hydroxyurea and/or anagrelide, were excluded from the study. Nineteen patients had received imatinib before study entry, 47 had received hydroxyurea, and four had received anagrelide. Study duration was 18 months; after 18 months, patients could continue therapy off study per their physician's clinical judgment. Response rate calculations included the number of patients who were still on study and those who had experienced treatment failure at previous time points. The protocol and informed consent form were reviewed by an institutional review board.
To be eligible, patients were required to be age ≥ 18 years with a diagnosis of CML-CP within 6 months and previously untreated or treated with imatinib for ≤ 1 month. Criteria for CP were as described. Ten patients were required to have an absolute neutrophil count of more than 1.5 × 109, a platelet count of more than 100 × 109, adequate end organ function with levels of aminotransferases less than 2.5× the upper limit of normal (ULN), and serum bilirubin and creatinine ≤ 1.5× ULN. Female patients must have had a negative pregnancy test within 7 days, and all patients with reproductive potential agreed to use birth control throughout the study and for up to 3 months following discontinuation of the study drug. Patients were excluded if their Eastern Cooperative Oncology Group performance status was ≥ 3; if they had a severe and/or uncontrolled medical disease, grade 3 or 4 cardiac problems, known chronic liver disease, or a significant history of nonadherence; or if they were pregnant or breastfeeding. All patients gave written informed consent according to institutional regulations.
Hematologic, Biochemical, and Cytogenetic Testing
Complete hematologic response was defined as absolute neutrophil count ≥ 1.5 × 109, platelet count ≥ 100,000 × 109, and no circulating blasts or extramedullary involvement. Bone marrow aspirate and cytogenetic analyses were performed at screening and at 12 and 18 months. In case of insufficient metaphases, fluorescence in situ hybridization was used. Cytogenetic responses (for both karyotype and fluorescent in situ hybridization) were classified as complete (0% Philadelphia chromosome–positive metaphases), partial (1% to 35%), major (0% to 35%), minor (36% to 65%), or minimal (66% to 95%).
Molecular Testing
Molecular testing by quantitative real-time polymerase chain reaction was performed by Quest Central Laboratory with validation at the Fred Hutchinson Cancer Center (FHCRC) in Seattle, WA. The Quest quantitative assay for BCR-ABL mRNA used ABL as the control gene, whereas the FHCRC assay used β2 microglobulin.11,12
The Quest assay was validated by one of the laboratories used in the IRIS trial. Of the 777 different PCR samples analyzed by Quest laboratory, 576 duplicate samples were also analyzed by the FHCRC laboratory. There was a strong correlation in the measured transcript levels between Quest Central Laboratory and FHCRC by both Pearson and Spearman coefficients (r2 = X and Y, respectively; P < .0001 for both analyses).
At the Quest laboratory, the baseline ratio of BCR-ABL/ABL was calculated from all prestudy samples in this trial in addition to other diagnostic samples assayed in this laboratory. A mean baseline BCR-ABL/ABL ratio of 3.797 was used to calculate log reductions of individual samples following therapy. The Quest assay was capable of detecting BCR-ABL transcripts down to a 4.5-log reduction from this baseline.
Statistical Considerations
Time to MMR and CMR was estimated by the Kaplan-Meier product-limit method. The relationship between dose-intensity and log reduction in quantitative BCR-ABL transcripts was assessed using Pearson's and Spearman's rank correlation. The relationship between median dose-intensity and log reduction at 18 months was determined using Fisher's exact test.
RESULTS
Patient Disposition
Baseline characteristics for the 115 enrolled patients are listed in Table 1. Median age was 50 years (range, 19 to 81 years), with 79% of patients younger than 65 years. Seventy percent had a low Sokal risk score; three patients had clonal evolution at baseline. Eighty-three patients (72%) completed the 18-month study. Median duration of exposure to imatinib 400 mg twice a day on study was 17.0 months (range, 0.2 to 19.8 months). Because imatinib was commercially available, expectations were that patients continued to receive imatinib after termination of the study, but no further information was collected after the final study visit. Of the 32 patients (28%) who discontinued the study, 10 (9%) discontinued because of adverse events (AEs), six (5%) because of unsatisfactory therapeutic effect, four (3%) because of protocol violation, and 10 (9%) because of withdrawal of consent. One patient (1%) died as a result of BP, and one patient (1%) was lost to follow-up (Table 2).
