Abstract
Purpose
Pertuzumab is a humanized monoclonal antibody inhibiting human epidermal growth factor receptor 2 (HER2) dimerization. The aim of this phase II trial was to assess the antitumor activity and safety profile of pertuzumab monotherapy in patients with HER2-negative metastatic breast cancer. The utility of biomarkers detected in paraffin-embedded tissue as predictors of response was also explored.
Patients and Methods
This was an international, multicenter, open-label, randomized phase II study. Patients (n = 79) with centrally confirmed HER2-negative metastatic breast cancer were randomly assigned to receive pertuzumab once every 3 weeks with a loading dose of 840 mg followed thereafter by either 420 mg (arm A) or 1,050 mg (arm B). Patients were stratified by country and prior taxane therapy.
Results
Of 79 patients who were randomly assigned, 78 were included in the intent-to-treat population. In arm A (n = 41), two patients had partial responses, and 18 patients (44%) experienced stable disease (SD) lasting ≥ 12 weeks. In arm B (n = 37), SD was observed in 14 patients (38%). Overall, six of 78 patients responded or had SD ≥ 6 months. Pertuzumab was generally well tolerated, and most adverse events were mild to moderate. Decline in left ventricular ejection fraction of ≥ 10% and/or to less than 50% was observed in eight patients, with one case of congestive heart failure in arm A. Pharmacokinetic data supported a fixed dose of pertuzumab once every 3 weeks.
Conclusion
The limited efficacy observed in this study, generally SD of relatively short duration, suggested little benefit of further investigation of single-agent pertuzumab in unselected patients with HER2-negative disease.
INTRODUCTION
The human epidermal growth factor family of receptors (HER1/EGFR, HER2, HER3, and HER4) mediates cell growth and is dysregulated in many types of cancer.1–3 Ligand-driven heterodimerization of HER2 with other HER family members leads to downstream signal transduction, which plays an important part in neoplastic transformation and disease progression.3–6 Receptor heterodimerization may have a critical role in breast cancers that overexpress multiple members of the HER family.7–11 Furthermore, dysregulation of HER signaling, including ligand overexpression or abnormal activity, decreased receptor turnover, or receptor mutation can also increase HER signaling activity and is associated with aggressive disease and poor prognosis in many solid tumor types, including breast cancer.3,12
Pertuzumab (rhuMAb 2C4; F. Hoffmann-La Roche, Basel, Switzerland), a recombinant humanized monoclonal antibody directed against the highly conserved dimerization domain of HER2, inhibits HER2 homo- and heterodimerization.13–15 Blockage of HER2 dimerization by pertuzumab inhibits HER family downstream signaling, such as activation of the AKT cell survival pathway and the mitogen-activated protein kinase pathway.15–17
Preclinical models have shown that pertuzumab inhibits tumor growth in the absence of HER2 overexpression, unlike trastuzumab, presumably by preventing ligand-stimulated HER2 heterodimer formation.18–20 A phase I study demonstrated that pertuzumab is generally well tolerated and clinically active in patients with locally advanced recurrent or metastatic solid tumors21 and that the pharmacokinetic profile of pertuzumab supports a dosing regimen of a fixed dose (420 or 1,050 mg) once every 3 weeks.20,22–25 On the basis of these observations, this phase II study was designed to evaluate the efficacy and safety of two dose levels of pertuzumab in patients with HER2-negative metastatic breast cancer.
PATIENTS AND METHODS
Study Design
This open-label, phase II, multicenter, randomized study was conducted at 14 centers in eight countries. The primary objective was response rate for each treatment regimen per Response Evaluation Criteria in Solid Tumors (RECIST).26 The secondary objectives were time to progression (TTP), time to treatment failure, time to response, duration of response, and rate of stable disease (SD). Characterizing the pharmacokinetics of pertuzumab and evaluating its safety and tolerability were secondary end points for both dose levels. An exploratory analysis evaluated the utility of biomarkers in predicting response to pertuzumab.
