Abstract
Purpose
Relapsed adult acute lymphoblastic leukemia (ALL) is associated with high reinduction mortality, chemotherapy resistance, and rapid progression leading to death. Vincristine sulfate liposome injection (VSLI), sphingomyelin and cholesterol nanoparticle vincristine (VCR), facilitates VCR dose-intensification and densification plus enhances target tissue delivery. We evaluated high-dose VSLI monotherapy in adults with Philadelphia chromosome (Ph) –negative ALL that was multiply relapsed, relapsed and refractory to reinduction, and/or relapsed after hematopoietic cell transplantation (HCT).
Patients and Methods
Sixty-five adults with Ph-negative ALL in second or greater relapse or whose disease had progressed following two or more leukemia therapies were treated in this pivotal phase II, multinational trial. Intravenous VSLI 2.25 mg/m2, without dose capping, was administered once per week until response, progression, toxicity, or pursuit of HCT. The primary end point was achievement of complete response (CR) or CR with incomplete hematologic recovery (CRi).
Results
The CR/CRi rate was 20% and overall response rate was 35%. VSLI monotherapy was effective as third-, fourth-, and fifth-line therapy and in patients refractory to other single- and multiagent reinduction therapies. Median CR/CRi duration was 23 weeks (range, 5 to 66 weeks); 12 patients bridged to a post-VSLI HCT, and five patients were long-term survivors. VSLI was generally well tolerated and associated with a low 30-day mortality rate (12%).
Conclusion
High-dose VSLI monotherapy resulted in meaningful clinical outcomes including durable responses and bridging to HCT in advanced ALL settings. The toxicity profile of VSLI was predictable, manageable, and comparable to standard VCR despite the delivery of large, normally unachievable, individual and cumulative doses of VCR.
INTRODUCTION
Adult Philadelphia chromosome (Ph)–negative acute lymphoblastic leukemia (ALL) is a B- or T-cell lineage hematologic malignancy with approximately 2,000 to 2,500 new patients diagnosed annually in the United States.1 In contrast to children with ALL, the prognosis for adults diagnosed with ALL is poor.2–4 Although initial rates of complete response (CR) in adult ALL can achieve 92% following first-line, curative-intent, multiagent chemotherapy including a corticosteroid, vincristine sulfate (VCR), and an anthracycline, most patients relapse while still on therapy.4–8 Second-line chemotherapy can achieve a second remission in approximately 40% of adults following first relapse; however, the vast majority of patients relapse again within months.4,5,9,10 Adult, Ph-negative ALL in second or greater relapse, that relapses and is refractory to one or more reinduction attempts, and/or relapsed following a hematopoietic cell transplantation (HCT) accounts for approximately 1,600 patients annually in the United States and is regarded as relatively chemotherapy resistant, incurable, and associated with reinduction mortality rates up to 30%.3 There are no established standards of care in this setting and limited treatment options.
Vincristine sulfate liposome injection (VSLI; Marqibo, Talon Therapeutics), a sphingomyelin- and cholesterol-based, nanoparticle formulation of the widely used anticancer drug, VCR, was designed to overcome the dosing and pharmacokinetic limitations of standard, nonliposomal VCR.11 In nonclinical experiments, VSLI had a larger maximum-tolerated dose than standard VCR and demonstrated enhanced mg-per-mg antileukemia activity without additional toxicity.11–13 VSLI enhanced VCR penetration and concentration in tissues and organs with fenestrated vasculature or involved in the mononuclear phagocyte system, including ALL target tissues (eg, bone marrow, lymph nodes, spleen) and implanted tumors.11–13
Based on a clear unmet medical need, the superiority of VSLI over standard VCR in nonclinical models, and encouraging activity attributed to VSLI in the phase 1 dose-ascending trial,14 we conducted a multinational, pivotal, phase II, single-arm, open-label trial of high-dose (2.25 mg/m2), once-per-week VSLI monotherapy in heavily pretreated adults with advanced, relapsed, and refractory B- or T-cell lineage Ph-negative ALL. This study served as the basis for accelerated approval in the United States granted on August 9, 2012.
PATIENTS AND METHODS
Patients
Adults (at least 18 years old) with confirmed Ph-negative ALL in second or greater relapse, or who had progressed following two or more lines of antileukemia chemotherapy (including HCT) were study eligible. Eligible patients had at least one prior CR with a leukemia-free interval of at least 90 days. Patients with residual persistent grade 1 or nonpersistent grade 2 or higher prior VCR-related neuropathy according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 were eligible. Patients with Burkitt's type leukemia, active and uncontrolled CNS ALL, and those eligible for immediate HCT (ie, readily available suitable donor, willingness to undergo HCT, and investigator belief that HCT was a better treatment option than VSLI) were excluded.
Study Oversight and Support
The institutional review board or independent ethics committee of each participating institution approved the study. This study was conducted in accordance with the principles of the World Medical Association's Declaration of Helsinki, the International Conference on Harmonisation Guideline for Good Clinical Practice, and the laws and regulations of the countries in which the research was conducted. All patients provided written, informed consent.
An independent data monitoring committee reviewed the study conduct and safety profile. An independent response review committee (IRRC) was unaware of principal investigator (PI) assessments, corroborated PI response assessments, and calculated response and survival durations. A central laboratory analyzed all bone marrow (BM) samples.
Study Design
Patients received once-per-week VSLI 2.25 mg/m2 monotherapy based on actual body-surface area and without dose capping. VSLI doses represent the VCR component of the drug. VSLI was administered intravenously via peripheral or central venous access over 60 minutes on days 1, 8, 15, and 22 of a 28-day course in an outpatient or inpatient treatment setting. Hydroxyurea and systemic corticosteroids to control leukocytosis were allowed through day 14 and day 5 of course 1, respectively. Patients received VSLI once per week until response achievement, leukemia progression, toxicity, or decision to pursue other therapy such as HCT. Patients with ongoing response could continue VSLI at the PI discretion.