Table 1.
Patient Characteristics (N = 115)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 50 | |
| Range | 19-81 | |
| < 65 | 91 | 79 |
| ≥ 65 | 24 | 21 |
| Sokal risk score | ||
| Low | 80 | 70 |
| Intermediate | 20 | 17 |
| High | 14 | 12 |
| Missing | 1 | 1 |
| Previously treated with imatinib* | 19 | 17 |
| Duration of imatinib, days | ||
| Mean | 19 | |
| Range | 1-38 | |
| Time from diagnosis to first study dose, months | ||
| Median | 0.96 | |
| Range | 0.13-9.69 | |
| Hemoglobin, g/dL | ||
| Median | 12.30 | |
| Range | 7.9-16 | |
| Platelets, ×109/L | ||
| Median | 373 | |
| Range | 46-4,284 | |
| Peripheral blasts | ||
| Median | 0 | |
| Range | 0-12 | |
| Clonal evolution† | 3 | 3 |
| Spleen size measured below the costal margin, > 0 cm | 34 | 30 |
| Philadelphia chromosome–positive metaphases < 100 | 21 | 18 |
Received imatinib for < 1 month per protocol inclusion criteria.
Clonal evolution is defined as the presence of additional chromosomal abnormalities other than the Philadelphia translocation.
Table 2.
Patient Disposition (N = 115)
| Variable | No. | % |
|---|---|---|
| Enrolled | 115 | 100 |
| Included in the safety population* | 115 | 100 |
| Included in the intent-to-treat population† | 115 | 100 |
| Included in the per-protocol population‡ | 101 | 88 |
| Completed the study | 83 | 72 |
| Discontinued | 32 | 28 |
| Adverse events | 10 | 9 |
| Unsatisfactory therapeutic effect§ | 6 | 5 |
| Protocol violation | 4 | 4 |
| Patient withdrawal of consent | 10 | 9 |
| Loss to follow-up | 1 | 1 |
| Death | 1 | 1 |
All enrolled patients who received at least one dose of study drug.
All enrolled patients who received study drug for at least 1 week.
All enrolled patients who were in the intent-to-treat population and did not violate any major inclusion or exclusion criteria.
Unsatisfactory therapeutic effect included two patients who were not evaluable.
Molecular Response
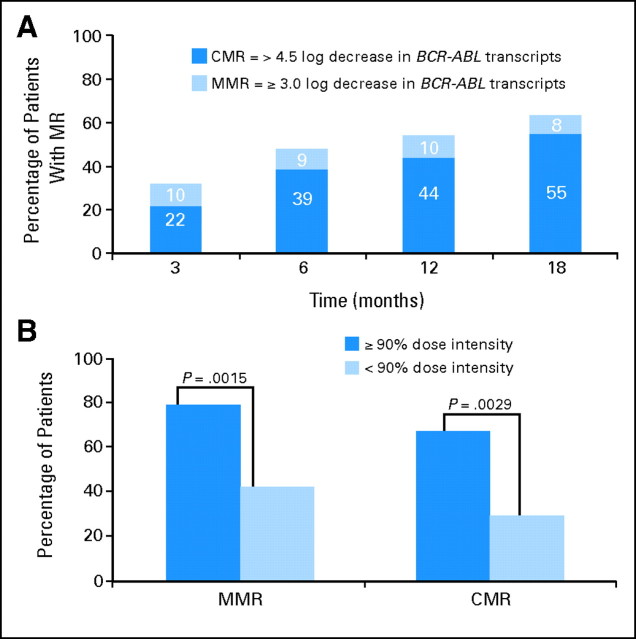
Molecular response rates in patients who received imatinib 400 mg twice a day increased over time, with MMR rates of 48% at 6 months, 54% at 12 months, and 63% at 18 months. CMR was achieved in 39%, 44%, and 55% of patients at 6, 12, and 18 months, respectively (Fig 1A). The median time to MMR was 8.3 months; median time to CMR was 9.7 months.