Patients
Women were enrolled who were age ≥ 18 years, had a prior histologically documented diagnosis of breast cancer with a measurable lesion according to RECIST, had a Karnofsky performance status ≥ 80%, had metastatic disease treated with up to two lines of chemotherapy, and had anthracycline-containing therapy either in an adjuvant setting or for metastatic disease. Central laboratory confirmation, using fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC), of the lack of HER2 amplification or high expression (FISH-negative and IHC HER2 0, 1+, or 2+) was required. Patients were required to have tumor blocks available for evaluation or to have a biopsy of lymph nodes or accessible tumor lesions. Adequate hematologic, renal, and hepatic function were required. Exclusion criteria included any major surgery, investigational drug or cytotoxic chemotherapy ≤ 28 days before starting pertuzumab, previous malignancy, pulmonary metastases with lymphangitis or dyspnea at rest, history or clinical evidence of CNS metastases, history of cardiac disease, poorly controlled hypertension or significant valvular disease, left ventricular ejection fraction (LVEF) less than 50%, prior cumulative dose of doxorubicin at more than 360 mg/m2 or epirubicin at more than 720 mg/m2, prior treatment with any agent targeting the HER family, or prior serious uncontrolled intercurrent illness. Women who were pregnant, lactating, or of childbearing age not using adequate contraception were also not eligible. The study was approved by the local ethics committees of all participating institutions. Written informed consent was obtained from all patients.
All patients had a screening assessment at baseline that included a physical examination, determination of performance status and vital signs, complete blood count, blood chemistry, pregnancy test for women of childbearing potential, electrocardiography (ECG), echocardiography (ECHO), chest x-ray, and tumor assessment.
Study Treatment
Patients were randomly assigned by a central randomization service in a 1:1 ratio to either arm A or arm B and stratified by country of origin and previous exposure to taxane therapy. Intravenous pertuzumab infusion was administered at two dose levels as previously described27: a loading dose of 840 mg on day 1 to rapidly achieve steady-state levels, followed by 420 mg every 3 weeks (arm A) or no loading dose and 1,050 mg every 3 weeks (arm B). Treatment was continued until disease progression, unacceptable toxicity, or death.
Tolerability Assessments
Adverse events (AEs) were assessed during all visits. The incidence, severity, and relationship to study drug of AEs were recorded using the National Cancer Institute Common Toxicity Criteria v2.0. ECHO with LVEF measurements was performed at baseline within 28 days before the first pertuzumab infusion, at cycles 2, 4, 8, 12, and 16, and at final visit. For patients with inadequate acoustic windows for ECHO, multiple-gated acquisition scans were used to assess LVEF. ECGs were required at baseline and on study exit. Copies of ECGs, ECHO recordings, or multiple-gated acquisition scans were sent to a central laboratory for independent review. Serum samples for cardiac troponin T and plasma samples for nitrogen terminal pro-brain natriuretic protein were collected at baseline and within 15 minutes of the end of pertuzumab infusions.
Response Assessments
Tumors were assessed using identical techniques every 6 weeks (2 cycles) until cycle 8 and every fourth cycle thereafter. Response to therapy was assessed according to RECIST. For tumor assessment, all films were duplicated and sent for centralized radiology evaluation.
Biomarker and Tumor Biopsy Analyses
The following tests were performed on tumor blocks (paraffin-embedded tissue) following random assignment: HER2 IHC and/or FISH estrogen receptor/progesterone receptor status; quantitative reverse transcriptase polymerase chain reaction for HER1 and HER ligand mRNA expression (amphiregulin, betacellulin, EGF, transforming growth factor-alpha [TGF-α], and neuregulin 1); IHC for HER1, HER3, and phosphorylated HER2; and IHC testing for components of downstream or alternative signal transduction pathway from HER family receptors (phosphorylated AKT, phophatase and tensin homolog [PTEN], insulin-like growth factor 1 receptor [IGF-1R]), and HER ligand expression (EGF, TGF-α, amphiregulin). Serum samples for biomarker analyses were collected at screening. Assessments included serum levels of HER2 extracellular domain and HER ligands (EGF, TGF-α, amphiregulin, and neuregulin 1).