A protocol-specified VSLI dose-modification algorithm (Appendix Table A1, online-only) was used to manage persistent grade 2 and any grade 3 or greater nonhematologic toxicity while attempting to maximize cumulative VSLI dosing and achievement of clinical benefit. Dose modification included 1-week dosing breaks and dose reduction.
End Points and Assessments
The protocol-specified primary efficacy end point was the proportion of patients who achieved CR or CR with incomplete recovery of peripheral blood neutrophil counts (< 1 × 109/L) or platelet counts (< 100 × 109/L; CRi) based on PI assessment. CRi was included because patients were expected to be heavily pretreated and unlikely to ever normalize their blood counts.
Secondary efficacy end points included CR/CRi duration, overall survival (OS), proportion of patients “bridged” to post-VSLI HCT, overall response rate (CR + CRi + partial remission [PR] + BM blast response [BMB]), and time to CR/CRi achievement. A BMB is a morphologic CR with incomplete recovery of both peripheral blood neutrophil and platelet counts. Efficacy end point criteria were adapted from published reporting standards.15,16
Exploratory analyses included assessment of response and survival as functions of baseline patient and disease characteristics. Postinduction minimal residual disease (MRD), based on site-specific flow cytometry analysis of BM, was evaluated posthoc in CR/CRi patients.
Patients underwent standard medical history and physical examination and a 15-point neurologic assessment to proactively identify peripheral neuropathy before each VSLI dose and 30 days after the last dose. BM examination and assessment of documented extramedullary disease were targeted for day 28 of study treatment courses 1 and 2 and every other active treatment course thereafter.
Statistical Analysis
This study was designed to detect a CR/CRi rate at least double the historical rate of 3.6% based on available data in the target population.3 Achievement of CR/CRi in nine (16%) of 56 patients (95% CI, 7.6 to 28.4) was the predefined threshold for a positive study. The study protocol was later amended to increase the sample size from 56 to 65 to accommodate the collection of pharmacokinetic data.
Efficacy and safety were assessed in patients who received at least one dose of VSLI. Exact two-sided 95% CI were constructed where appropriate.17 Multivariate logistic regression was applied to analyze CR/CRi prognostic factors. Standard multivariate Cox proportional hazards regression analysis was used to evaluate OS prognostic factors. Analyses were carried out using SAS version 9.1 or higher (SAS Institute, Cary, NC).
RESULTS
Patient and Disease Characteristics
Baseline demographic and disease characteristics are listed in Table 1. The patient population was clinically advanced and heavily pretreated. Most presented with a large disease burden as reflected by the median BM and peripheral blood blast contents of 82%, more than half had already received three or more lines of therapy, and 45% were refractory to their immediate prior line of antileukemia therapy. Patients had been exposed to a median of nine and as many as 15 different antileukemia drugs before enrollment. All patients had previously received one or more standard VCR therapies. Ongoing, pre-VSLI, neuropathy-related signs or symptoms including extremity pain (31%), areflexia (28%), constipation (17%), and hypoesthesia (14%) were present in 77% of patients, consistent with the prior VCR exposure. Four patients received at least one dose of hydroxyurea and 10 received a corticosteroid for leukocytosis control.
Table 1.
Baseline Demographic and Disease Characteristics of the Study Patients (N = 65)
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Women | 32 | 49 |
| Men | 33 | 51 |
| Race | ||
| White | 56 | 86 |
| Black or African American | 4 | 6 |
| Asian | 2 | 3 |
| Other | 3 | 5 |
| Ethnicity | ||
| Hispanic | 17 | 26 |
| Not Hispanic | 48 | 74 |
| Age, years | ||
| Median | 31 | |
| Range | 19 to 83 | |
| 18-29 | 29 | 45 |
| 30-59 | 28 | 43 |
| ≥ 60 | 8 | 12 |
| ECOG performance status at baseline | ||
| 0 or 1 | 50 | 77 |
| 2 or 3 | 15 | 23 |
| Prior VCR exposure | ||
| Yes | 65 | 100 |
| No | 0 | 0 |
| Prior HCT | ||
| Yes | 31 | 48 |
| No | 34 | 52 |
| Ongoing neuropathy sign or symptom at baseline | ||
| Yes | 50 | 76.9 |
| No | 15 | 23.1 |
| ALL lineage | ||
| B cell | 55 | 85 |
| T cell | 10 | 15 |
| Extramedullary disease at baseline | ||
| Yes | 10 | 15 |
| No | 55 | 85 |
| Number of prior lines of anti-ALL therapy | ||
| 2 | 32 | 49 |
| 3 | 24 | 37 |
| ≥ 4 | 9 | 14 |
| Refractory to immediate prior line of therapy | ||
| Yes | 29 | 45 |
| No | 36 | 55 |
| Baseline bone marrow leukemic blasts, % | ||
| Median | 82 | |
| Range | 1 to 99 | |
| > 50 | 52 | 80 |
| ≤ 50 | 13 | 20 |
| Baseline bone marrow cytogenetics | ||
| Unfavorable | 33 | 51 |
| Intermediate | 23 | 35 |
| Favorable | 0 | 0 |
| Not available | 9 | 14 |
Abbreviations: ALL, acute lymphoblastic leukemia; ECOG, Eastern Cooperative Oncology Group; HCT, hematopoietic cell transplantation; VCR, vincristine.
Patients received a median of four doses of VSLI (range, 1 to 18) with a median individual dose size of 4.12 mg (range, 3.14 to 5.51 mg). Median dose density was 2.25 mg/m2/week (range, 0.94 to 2.29 mg/m2/week) and median cumulative VSLI exposure was 18.84 mg (range, 3.50 to 70.12 mg). Seventy-two percent of VSLI doses were administered in an outpatient setting.
Response
CR/CRi was achieved in 20% of patients (Table 2). An additional 15% of patients achieved a PR or BMB resulting in an overall response rate of 35%. Table 3 lists the 13 patients who achieved CR/CRi.