Fig 1.
Molecular response (MR) rates with high-dose imatinib. (A) Newly diagnosed patients with chronic myeloid leukemia–chronic phase receiving imatinib 800 mg/d achieved major MR (MMR) and complete molecular response (CMR) rates that increased over time. (B) The relationship between median dose-intensity and log reduction at 18 months was determined using Fisher's exact test.
Exposure, Dose-Intensity, and Molecular Response
The planned daily dose of imatinib was 400 mg twice a day. Median dose duration was 17 months (range, 0.2 to 19.8 months), and 89 patients (77%) had more than 12 months of exposure. Dose-intensity was calculated as follows: [total dose (mg) of imatinib taken during the study × 100%]/[800 × (last dose date − first dose date + 1)].
The dose-intensity for imatinib was correlated with the molecular response (Fig 1B). The median dose-intensity (ie, actual dose delivered/dose planned) was 98%, and the median average daily dose was 784 mg. A dose-intensity of ≥ 90% was attained by 74 (64%) of 115 patients. Dose-intensity was similar for patients younger than 65 years and those ≥ 65 years (dose-intensity ≥ 90% in 67% and 50% of patients, respectively; P = .1232).
Overall, 74 patients (64%) required a treatment interruption of at least 1 day, and 73 patients (63%) required a dose reduction. The median duration of dose interruptions was 5 days (range, 0 to 120 days), and the median duration of dose reductions was 4 days (range, 0 to 568 days). Fifty-eight patients (50%) had 5 or fewer days of missed doses. Patients who were dose reduced were re-escalated, when possible, after resolution of toxicity. At month 18, 74% of the patients on study with dose data available were still taking imatinib 400 mg twice a day.
Rates of molecular response at 18 months were correlated with dose-intensity. Seventy-nine percent of patients who received ≥ 90% dose-intensity achieved an MMR, compared with 42% of patients who received less than 90% dose-intensity (P = .0015; Fig 1B). Similarly, CMR was achieved by 67% of patients who received ≥ 90% dose-intensity, compared with 29% of patients who received less than 90% dose-intensity (P = .0029; Fig 1B).
Hematologic, Cytogenetic, and Clinical Response
Complete hematologic response at 6, 12, and 18 months was achieved and maintained in 93%, 94%, and 93% of evaluable patients, respectively (Table 3). Cytogenetic response was first assessed at 12 months. The actual rate of major cytogenetic response (MCyR) was 90% at 12 months and 96% at 18 months, and the rate of CCyR was 85% at 12 months and 83% at 18 months. One patient transformed to advanced phase (BP) and subsequently died. Four other patients remained in CP but had increasing transcript levels (> 1 log) on PCR testing without changes in hematologic or cytogenetic tests, for which their physician discontinued them from study (“unsatisfactory therapeutic effect”). Of these four patients, two were tested for mutations, one had an E255V mutation, and the other had no mutations.
Table 3.
Hematologic and Cytogenetic Response Rates of Imatinib 800 mg/d in Patients With Newly Diagnosed Chronic Myeloid Leukemia–Chronic Phase
| Response | 6 Months |
12 Months |
18 Months |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All(N = 115) |
Evaluable*(n = 104) |
All(N = 115) |
Evaluable(n = 89) |
All(N = 115) |
Evaluable(n = 84) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| CHR | 97 | 90 | 97 | 93 | 84 | 73 | 84 | 94 | 78 | 68 | 78 | 93 |
| MCyR | NA | NA | 80 | 70 | 80 | 90 | 81 | 70 | 81 | 96 | ||
| CCyR | NA | NA | 76 | 66 | 76 | 85 | 70 | 61 | 70 | 83 | ||
Abbreviations: CHR, complete hematologic response; MCyR, major cytogenetic response; NA, cytogenetics not performed at 6 months; CCyR, complete cytogenetic response.