Pharmacokinetic Analyses
Serum pharmacokinetic samples were collected at cycles 1 and 2 immediately before infusion and 15 minutes following the end of infusion on days 1, 8, and 15. Additional samples were collected before drug administration on day 1 of subsequent cycles.
Pertuzumab concentrations were determined by a receptor-binding, enzyme-linked immunosorbent assay, using the antigen p185HER2 extracellular domain to capture pertuzumab from serum samples. Validation data show that p185HER2 extracellular domain at 100 ng/mL interferes with pertuzumab quantitation at 60 ng/mL. Bound pertuzumab was detected with mouse antihuman Fc-horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA), and tetramethyl benzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was used as the substrate for color development to quantify serum pertuzumab against a standard curve. The assay had a minimum quantifiable concentration of 0.25 μg/mL for pertuzumab in human serum. Serum pharmacokinetics of pertuzumab were summarized by arm (mean, standard deviation, and range) for area under the plasma concentration-time curve, maximum plasma concentration, clearance, volume of distribution, elimination half-life, and mean residence time.
Statistical Analyses
A sample size of 56 evaluable patients per arm was used, with a Simon's two-stage design allowing for stopping the study due to lack of treatment activity (≤ one response in 23 patients per arm) after the first stage. The type I error rate was 4.12% and the power to reject the null hypothesis (objective response rate ≤ 5%) was approximately 57% in both study arms when the true response rate in each arm was 15%. The null hypotheses were rejected after the second stage if there were more than six responses from 56 patients. To account for a possible unevaluable rate of less than 10%, four additional patients were added to each arm, bringing the total patient number per arm to 60.
The intent-to-treat population included all patients who received at least one dose of pertuzumab. All patients who received at least two cycles of therapy and had their disease re-evaluated were considered evaluable for efficacy analysis, including patients who exhibited objective disease progression before the end of cycle 1 as long as they had received at least one dose of pertuzumab. Kaplan-Meier estimates of TTP and survival time were calculated. All patients who received at least one dose of the study drug were included in safety analyses.
RESULTS
Patient Characteristics
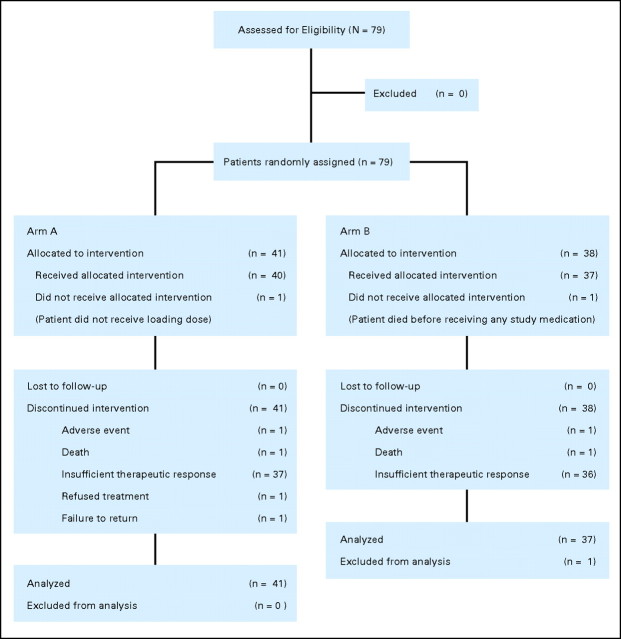