Table 2.
Primary, Secondary, and Key Subgroup Efficacy Outcomes(intention-to-treat population; N = 65)
| Outcome | No. of Patients | % |
|---|---|---|
| Overall complete response | 13 | 20 |
| 95% CI | 11.1 to 31.8 | |
| CR | 7 | 11 |
| 95% CI | 4.4 to 20.9 | |
| CRi | 6 | 9 |
| 95% CI | 3.5 to 19.0 | |
| PR | 6 | 9 |
| 95% CI | 3.5 to 19.0 | |
| BMB | 4 | 6 |
| 95% CI | 1.7 to 15.0 | |
| Overall response (CR, CRi, PR, and BMB) | 23 | 35 |
| 95% CI | 23.8 to 48.3 | |
| CR and CRi, based on baseline disease status | ||
| Relapsed ALL (n = 36) | 9 | 25 |
| Relapsed and refractory ALL (n = 29) | 4 | 14 |
| CR and CRi based on ALL lineage | ||
| B-cell lineage (n = 55) | 11 | 20 |
| T-cell lineage (n = 10) | 2 | 20 |
| CR and CRi based on VSLI line of therapy | ||
| 3rd line (n = 32) | 6 | 19 |
| 4th line (n = 24) | 5 | 21 |
| 5th or greater line (n = 9) | 2 | 22 |
| CR and CRi based on prior HCT | ||
| Prior HCT (n = 31) | 8 | 26 |
| No prior HCT (n = 34) | 5 | 15 |
| CR and CRi based on prior clofarabine treatment | ||
| Prior clofarabine in a multidrug regimen (n = 3) | 0 | 0 |
| Prior clofarabine as a single agent (n = 4) | 2 | 50 |
| No prior clofarabine treatment (n = 58) | 11 | 19 |
| CR and CRi based on baseline bone marrow cytogenetics | ||
| Unfavorable (n = 33) | 5 | 15 |
| Intermediate (n = 23) | 6 | 26 |
| CR and CRi based on baseline age group, years | ||
| 18 to 29 (n = 29) | 10 | 34 |
| 30 to 59 (n = 28) | 2 | 7 |
| ≥ 60 (n = 8) | 1 | 13 |
| CR and CRi based on dose reduction | ||
| Required dose reduction (n = 15) | 7 | 47 |
| No required dose reduction (n = 50) | 6 | 12 |
| Time to CR and CRi, days | ||
| Median | 54 | |
| Range | 25 to 81 | |
| CR and CRi duration (uncensored), weeks | ||
| Median | 23 | |
| Range | 5 to 66 | |
| Overall survival, months | ||
| Median | 4.6 | |
| Range | < 1 to > 25 | |
| Post-VSLI HCT | 12 | 18.5 |
Abbreviations: ALL, acute lymphoblastic leukemia; BMB, bone marrow blast response; CR, complete response; CRi, CR with incomplete hematologic recovery; HCT, hematopoietic cell transplantation; PR, partial remission; VSLI, vincristine sulfate liposome injection.
Table 3.
Disease Characteristics and Outcomes in Complete Responders
| CR/CRi Patient | Lineage | Baseline BM Blast (%) | Baseline PB Blast (%) | Relapse Status | Pre-VSLI HCT | Refractory to | VSLI Line of Therapy | VSLI Cumulative Exposure (mg) | VSLI Response (per PI) | Post-VSLI MRD | Post-VSLI HCT | CR/CRi Duration (days) | OS (days) | Post-VSLI Grade 3/4 Peripheral Neuropathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B | 99 | 96 | 2nd | + | 3rd | 15.8 | CRi | + | + | 132 | 327 | None | |
| 2 | T | 25 | 51 | 2nd | + | Cytarabine and methotrexate | 5th | 20.6 | CR | + | − | 35 | 139 | + (3) |
| 3 | B | 90 | 95 | 2nd | + | 4th | 19.6 | CRi | + | − | 42 | 71 | None | |
| 4 | B | 95 | 94 | 2nd | − | 3rd | 27.2 | CR | − | − | 162 | 321 | None | |
| 5 | B | 98 | 95 | 3rd | + | 5th | 17.4 | CRi* | − | − | 89 | 137 | None | |
| 6 | B | 40 | 1 | 2nd | + | 4th | 43.2 | CR | − | − | 166 | 230 | + (4) | |
| 7 | T | 84 | 84 | 1st | + | 3rd | 33.4 | CRi | + | + | 210 | 267 | + (3) | |
| 8 | B | 75 | 40 | 1st | + | 3rd | 41.4 | CR | − | + | 162 | 230 | None | |
| 9 | B | 93 | 93 | 2nd | − | 3rd | 30.3 | CR | − | + | 135 | 163 | None | |
| 10 | B | 90 | 41 | 2nd | − | Clofarabine | 4th | 49.3 | CRi | − | − | 32 | 232 | + (3) |
| 11 | B | 63 | 63 | 2nd | + | 4th | 58.6 | CR | − | − | 144 | 229 | None | |
| 12 | B | 81 | 81 | 2nd | − | Vinorelbine, mitoxantrone, and cytarabine | 4th | 25.2 | CR | N/A | + | 463 | 699 | None |
| 13 | B | 30 | 54 | 1st | − | Clofarabine | 3rd | 26.1 | CRi* | − | − | 33 | 121 | + (3) |
NOTE. Details regarding the drugs used in prior lines of therapy are available in Appendix Table A2 (online only).
Abbreviations: B, B cell; BM, bone marrow; CR, complete response; CRi, CR with incomplete hematologic recovery; HCT, hematopoietic cell transplantation; IRRC, independent response review committee; MRD, minimal residual disease; N/A, not applicable; OS, overall survival; PB, peripheral blood; PI, principal investigator; T, T cell; VSLI, vincristine sulfate liposome injection; +, affirmative; −, negative.
BMB per the IRRC.