Evaluable patients are those with available data and those who were treatment failures.
Effect of CCyR on Molecular Response
Of 76 patients who had a CCyR at 12 months, 49 (64%) had an MMR including 34 (45%) who had CMR. At 18 months, 54 (71%) and 44 (58%) patients had MMR and CMR, respectively. Of the 70 patients who had a CCyR at 18 months, 55 patients (79%) had MMR including 45 (64%) with CMR.
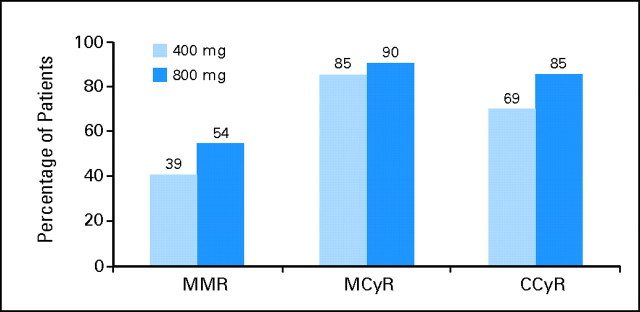
Results from the RIGHT trial were then evaluated in the context of data obtained in a similar patient population from the IRIS trial,3,9 who received imatinib 400 mg/d. Molecular response rates at 12 months with high-dose imatinib (400 mg twice a day) were higher compared with those reported for the historical control group (Fig 2). At 12 months, MMR was 54% for patients in the RIGHT trial compared with an estimated 39% for the 400 mg/d historical control group.9 Similarly, cytogenetic response rates at 12 months with high-dose imatinib were higher compared with those for the historical control group (Fig 2). At 18 months, MCyR and CCyR rates were 90% and 85%, respectively, in the RIGHT trial compared with an estimated 85% and 69%, respectively, in the historical control group that received imatinib 400 mg/d.
Fig 2.
Comparison of molecular and cytogenetic response rates at 12 months with imatinib 800 and 400 mg/d. Molecular and cytogenetic response rates were compared between patients on the Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) trial receiving imatinib 800 mg/d and historical controls from the International Randomized Study of Interferon and STI571 (IRIS) trial receiving imatinib 400 mg/d. Values from the IRIS trial (400 mg/d) reflect the estimated responses as published in the references listed; those from the RIGHT trial (800 mg/d) reflect actual response rates. In the IRIS trial, a substudy analysis of major molecular response (MMR) was performed for a group of patients (n = 124) who achieved a complete cytogenetic response (CCyR). MCyR, major cytogenetic response.
AEs
High-dose imatinib was associated with a low frequency of grade 3 to 4 treatment-related hematologic and nonhematologic toxicity (Table 4). Of hematologic AEs related to imatinib therapy, anemia was the most frequent (41%, any grade; 5%, grade 3/4), whereas thrombocytopenia was the most frequently reported grade 3 or 4 AE (18%). Of the nonhematologic imatinib-related AEs (any grade), nausea (65%), diarrhea (57%), fatigue (57%), edema (52% periorbital, 40% peripheral), vomiting (38%), and rash (33%) were the most frequent.
Table 4.