Because enrollment was stopped following a protocol-defined interim analysis, 79 patients enrolled in the study, 78 of whom were evaluable for intent-to-treat and efficacy analyses (arm A, n = 41; arm B, n = 37; Fig 1). One patient was not evaluable because she did not receive study medication. Baseline characteristics of patients were well balanced between the two arms (Table 1). Patients in both arms received a median of two cycles of pertuzumab. The majority of patients (92.4%) discontinued study treatment because of disease progression.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Characteristics of Patients (N = 78) and Characteristics of Treatment
| Characteristic | Arm A (n = 41) |
Arm B (n = 37) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 54 | 55 | ||
| Range | 33-76 | 36-78 | ||
| Karnofsky performance status, % | ||||
| 100 | 44 | 46 | ||
| 90 | 37 | 35 | ||
| 80 | 20 | 19 | ||
| Total number of lesions | ||||
| Median | 5 | 5 | ||
| Range | 1-16 | 1-14 | ||
| Number of tumor sites | ||||
| 1 | 1 | 2 | 1 | 3 |
| 2-5 | 25 | 61 | 25 | 68 |
| 6-10 | 14 | 34 | 9 | 24 |
| 11-15 | 0 | 0 | 2 | 5 |
| > 15 | 1 | 2 | 0 | 0 |
| Metastatic sites | ||||
| Visceral | 32 | 78 | 31 | 84 |
| Liver | 19 | 46 | 19 | 51 |
| Bone | 14 | 34 | 16 | 43 |
| Lung | 13 | 32 | 17 | 46 |
| Soft tissue | 11 | 27 | 2 | 5 |
| Other | 33 | 80 | 25 | 68 |
| ER/PgR status, %/% | ||||
| Positive | 71/51 | 76/57 | ||
| Negative | 24/32 | 24/35 | ||
| Unknown | 5/17 | 0/8 | ||
| HER2 status by IHC* | ||||
| 0 | 3 | 7 | 1 | 3 |
| 1+ | 19 | 46 | 14 | 38 |
| 2+ | 19 | 46 | 22 | 60 |
| 3+ | 0 | 0 | 0 | 0 |
| Prior treatment for metastatic disease | ||||
| Anthracycline | 41 | 100 | 37 | 100 |
| Radiotherapy | 36 | 88 | 32 | 87 |
| Hormone therapy | 30 | 73 | 31 | 84 |
| Taxane | 26 | 63 | 24 | 6 |
| Capecitabine | 8 | 20 | 7 | 19 |
| Pertuzumab cycles completed | ||||
| Median | 2 | 2 | ||
| Range | 1-24 | 1-12 | ||
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor-2; IHC, immunohistochemistry.
Assessed in the central laboratory.
Response to Treatment
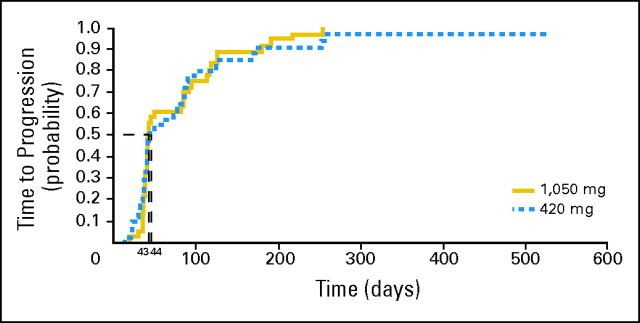
Of the 78 patients evaluable for efficacy (using independent review as described in Patients and Methods), six patients (7.7%) had either a partial response (PR) or SD ≥ 24 weeks. Two patients (4.9%) who received the pertuzumab 420 mg dose experienced a PR lasting 18 weeks in one patient with target lesions in the liver (HER2 IHC 2+) and 31 weeks in the other, who had target lesions on the skin (posterior axillary pillar; HER2 IHC 1+), with time to response of 18 and 6.1 weeks, respectively (Table 2). SD lasting ≥ 24 weeks was reported in two patients in each arm. The median duration of clinical benefit (complete response, PR, or SD ≥ 24 weeks) was 36.5 weeks (range, 22.1 to 74.9 weeks) in arm A and 33.6 weeks (range, 31.0 to 36.3 weeks) in arm B. SD lasting ≥ 12 weeks was observed in 18 patients (43.9%) in arm A and 14 (37.8%) in arm B. Median time to treatment failure was 6.3 weeks (range, 2 to 80.1 weeks) in arm A and 6.0 weeks (range, 2.7 to 36.3 weeks) in arm B. Median TTP (Fig 2) was 44 days in arm A (95% CI, 38 to 82 days) and 43 days in arm B (95% CI, 39 to 85 days).
Table 2.