Younger age and higher baseline platelet count were independently associated with a favorable response (P < .05). Patients with a baseline platelet count of greater than 50 × 109/L achieved a significantly higher CR/CRi rate (odds ratio, 5.5; 95% CI, 1.04 to 29.2). Time interval between last standard VCR exposure and VSLI treatment did not correlate with likelihood of response.
CR/CRi was achieved in 25% of patients with an untreated relapse and 14% of those with relapse that was refractory to one or more lines of single-agent (ie, clofarabine) or multiagent (eg, high-dose cytarabine plus methotrexate and vinorelbine, mitoxantrone, plus cytarabine) antileukemia therapy. The IRRC corroborated 11 CR/CRi and assessed the remaining two as BMB, based on available laboratory data.
The majority of patients (nine [69%] of 13) with a CR/CRi from VSLI had a response duration that exceeded the duration of response to their immediate prior line of ALL therapy. One patient who achieved CR/CRi relapsed approximately 15 months later and achieved a second CR following re-treatment with VSLI monotherapy on a compassionate basis.
Survival
As illustrated in Figure 1, the OS rates were 89% (95% CI, 82 to 97) at 1 month, 35% (95% CI, 23 to 47) at 6 months, 8% (95% CI, 1 to 15) at 12 to 18 months, and 4% (95% CI, 0 to 9) at 24 months. Median OS, overall, was 4.6 months (range, < 1 month to > 25 months) and 7.7 months (range, 2.4 months to > 23.3 months) in those achieving CR/CRi. Five patients had an OS longer than 1 year. Two patients are still alive. Prognostic factors associated independently with more prolonged OS were younger age, Eastern Cooperative Oncology Group performance status 0 or 1, duration of first CR of 36 months or longer, baseline serum albumin of 3 g/L or higher, and baseline hemoglobin level of 10 g/dL or higher.
Fig 1.
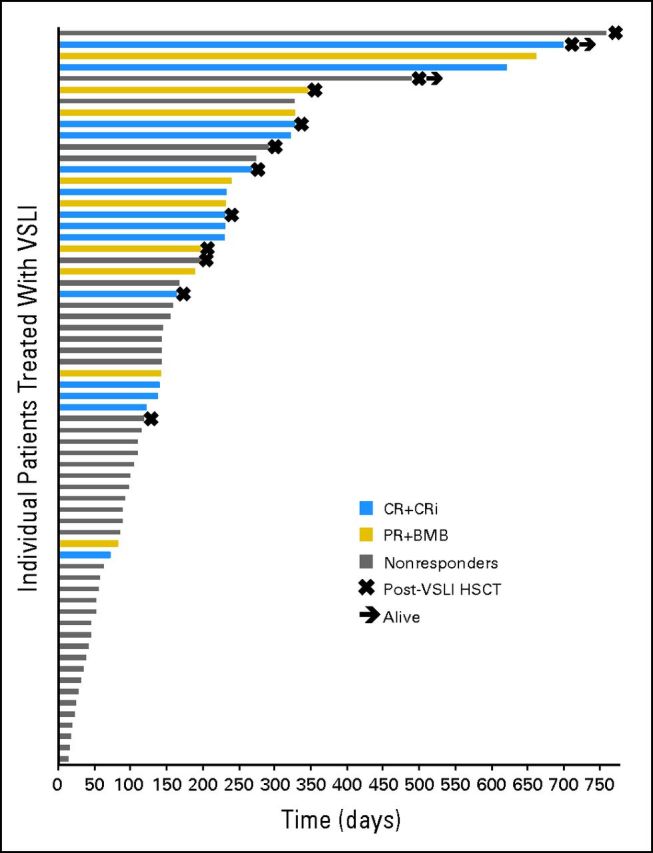
Overall survival and hematopoietic cell transplantation after vincristine sulfate liposome injection (post-VSLI HCT) among the 65 patients treated with VSLI monotherapy. The median overall survival in the 65 patients treated with VSLI monotherapy was 4.6 months, with 34% of patients living longer than 6 months. There were five long-term survivors (ie, survival for longer than 1 year) and two patients who remain alive and potentially represent cures. The median overall survival in responders (patients experiencing complete response, complete response with incomplete hematologic recovery, partial remission, and bone marrow blast response [CR + CRi + PR + BMB]) was 7.7 months, with 70% of patients alive for longer than 6 months. Nonresponders had a median overall survival of 3.0 months, with 14% alive for longer than 6 months. The median overall survival in the 12 patients who underwent post-VSLI HCT was 9.3 months, with 83% alive for longer than 6 months.
Stem-Cell Transplantation
Twelve (19%) patients underwent HCT after being treated with VSLI monotherapy; five patients after achieving CR/CRi, one after achieving a PR, one after a BMB, and five patients without a documented response. The seven responders with a post-VSLI HCT had a median OS of 8.9 months (range, 5.4 to > 23.3 months) with 86% surviving longer than 6 months, whereas the 16 responders without post-VSLI HCT had a median OS of 7.7 months (range, 2.4 to 22.0 months) with 63% surviving longer than 6 months. Overall, patients receiving transplantations had a median OS of 9.3 months (range, 4.0 to 25.3 months). Only 5% of VSLI nonresponders without post-VSLI HCT survived longer than 6 months.
MRD Assessment
Post-VSLI induction MRD data were available for 12 of the 13 patients who achieved CR/CRi. Molecular remission, based on MRD negativity, was confirmed in 67% (eight of 12 patients), including both CRi patients assessed as BMBs by the IRRC. Median response duration was 140 days (range, 32 to 162 days) in the MRD-negative group and 87 days (range, 35 to 210 days) in the MRD-positive group.
Safety
Treatment-related adverse events (AEs) occurring in at least 5% of patients are presented in Table 4. Overall, 86% of patients had a neuropathy-associated AE. There was no significant pattern in the frequency or type of AE when evaluated by sex, race, or age.
Table 4.