Adverse Events Occurring With Imatinib 800 mg/d Experienced by at Least 5% of Patients, Regardless of Causality
| Adverse Event | Patients, % (N = 115) |
||||
|---|---|---|---|---|---|
| Any | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Nonhematologic | |||||
| Nausea | 65 | 47 | 14 | 4 | 1 |
| Diarrhea | 57 | 44 | 9 | 4 | 1 |
| Fatigue | 57 | 36 | 15 | 6 | 0 |
| Edema | |||||
| Periorbital | 52 | 44 | 8 | 0 | 0 |
| Peripheral | 40 | 30 | 8 | 3 | 0 |
| Vomiting | 38 | 24 | 10 | 3 | 1 |
| Rash | 33 | 20 | 8 | 4 | 1 |
| Headache | 30 | 26 | 4 | 0 | 0 |
| Musculoskeletal events | |||||
| Pain | 8 | 5 | 3 | 1 | 0 |
| Muscle spasms | 25 | 23 | 2 | 0 | 0 |
| Arthralgia | 28 | 18 | 7 | 3 | 0 |
| Myalgia | 23 | 14 | 6 | 3 | 0 |
| Abdominal pain | 24 | 14 | 9 | 1 | 0 |
| Nasopharyngitis | 9 | 7 | 2 | 0 | 0 |
| Pyrexia | 23 | 16 | 5 | 2 | 0 |
| Hematologic | |||||
| Anemia | 41 | 18 | 17 | 5 | 0 |
| Neutropenia | 26 | 3 | 10 | 13 | 1 |
| Thrombocytopenia | 25 | 3 | 4 | 16 | 2 |
The most common grade 3 or 4 nonhematologic toxicities were fatigue (6%), diarrhea, nausea, and rash (5% each). One patient with extensive previous cardiac history (coronary artery disease, myocardial infarction, and cardiac stents) had grade 4 congestive heart failure after the occurrence of a second myocardial infarction while on study. Ten patients discontinued from the study because of one or more AEs. AEs leading to premature discontinuation included weight loss, liver function abnormalities, secondary carcinomas, nausea, vomiting, fatigue, musculoskeletal pain, pulmonary embolism, pleural effusion, rash, thrombocytopenia, edema, and hand-foot syndrome. All 115 patients experienced at least one AE; 74 required either a dose adjustment or a transient treatment interruption. The most common AEs leading to dose adjustment or treatment interruption were thrombocytopenia (17%), vomiting (12%), neutropenia (11%), and nausea (11%).
When the incidence of AEs with imatinib 800 mg/d in the RIGHT trial was compared with that seen in the IRIS trial,3 nonhematologic grade 3/4 AEs occurred at a somewhat higher rate among high-dose imatinib patients versus those who received standard-dose imatinib (Table 5). Grade 3/4 neutropenia and anemia were similar in both groups, whereas the incidence of thrombocytopenia was higher among patients who received imatinib 400 mg twice a day versus patients from the IRIS trial who received imatinib 400 mg/d. Some grade 1/2 toxicities (eg, GI, edema, rash) were more frequent among patients treated with high-dose imatinib.
Table 5.
Adverse Events Occurring With Imatinib 400 mg/d From the International Randomized Study of Interferon and STI571 (IRIS) Trial2 Experienced by at Least 5% of Patients, Regardless of Causality
| Adverse Event | Patients, % (N = 553) |
||
|---|---|---|---|
| Any | Grade 1/2 | Grade 3/4 | |
| Nonhematologic | |||
| Nausea | 44 | 43 | 1 |
| Diarrhea | 33 | 31 | 2 |
| Fatigue | 35 | 34 | 1 |
| Superficial edema | 56 | 55 | 1 |
| Vomiting | 17 | 15 | 2 |
| Rash | 34 | 32 | 2 |
| Headache | 31 | 31 | 0 |
| Musculoskeletal events | |||
| Pain | 37 | 34 | 3 |
| Muscle spasms | 38 | 37 | 1 |
| Arthralgia | 28 | 26 | 2 |
| Myalgia | 21 | 19 | 2 |
| Abdominal pain | 27 | 25 | 2 |
| Nasopharyngitis | 22 | 22 | 0 |
| Pyrexia | 13 | 12 | 1 |
| Hematologic | |||
| Anemia | 45 | 42 | 3 |
| Neutropenia | 61 | 47 | 14 |
| Thrombocytopenia | 57 | 49 | 8 |
DISCUSSION
The rationale for this multicenter study of high-dose initial therapy in CML-CP is based on improved rates and earlier occurrence of both molecular and cytogenetic responses seen with higher doses of imatinib in single-institution trials, and the anticipated benefit of achieving early molecular and cytogenetic responses with imatinib. Therefore, the RIGHT trial was conducted to evaluate the effect of imatinib 800 mg/d, given as 400 mg twice a day, in achieving early molecular and cytogenetic responses. Although it is difficult to establish the real value of high-dose versus standard-dose imatinib without a head-to-head, randomized clinical trial, comparisons with historical data strongly suggest that higher dose may be associated with improved clinical benefit. At 12 months from start of