Efficacy End Points (N = 78)
| Variable | Arm A (n = 41) |
Arm B (n = 37) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| PR | 2 | 4.9 | 0 | |
| SD ≥ 12 weeks | 18 | 43.9 | 14 | 37.8 |
| SD ≥ 24 weeks | 2 | 4.9 | 2 | 5.4 |
| Progressive disease | 21 | 51.2 | 22 | 59.5 |
| Missing | 0 | 1 | 2.7 | |
| Clinical benefit (CR + PR + SD ≥ 24 weeks) | 4 | 9.8 | 2 | 5.4 |
| Duration of clinical benefit, weeks | ||||
| Median | 36.5 | 33.6 | ||
| Range | 22.1-74.9 | 31.0-36.3 | ||
| Time to progression, weeks | ||||
| Median | 6.1 | 6.1 | ||
| Range | 2.0-37.0 | 2.7-36.3 | ||
Abbreviations: PR, partial response; SD, stable disease; CR, complete response.
Fig 2.
Progression-free survival.
Safety
Both dose levels of pertuzumab were generally well tolerated. Most frequent toxicities in both arms were grade 1 to 2 diarrhea, asthenia, nausea, and vomiting. Table 3 summarizes the most frequent drug-related AEs. Only two toxicities led to drug discontinuation; one congestive heart failure (CHF; arm A), and one grade 3 diarrhea (arm B). Drug-related serious AEs in arm A included the previously mentioned CHF, two episodes of sepsis in one patient, and two patients with asymptomatic decreases in LVEF; serious AEs in arm B included grade 3 diarrhea and an infusion-associated reaction (urticaria).
Table 3.
Drug-Related Adverse Events: Grade 1-2 (≥ 10%) and All Grade 3-4
| Adverse Event | Arm A (n = 41) |
Arm B (n = 37) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Total |
Grade 1 |
Grade 2 |
Grade 3 |
Total |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Any event | 26 | 63.4 | 19 | 46.3 | 6 | 14.6 | 31 | 75.6 | 28 | 75.7 | 19 | 51.4 | 5 | 13.5 | 32 | 86.5 |
| Diarrhea | 10 | 24.4 | 8 | 19.5 | 3 | 7.3 | 21 | 51.2 | 9 | 24.3 | 8 | 21.6 | 2 | 5.4 | 19 | 51.4 |
| Nausea | 8 | 19.5 | 2 | 4.9 | 0 | 10 | 24.4 | 6 | 16.2 | 4 | 10.8 | 0 | 10 | 27.0 | ||
| Fatigue/asthenia | 5 | 12.2 | 3 | 7.3 | 1 | 2.4 | 9 | 22.0 | 5 | 13.5 | 4 | 10.8 | 0 | 9 | 24.3 | |
| Rash* | 8 | 19.5 | 0 | 0 | 8 | 19.5 | 5 | 13.5 | 3 | 8.1 | 0 | 8 | 21.6 | |||
| Vomiting | 3 | 7.3 | 2 | 4.9 | 1 | 2.4 | 6 | 14.6 | 3 | 8.1 | 3 | 8.1 | 0 | 6 | 16.2 | |
| Abdominal pain | 2 | 4.9 | 3 | 7.3 | 0 | 5 | 12.2 | 4 | 10.8 | 1 | 2.7 | 0 | 5 | 13.5 | ||
| Headache | 2 | 4.9 | 3 | 7.3 | 0 | 5 | 12.2 | 1 | 2.7 | 0 | 0 | 1 | 2.7 | |||
| Stomatitis | 1 | 2.4 | 3 | 7.3 | 0 | 4 | 9.8 | 1 | 2.7 | 3 | 8.1 | 0 | 4 | 10.8 | ||
| Anorexia | 1 | 2.4 | 1 | 2.4 | 0 | 2 | 4.9 | 3 | 8.1 | 2 | 5.4 | 0 | 5 | 13.5 | ||
| Anemia | 1 | 2.4 | 0 | 0 | 1 | 2.4 | 2 | 5.4 | 2 | 5.4 | 0 | 4 | 10.8 | |||
Includes rash, erythema, eczema, macular rash, maculopapular rash, pruritic rash, and urticaria.