Treatment-Related Adverse Events by System Organ Class, Preferred Term, and Maximum CTCAE Grades 3 and 4 in ≥ 5%of Patients
| System Organ Class Preferred Term | ITT Population (N = 65) |
|||||
|---|---|---|---|---|---|---|
| All Grades |
Grade 3 |
Grade 4 |
||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Any treatment-related adverse event | 53 | 82 | 25 | 39 | 12 | 19 |
| Nervous system disorders | 41 | 63 | 12 | 19 | 1 | 2 |
| Neuropathy peripheral | 19 | 29 | 10 | 15 | 0 | 0 |
| Hypoesthesia | 16 | 25 | 1 | 2 | 0 | 0 |
| Paraesthesia | 13 | 20 | 1 | 2 | 0 | 0 |
| Areflexia | 6 | 9 | 1 | 2 | 0 | 0 |
| Hyporeflexia | 5 | 8 | 0 | 0 | 0 | 0 |
| GI disorders | 33 | 51 | 8 | 12 | 0 | 0 |
| Constipation | 22 | 34 | 2 | 3 | 0 | 0 |
| Nausea | 14 | 22 | 0 | 0 | 0 | 0 |
| Vomiting | 7 | 11 | 0 | 0 | 0 | 0 |
| Abdominal pain | 6 | 9 | 2 | 3 | 0 | 0 |
| Diarrhea | 4 | 6 | 1 | 2 | 0 | 0 |
| Blood and lymphatic system disorders | 19 | 29 | 6 | 9 | 8 | 12 |
| Neutropenia | 11 | 17 | 5 | 8 | 5 | 8 |
| Anemia | 8 | 12 | 3 | 5 | 0 | 0 |
| Thrombocytopenia | 6 | 9 | 1 | 2 | 3 | 5 |
| Febrile neutropenia | 5 | 8 | 2 | 3 | 0 | 0 |
| General disorders and administration site conditions | 16 | 25 | 5 | 8 | 0 | 0 |
| Fatigue | 7 | 11 | 2 | 3 | 0 | 0 |
| Asthenia | 6 | 9 | 2 | 3 | 0 | 0 |
| Pyrexia | 6 | 9 | 1 | 2 | 0 | 0 |
| Metabolism and nutrition disorders | 14 | 22 | 4 | 6 | 2 | 3 |
| Decreased appetite | 8 | 12 | 1 | 2 | 0 | 0 |
| Tumor lysis syndrome | 5 | 8 | 1 | 2 | 2 | 3 |
| Investigations | 12 | 19 | 5 | 8 | 1 | 2 |
| Weight decreased | 7 | 11 | 0 | 0 | 0 | 0 |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; ITT, intention-to-treat.
Twenty-five patients (39%) experienced at least one grade 3 treatment-related AE, and 12 patients (19%) experienced at least one grade 4 treatment-related AE. Grade 3 peripheral neuropathy-related events combined (eg, hypoesthesia, hyporeflexia, neuropathy peripheral, limb pain, and motor weakness) were reported in 23% of patients. There was only one grade 4 peripheral neuropathy-related AE (sensory peripheral neuropathy) and no incidents of grade 4 constipation. There was no grade 3 or grade 4 nausea or vomiting.
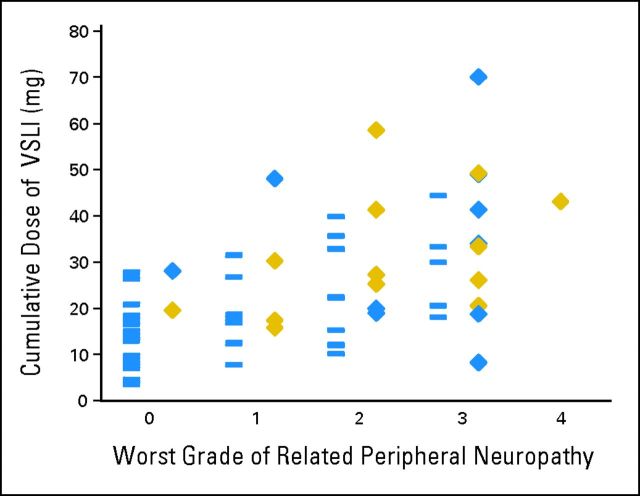
Figure 2demonstrates the trend toward higher VSLI exposure in patients with higher-grade related peripheral neuropathy and more responses. However, peripheral neuropathy development was not required for a patient to achieve CR/CRi. Fifty-two percent of responders and 62% of patients with CR/CRi developed grade 2 or less related peripheral neuropathy.
Fig 2.
Relationship between cumulative vincristine sulfate liposome injection (VSLI) exposure, worst grade of related peripheral neuropathy adverse events, and response. Each grade of VSLI-related peripheral neuropathy has two columns of data. The blue rectangles in the left-hand columns of data represent nonresponders. The blue diamonds in the right-hand columns of data represent patients who achieved a partial remission or bone marrow blast response. The gold diamonds represent patients who achieved complete response or complete response with incomplete hematologic recovery. Responses, including complete response or complete response with incomplete hematologic recovery, were achieved at each grade of related peripheral neuropathy. Application of the dose reduction algorithm was intended to manage grade 3 toxicity and continue dosing to achieve response.
Approximately one third of patients (32%) experienced at least one serious adverse event that was assessed as treatment-related. The most frequently occurring treatment-related serious adverse events were neuropathy peripheral (6%), febrile neutropenia (5%), tumor lysis syndrome (5%), and constipation (3%).
Sixty patients (92%) died during the study; 15 patients (23%) died during the treatment period and 45 patients (69%) died during the follow-up period (ie, more than 30 days following the last VSLI dose). The 30-day induction mortality rate was 12%. The most frequently occurring AEs resulting in death were ALL (12%), respiratory distress (5%), septic shock (3%), and cardiac arrest (3%). No neuropathy-associated AE led to death.