therapy, molecular and cytogenetic response rates were higher among patients enrolled onto the RIGHT trial (400 mg twice a day) compared with historical data from the IRIS trial (400 mg once daily). Molecular response occurred at a faster rate in patients receiving high-dose imatinib, with 12-month MMR of 54% compared with 39% receiving standard-dose imatinib (Fig 2).9 The observed 12-month MCyR rate for evaluable patients in the RIGHT trial was higher than the estimated best MCyR rate in the IRIS trial (90% v 84%, respectively).2 The difference was more significant for 12-month CCyR rates (85% v 65%, respectively). Although response rates are lower on an intention-to-treat analysis, there is no historical data available for comparison. Results from the RIGHT trial were consistent with a previous study of patients with newly diagnosed CML-CP conducted at a single institution comparing imatinib 800 mg/d with historical controls given imatinib 400 mg/d,8 which demonstrated MMR rates statistically significantly higher with imatinib 800 mg/d than with 400 mg/d historical controls. CCyR rates in this study were also significantly greater in patients receiving high-dose imatinib (90% v 74%; P = .01) with 15-month follow-up. These data suggest that high-dose imatinib may induce higher rates of cytogenetic and molecular responses compared with standard-dose imatinib.
Prior reports using high-dose imatinib have come from single-institution studies in which maintaining dose-intensity could be more feasible compared with maintaining dose-intensity in multicenter trials. However, in this multicenter study including both academic and community institutions, dose-intensity of more than 90% was achieved in most patients and was not influenced by age. Furthermore, dose-intensity significantly correlated with a reduction in BCR-ABL transcripts. Patients who received higher dose-intensity had a statistically significant increase in achievement of MMR and CMR (Fig 1B). This supports the concept that higher exposure to imatinib is associated with a more dramatic disease response by cytogenetic and molecular parameters. Most patients who achieved CCyR at 12 and 18 months also achieved MMR, reinforcing the clinical benefit of imatinib 800 mg/d by both molecular and cytogenetic assessments.
Patients receiving imatinib 800 mg/d had a relatively low frequency of grade 3/4 toxicities irrespective of age. The incidence of AEs in the RIGHT trial was comparable with that seen in the IRIS trial2 among patients who received the standard dose of imatinib (400 mg/d). The RIGHT and IRIS trials had similarly low rates of grade 3/4 AEs except for a slightly higher rate of GI toxicities in the RIGHT trial, suggesting that high-dose imatinib had tolerability comparable to that of standard-dose imatinib. Additionally, the incidence of nonhematologic AEs in the RIGHT trial was consistent with that previously reported in a single-institution study that also investigated 800 mg/d, given as 400 mg twice a day, in newly diagnosed patients.8 These results suggest that high-dose imatinib could be reliably delivered and consistently maintained for prolonged periods across a variety of patient care settings. Still, fully assessing patient compliance remains a challenge for oral drugs because investigators cannot account for variations not reported to physicians. Ongoing randomized trials comparing standard- and high-dose imatinib will definitively address and may confirm these results.
In conclusion, patients with CML-CP treated with imatinib 400 mg twice a day as initial therapy achieved rapid and deep responses. Median dose-intensity was high, with no difference in dose-intensity among patients younger or older than age 65 years and no difference in the types or rates of AEs at any severity in these age groups. Thus, imatinib 800 mg/d is a feasible and effective dosing strategy for achieving early cytogenetic and molecular responses in patients with newly diagnosed CML-CP. Whether these early responses were influenced by the high proportion of Sokal low-risk patients and will translate into an improved long-term outcome for patients remains to be determined in ongoing randomized clinical trials.