Eight patients (three in arm A and five in arm B) experienced drops in LVEF of ≥ 10% such that LVEF fell to less than 50%. All cases were asymptomatic, except in two patients in arm A including the previously mentioned patient with CHF who had received prior doxorubicin therapy and withdrew from the study. The second patient, who had previously received epirubicin in the metastatic setting, experienced syncope, dyspnea, and blackouts (LVEF, 25% of absolute value) that resolved and treatment was continued. A follow-up LVEF assessment 3 weeks later showed a recovery to baseline (60%). The median time to lowest LVEF in these eight patients was 100 days (range, 41 to 175 days) and, of the patients with a drop in LVEF that required a repeat assessment after 3 weeks, all LVEFs recovered to allow treatment to continue (LVEF, 40% to 45% absolute value and < 10 percentage points from that at baseline or LVEF > 45% absolute value) in the absence of disease progression or unacceptable toxicity. Because baseline LVEF was ≤ 55% at study entry in all patients who experienced cardiac dysfunction, this may be a subgroup of susceptible patients. No correlation was observed between NT-pBMP and cardiac events (data not shown). Furthermore, no cardiac events occurred in the two patients who had increased troponin T levels.
Tumor and Blood Biomarkers
Analysis by quantitative reverse transcriptase polymerase chain reaction was performed in 46 of the 78 tumor blocks that met test requirements. IHC analyses were performed on between 37 and 49 tumor samples for the following analytes: pHER2, EGFR, HER3, EGF, phosphorylated AKT, PTEN, IGF-1R, and TGF-α. There was no relevant correlation between clinical end points and immunoreactive scores.
Blood serum markers were assessed for all patients. Multivariate analyses showed that combinations of serum markers may improve prediction of benefit from pertuzumab in this patient population, though in general little benefit was observed.
Pharmacokinetic Results
Plasma pharmacokinetics were assessed in 40 patients in arm A and 37 patients in arm B (Table 4). The mean terminal half-life obtained in the study appears to be short (15.6 days in arm A and 19.5 days in arm B) compared with those of other immunoglobulin G monoclonal antibodies (3 to 4 weeks).
Table 4.
Pharmacokinetic Parameters of Pertuzumab Following First Infusion of Pertuzumab
| Parameter | Arm A |
Arm B |
||||
|---|---|---|---|---|---|---|
| No. | Mean | CV (%) | No. | Mean | CV (%) | |
| t1/2, days | 38 | 15.6 | 32 | 36 | 19.5 | 71 |
| Cmax, μg/mL | 40 | 289 | 37 | 37 | 409 | 39 |
| AUClast, μg.day/mL | 40 | 2,520 | 35 | 37 | 3,470 | 30 |
| AUC∞, μg.day/mL | 38 | 3,880 | 38 | 36 | 6,350 | 61 |
| Cl, mL/d | 38 | 243 | 45 | 36 | 213 | 49 |
| Vc, mL | 38 | 5,120 | 39 | 36 | 4,710 | 36 |
| MRT, days | 38 | 19.5 | 32 | 36 | 25.4 | 74 |
Abbreviations: CV, coefficient of variation; t1/2, terminal half-life; Cmax, maximum concentration; AUC, area under the curve; Cl, clearance; Vc, volume of distribution; MRT, mean residence time.
DISCUSSION
This trial was designed to evaluate the efficacy and safety of two dose levels of pertuzumab administered every 3 weeks in patients with metastatic breast cancer without HER2 overexpression or amplification. The data presented here show that single-agent pertuzumab generally caused SD of relatively short duration in approximately 40% of patients with HER2-negative breast cancer, with 7.7% of patients experiencing either PR or SD ≥ 6 months (clinical benefit).