DISCUSSION
High-dose, once-per-week VSLI (2.25 mg/m2) monotherapy delivered as third-, fourth-, fifth-, or seventh-line therapy to heavily pretreated and immunocompromised, adult patients with previously responsive, advanced, relapsed and refractory Ph-negative ALL resulted in an overall response rate of 35%, CR/CRi rate of 20%, and HCT bridging rate of 19% in conjunction with a predictable and manageable toxicity profile. Better than anticipated survival, consistent with that expected in the second-line therapy setting and not the third or greater line therapy, was attributed to a combination of meaningful single-agent efficacy and relatively low 30-day induction mortality rate. Demonstrated efficacy in patients with relapsed ALL refractory to clofarabine or vinca-alkaloid containing multiagent therapy and patients with relapse post-HCT is testament to the antileukemia activity of VSLI. High-dose VSLI provided a generally well-tolerated and effective inpatient or outpatient salvage treatment option for patients with little else to be offered other than a purely comfort care management approach.18
In addition to optimizing pharmacokinetics19 and fostering enhanced target-tissue delivery,11–13 the VSLI formulation facilitated VCR dose-intensification from 1.4 mg/m2, with a widely employed 2.0 mg cap, to 2.25 mg/m2, without a cap, without apparent toxicity exacerbation. VSLI delivered 1.57- to 2.76-fold as much VCR per dose as would have been delivered by a 2-mg dose of standard VCR. Combined with prolonged and sustained delivery to ALL target tissues, these increased doses were likely instrumental in overcoming any relative VCR resistance and inducing remissions.20
Based on landmark investigations, primarily in lymphoma patients, VCR dose (ie, individual dose, cumulative dose, and dose density), clinical efficacy, and neurotoxicity have been inextricably linked.21–27 Doses of standard VCR comparable to the doses of VSLI achieved in this study have been associated with almost universal grade 3 or greater neurotoxicity.21 DeVita and Hubbard24 reported that standard VCR at 1.4 mg/m2, without a dose cap (2.5 mg/dose average), delivered on days 1 and 8 of a 28-day cycle resulted in loss of deep tendon reflexes in 100% of patients. Dose reductions reduced the incidence and severity of toxicity but with a parallel reduction in CR and cure rates. The resultant practice of dose capping at 2.0 mg per infusion limits the dose, and potentially the efficacy, of standard VCR in adults with body-surface area greater than 1.43 m2.28 The standard VCR 2.0 mg dose cap remains today and is espoused in practice guidelines and employed in major cancer clinical trials.
The dose-reduction algorithm facilitated continued dosing, instead of treatment discontinuation, to achieve the highest possible cumulative VSLI dose and presumably a higher response rate. In striking contrast with past observations with standard VCR, individual dose reduction did not preclude nor reduce efficacy. In fact, of the 15 patients who underwent a dose reduction, seven patients (47%) achieved CR/CRi. MRD assessment may be of particular utility in the advanced, relapsed, and refractory ALL setting, in which CR determination may be challenging in patients with morphologic remission and incomplete recovery of either (ie, CRi) or both (ie, BMB) platelet count and neutrophil count because of extensive prior myelotoxic therapy.
In our study, once-per-week VSLI 2.25 mg/m2 produced no new or unexpected toxicities and had a toxicity profile comparable to standard VCR at its labeled dose. This is noteworthy given the markedly higher VCR dosing afforded by VSLI and the high prevalence of baseline residual neuropathy. In a comprehensive evaluation of standard VCR-related neuropathy, Haim et al29 reported a 92% neuropathy AE rate, 78% paraesthesia rate, and 16% grade 3 motor weakness rate as a result of standard VCR 1.4 mg/m2 dosed every 21 days as part of a multidrug regimen. In our study, once-per-week VSLI 2.25 mg/m2 monotherapy was related to a 63% neuropathy rate, 20% paraesthesia rate, and no incidence of grade 3 peripheral motor neuropathy. These AE rates likely reflect the successful use of the dose reduction algorithm.
There are no comparative phase 3 studies in advanced, relapsed, and refractory Ph-negative adult ALL patients. Experience is primarily limited to analyses of comparable populations from three small, phase II prospective studies and one large, single-institution, retrospective study. In a study of single-agent clofarabine, one (13%) of eight patients in need of third-line therapy achieved a short-lived CR.30 A study of single-agent nelarabine reported CR in five (18%) of 28 patients exclusively with T-cell lineage ALL or lymphoblastic lymphoma.31 An additional patient achieved a “CR*” which was equivalent to a BMB in this study. In a study of clofarabine plus cytarabine,32 three (19%) of 16 patients in need of third-line or greater therapy achieved CR/CRi.
The retrospective study included a 56 patient subgroup with Ph-negative ALL treated with non-VSLI single-agents that represents the best historical comparison group for our study population.3,33 Single-agent therapy is often warranted because of a lack of standard multiagent regimens, poor response to prior multiagent therapy and HCT, or inability to tolerate additional multiagent therapy. This subgroup had a 4% (two of 56 patients) CR/CRi rate at the expense of a 30% (17 of 56 patients) 30-day mortality rate.
In summary, high-dose VSLI monotherapy resulted in meaningful clinical outcomes including durable responses and ability to bridge to HCT in a near end-stage adult ALL population desperately in need of new therapies. The toxicity profile of high-dose VSLI was predictable, manageable, and comparable to that of standard dose and formulation VCR despite the delivery of large, normally unachievable, individual and cumulative doses of VCR. Phase 3 studies in frontline adult ALL and frontline aggressive non-Hodgkin lymphoma, predicated on the current study and the successful combination of VSLI with other chemotherapies and rituximab in other phase II studies,34,35 are currently enrolling.
Appendix
Table A1.