Acknowledgment
We thank the patients and their families; coinvestigators; the members of the medical, nursing, and research staff at the trial centers; the clinical trial monitors and the data managers and programmers at PPD for their contributions; and Ghulam Warsi for his statistical collaboration. RIGHT (Rationale and Insight for Gleevec High-Dose Therapy) trial enrolling investigators: Luke Akard, Asad Bashey, Laszlo Boros, James Bradof, John Burfeind, John Burke, Robert Collins, Paul Conkling, Siddhartha Ganguly, Jonathan Glass, Stuart Goldberg, Richard Greenberg, Susan Guba, John Hainsworth, David Irwin, C. Michael Jones, Leonard Kalman, Robert Kerr, Jeffrey E. Lancet, Leon Landau, Mary Laughlin, Lance Mandell, Carole Miller, Ira Oliff, K. Philip, Bayard Powell, Saleh Mansoor, Jasotha Sanmugarajah, Richard Shadduck, Roger Strair, Marjorie Vukelich, and Meir Wetzler. Medical writing assistance was funded by and editorial support was provided by Novartis Pharmaceuticals.
Footnotes
Written on the behalf of the Rationale and Insight for Gleevec High-Dose Therapy (RIGHT) Trial Study Group.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00081926.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: August Salvado, Novartis (C); Karen McDougall, Novartis (C) Consultant or Advisory Role: Stuart L. Goldberg, Novartis (U); Luke Akard, Novartis (U); Jerald Radich, Novartis (U) Stock Ownership: August Salvado, Novartis; Karen McDougall, Novartis Honoraria: Meir Wetzler, Novartis Research Funding: Jorge E. Cortes, Novartis; Hagop M. Kantarjian, Novartis; Stuart L. Goldberg, Novartis; Bayard L. Powell, Novartis; Francis J. Giles, Novartis; Luke Akard, Novartis; Jerald Radich, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jorge E. Cortes, Francis J. Giles, Luke Akard, Robert Kerr, Mansoor Saleh, August Salvado, Jerald Radich
Administrative support: Karen McDougall
Provision of study materials or patients: Meir Wetzler, Luke Akard
Collection and assembly of data: Hagop M. Kantarjian, Stuart L. Goldberg, Bayard L. Powell, Francis J. Giles, Meir Wetzler, John M. Burke, Karen McDougall, Maher Albitar, Jerald Radich
Data analysis and interpretation: Jorge E. Cortes, Hagop M. Kantarjian, Stuart L. Goldberg, Francis J. Giles, Luke Akard, Jerald Radich
Manuscript writing: Jorge E. Cortes, Hagop M. Kantarjian, Stuart L. Goldberg, Francis J. Giles, Karen McDougall, Jerald Radich
Final approval of manuscript: Bayard L. Powell, Francis J. Giles, Meir Wetzler, Luke Akard, John M. Burke, Robert Kerr, Mansoor Saleh, August Salvado, Maher Albitar
REFERENCES
- 1.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European Leukemia Net. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Talpaz M, Goldman JM, Sawyers CL, et al. High dose imatinib (STI571, Gleevec) provides long-term outcomes for patients with chronic myeloid leukemia (CML) in accelerated phase or myeloid blast crisis: Follow-up of the phase 2 studies. Blood. 2003;102:905a–906a. abstr 3369. [Google Scholar]
- 5.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J, Giles F, O'Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 9.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 10.Cortes JE, Talpaz M, Kantarjian H. Chronic myelogenous leukemia: A review. Am J Med. 1996;100:555–570. doi: 10.1016/s0002-9343(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 11.Ma W, Tseng R, Gorre M, et al. Plasma RNA as an alternative to cells for monitoring molecular response in patients with chronic myeloid leukemia. Haematologica. 2007;92:170–175. doi: 10.3324/haematol.10360. [DOI] [PubMed] [Google Scholar]
- 12.Radich JP, Gooley T, Bryant E, et al. The significance of bcr-abl molecular detection in chronic myeloid leukemia patients “late,” 18 months or more after transplant. Blood. 2001;98:1701–1707. doi: 10.1182/blood.v98.6.1701. [DOI] [PubMed] [Google Scholar]