Recommended Dose Modifications for VSLI-Related Peripheral Neuropathy
| Severity of Peripheral Neuropathy Signs and Symptoms* | Modification of Dose and Regimen |
|---|---|
| If the patient develops grade 3 (severe symptoms; limiting self-care ADL†) or persistent grade 2 (moderate symptoms; limiting instrumental ADL‡) peripheral neuropathy | Interrupt VSLI. |
| If the peripheral neuropathy remains at grade 3 or 4, discontinue VSLI. | |
| If the peripheral neuropathy recovers to grade 1 or 2, reduce the VSLI dose to 2 mg/m2. | |
| If the patient has persistent grade 2 peripheral neuropathy after the first dose reduction to 2 mg/m2 | Interrupt VSLI for up to 7 days. |
| If the peripheral neuropathy increases to grade 3 or 4, discontinue VSLI. | |
| If peripheral neuropathy recovers to grade 1, reduce the VSLI dose to 1.825 mg/m2. | |
| If the patient has persistent grade 2 peripheral neuropathy after the second dose reduction to 1.825 mg/m2 | Interrupt VSLI for up to 7 days. |
| If the peripheral neuropathy increases to grade 3 or 4, discontinue VSLI. | |
| If the toxicity recovers to grade 1, reduce the VSLI dose to 1.5 mg/m2. |
Abbreviations: ADL, activities of daily living; VSLI, vincristine sulfate liposome injection.
Grading based on the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.
Self-care ADL refers to bathing, dressing and undressing, feeding self, using the toilet, and taking medications (not bedridden).
Instrumental ADL refers to preparing meals, shopping for groceries and clothes, using telephone, managing money, etc.
Table A2.
Prior Lines of Therapy in Complete Responders
| CR/CRi Patient | Treatment Line No. 1 | Treatment Line No. 2 | Treatment Line No. 3 | Treatment Line No. 4 | Treatment Line No. 5 |
|---|---|---|---|---|---|
| 1 | CVAPM | ELann and HCT | VSLI | ||
| 2 | CVAD | CVAAsp | BMe and HCT | AracM | VSLI |
| 3 | CVADMpMP | CVDAracAsp | BMe and HCT | VSLI | |
| 4 | IdaAracCDaunPVAspMMp | CAracMMp | VSLI | ||
| 5 | CVAAspDaunAracMMp | DaunVArac | EIDR | F and HCT | VSLI |
| 6 | VPAracAspMMp | VAPAracAsp | E and HCT | VSLI | |
| 7 | CVADAracM | C and HCT | VSLI | ||
| 8 | CVPDaunAspAracA | AracCM and HCT | VSLI | ||
| 9 | CVAD | Unknown | VSLI | ||
| 10 | VAspDaunMMp | CVDDaunAracM | Cl | VSLI | |
| 11 | CVA | CVADAspM | Unknown and HCT | VSLI | |
| 12 | VDAspDaunMMp | AspVCDaunAracIMpVb | VnMiArac | VSLI | |
| 13 | CVADMMtAracPMp | Cl | VSLI |
Abbreviations: A, doxorubicin; Arac, cytarabine arabinoside; Asp, asparaginase; B, busulfan; C, cyclophosphamide; Cl, clofarabine; CR, complete response; CRi, CR with incomplete hematologic recovery; D, dexamethasone; Daun, daunorubicin; E, etoposide; F, fludarabine; HCT, hematopoietic cell transplantation; I, ifosphamide; Ida, idarubicin; Lann, L-annamycin; M, methotrexate; Me, melphalan; Mi, mitoxantrone; Mp, 6-mercaptopurine; Mt, methylprednisolone; P, prednisone; r, rituximab; V, vincristine; Vb, vinblastine; Vn, vinorelbine; VSLI, vincristine sulfate liposome injection.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 657
Supported by Talon Therapeutics.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00495079.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships markedwith a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors
Employment or Leadership Position: Jeffrey A. Silverman, Talon Therapeutics (C); Steven R. Deitcher, Talon Therapeutics (C) Consultant or Advisory Role: Susan O'Brien, Talon Therapeutics (C); Wendy Stock, Talon Therapeutics (C), Amgen (C), Sigma Tau (C); Dan Douer, Talon Therapeutics (C); Leonard T. Heffner, Talon Therapeutics (C); Deborah Thomas, Talon Therapeutics (C) Stock Ownership: Jeffrey A. Silverman, Talon Therapeutics; Steven R. Deitcher, Talon Therapeutics Honoraria: None Research Funding: Susan O'Brien, Talon Therapeutics; Gary Schiller, Talon Therapeutics; Lloyd Damon, Talon Therapeutics; Stuart Goldberg, Talon Therapeutics; Wendy Stock, Sigma Tau; Dan Douer, Talon Therapeutics; Leonard T. Heffner, Talon Therapeutics; Karen Seiter, Talon Therapeutics; Scott Smith, Talon Therapeutics; Lori Maness, Talon Therapeutics; Markus Schaich, Talon Therapeutics; Ofer Shpilberg, Talon Therapeutics; Karen Yee, Talon Therapeutics; Hagop Kantarjian, Talon Therapeutics Expert Testimony: None Other Remuneration: Gary Schiller, Talon Therapeutics
AUTHOR CONTRIBUTIONS
Conception and design: Susan O'Brien, Deborah Thomas, Steven R. Deitcher, Hagop Kantarjian
Provision of study materials or patients: Susan O'Brien, Gary Schiller, John Lister, Lloyd Damon, Stuart Goldberg, Walter Aulitzky, Dina Ben-Yehuda, Wendy Stock, Steven Coutre, Dan Douer, Leonard T. Heffner, Melissa Larson, Karen Seiter, Sarit Assouline, Lori Maness, Markus Schaich, Ofer Shpilberg, Karen Yee, Deborah Thomas,Hagop Kantarjian
Collection and assembly of data: Susan O'Brien, Gary Schiller, John Lister, Lloyd Damon, Stuart Goldberg, Walter Aulitzky, Dina Ben-Yehuda, Wendy Stock, Dan Douer, Leonard T. Heffner, Melissa Larson, Karen Seiter, Scott Smith, Philip Kuriakose, Lori Maness, Arnon Nagler, Markus Schaich, Ofer Shpilberg, Guenter Schmieder, Jeffrey A. Silverman, Deborah Thomas, Steven R. Deitcher, Hagop Kantarjian
Data analysis and interpretation: Susan O'Brien, Stuart Goldberg, Wendy Stock, Steven Coutre, Leonard T. Heffner, Sarit Assouline, Jacob Rowe, Karen Yee, Guenter Schmieder, Jeffrey A. Silverman, Deborah Thomas, Steven R. Deitcher, Hagop Kantarjian
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2012. Cancer Facts & Figures 2012. [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: Characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 6.Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood. 1995;85:2025–2037. [PubMed] [Google Scholar]
- 7.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 8.Larson S, Stock W. Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol. 2008;15:400–407. doi: 10.1097/MOH.0b013e3283034697. [DOI] [PubMed] [Google Scholar]
- 9.Welborn JL. Impact of reinduction for relapsed and refractory acute lymphoblastic leukemia in adults. Am J of Hematol. 1994;45:341–344. doi: 10.1002/ajh.2830450413. [DOI] [PubMed] [Google Scholar]
- 10.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): An MRC UKALL 12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 11.Webb MS, Harasym TO, Masin D, et al. Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br J Cancer. 1995;72:896–904. doi: 10.1038/bjc.1995.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna R, Webb MS, St Onge G, et al. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther. 2001;298:1206–1212. [PubMed] [Google Scholar]
- 13.Webb MS, Logan P, Kanter PM, et al. Preclinical pharmacology, toxicology and efficacy of sphingomyelin/cholesterol liposomal vincristine for therapeutic treatment of cancer. Cancer Chemother Pharmacol. 1998;42:461–470. doi: 10.1007/s002800050846. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DA, Kantarjian HM, Stock W, et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer. 2009;115:5490–5498. doi: 10.1002/cncr.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelbaum FR, Rosenblum D, Arceci RJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 18.US Food and Drug Administration. Center for Drug Evaluation and Research: Oncologic Drugs Advisory Committee meeting, March 21, 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM304480.pdf.
- 19.Bedikian AY, Silverman JA, Papadopoulos NE, et al. Pharmacokinetics and safety of Marqibo (vincristine sulfate liposomes injection) in cancer patients with impaired liver function. J Clin Pharmacol. 2011;51:1205–1212. doi: 10.1177/0091270010381499. [DOI] [PubMed] [Google Scholar]
- 20.Leonetti C, Scarsella M, Semple SC, et al. In vivo administration of liposomal vincristine sensitizes drug-resistant human solid tumors. Int J Cancer. 2004;110:767–774. doi: 10.1002/ijc.20174. [DOI] [PubMed] [Google Scholar]
- 21.Carbone PP, Bono V, Frei E, III, et al. Clinical studies with vincristine. Blood. 1963;21:640–647. [PubMed] [Google Scholar]
- 22.Devita VT, Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970;73:881–895. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- 23.Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin's disease. J Clin Oncol. 1986;4:1295–1306. doi: 10.1200/JCO.1986.4.9.1295. [DOI] [PubMed] [Google Scholar]
- 24.DeVita VT, Jr, Hubbard SM. Hodgkin's disease. N Engl J Med. 1993;328:560–565. doi: 10.1056/NEJM199302253280808. [DOI] [PubMed] [Google Scholar]
- 25.Carde P, MacKintosh FR, Rosenberg SA. A dose and time response analysis of the treatment of Hodgkin's disease with MOPP chemotherapy. J Clin Oncol. 1983;1:146–153. doi: 10.1200/JCO.1983.1.2.146. [DOI] [PubMed] [Google Scholar]
- 26.DeVita VT., Jr The evolution of chemotherapy of lymphomas of adults. Leukemia. 1987;1:467–485. [PubMed] [Google Scholar]
- 27.Epelbaum R. Non-Hodgkin's lymphoma: Long-term survivors and adverse effects. Ann Oncol. 2000;11(suppl 3):123–128. doi: 10.1093/annonc/11.suppl_3.123. [DOI] [PubMed] [Google Scholar]
- 28.Moore MR, Jones SE, Bull JM, et al. MOPP chemotherapy for advanced Hodgkin's disease: Prognostic factors in 81 patients. Cancer. 1973;32:52–60. doi: 10.1002/1097-0142(197307)32:1<52::aid-cncr2820320107>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Haim N, Epelbaum R, Ben-Shahar M, et al. Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer. 1994;73:2515–2519. doi: 10.1002/1097-0142(19940515)73:10<2515::aid-cncr2820731011>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MH, Johnson JR, Justice R, et al. FDA drug approval summary: Nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008;13:709–714. doi: 10.1634/theoncologist.2006-0017. [DOI] [PubMed] [Google Scholar]
- 32.Advani AS, Gundacker HM, Sala-Torra O, et al. Southwest Oncology Group Study S0530: A phase 2 trial of clofarabine and cytarabine for relapsed or refractory acute lymphocytic leukaemia. Br J Haematol. 2010;151:430–434. doi: 10.1111/j.1365-2141.2010.08387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deitcher OR, O'Brien S, Deitcher SR, et al. Single-agent vincristine sulfate liposomes injection (Marqibo®) compared to historical single-agent therapy for adults with advanced, relapsed and/or refractory philadelphia chromosome negative acute lymphoblastic leukemia. Blood. 2011;118:2592. [Google Scholar]
- 34.Rodriguez MA, Dang N, Fayad L, et al. Phase II study of sphingosomal vincristine in CHOP +/− rituximab for patients with aggressive non-Hodgkin's lymphoma (NHL): Promising 3 year follow-up results in elderly patients. Blood. 2005;106:943. [Google Scholar]
- 35.Ashcroft A, Kaplan L, Damon L, et al. Sphingosomal vincristine plus rituximab for treatment of large B-cell lymphoma. Blood. 2003;102:1454. [Google Scholar]