Pertuzumab was generally well tolerated at both dose levels in this study, with a broadly similar toxicity profile to that which has previously been reported.21,27–30 The most common AEs were mild to moderate GI toxicities and rash, which would be expected from an agent inhibiting HER family dimerization and therefore HER1 signaling.31–35 The low rate of cardiac dysfunction observed suggests that pertuzumab-associated cardiotoxicity occurred only in a subset of susceptible individuals shortly after first exposure. While the limited number of cardiac events means these data should be interpreted with caution, they are consistent with LVEF results from four pertuzumab monotherapy phase II studies.27,28,30,36 The rate of cardiac events reported in this study is similar to that described in the pivotal trastuzumab trials; a recent pooled analysis demonstrated that 11% of patients who received trastuzumab experienced an LVEF decrease to less than 50%.37
Pertuzumab interferes with HER2 heterodimer formation and downstream signaling, while trastuzumab is unable to block HER dimer formation. Additionally pertuzumab, but not trastuzumab, inhibited the growth of cancer cells expressing both low/normal and high levels of HER2 in the preclinical setting.19,38 However, in this study, the measurable therapeutic benefit of pertuzumab in women with HER2-negative breast cancer was mostly in the form of SD of relatively short duration. Other preclinical studies have shown that a trastuzumab and pertuzumab combination may act in synergy to induce tumor regression in xenograft models of HER2-positive tumors, even after the tumor progressed on trastuzumab.39 This strongly suggests that pertuzumab might have the greatest clinical benefit among patients with HER2-positive disease. Trastuzumab in combination with chemotherapy is associated with a survival benefit in patients with both early and metastatic HER2-positive breast cancer.40–42 However, some patients with HER2-overexpressing breast cancer who progress on trastuzumab may benefit from the addition of pertuzumab to trastuzumab therapy. Indeed, clinical data from trials investigating trastuzumab and pertuzumab for treatment beyond first-line trastuzumab therapy have shown good response data and long progression-free survival.43
The relatively short half-life for pertuzumab reported in this study can be explained by a number of factors; both the dosing schedule and the noncompartmental analysis (NCA) contribute to the underestimate of the terminal half-life. The sampling schedule of 3 weeks, at which point the next dose was administered, prevents a detailed assessment of the terminal elimination phase. In addition, the nature of the NCA and the inherent interindividual variability of the data across studies are also factors in this underestimate. Thus, these pharmacokinetic data do not contradict dosing once every 3 weeks. NCA is model-independent and relies on the number and timing of the plasma samples, typically yielding different values than a compartment modeling approach. The latter gives the best fit for the data for pertuzumab using a two-compartment model and is therefore the one used for the population pharmacokinetic analyses which are reported separately.
As a comparison, in the phase I dose-escalation study, the terminal half-life ranged from 15.3 to 27.6 days with a mean terminal half-life of 18.9 days (standard deviation, 8 days),21 and in a similar phase I study in Japan, the half-life range was 11 to 18 days,44 both of which support dosing once every 3 weeks.
In this study, only two of the 41 patients who received the 420-mg dose experienced a PR, and the rate of SD ≥ 12 weeks was 44% for patients in arm A and 38% for patients in arm B. The rate of clinical benefit at 6 months in this trial, while limited, was similar to that reported with pertuzumab in advanced ovarian cancer.27 It is notable that the durations of response observed in the two patients with PR were 18 and 31 weeks and, in contrast to observations made with cytotoxic therapy, they occurred after a median of 18 and 6 weeks, respectively. Prolonged progression-free survival without tumor response has also been observed in trials of pertuzumab in non–small-cell lung cancer and castration-resistant prostate cancer.28,30 The RECIST and WHO criteria for response were designed primarily for cytotoxic agents and, hence, these criteria may not translate to targeted therapies. For example, the EGFR tyrosine kinase inhibitor erlotinib as second-line therapy for non–small-cell lung cancer produced a PR in less than 10% of patients, although both progression-free and overall survival were significantly increased.45 However, in our study, median TTP was reported as 44 and 43 days in arms A and B, respectively, suggesting that the majority of the patients with HER2-negative disease derived minimal benefit from HER2-targeted therapy.
In conclusion, the use of single-agent pertuzumab in HER2-negative breast cancer was justified by preclinical evidence but did not meet expectations of clinical benefit in the metastatic setting. Biologic effects were measured and were possibly associated with clinical benefit that supported investigation of pertuzumab in combination with trastuzumab in women with breast cancer characterized by HER2 overexpression or amplification.
Acknowledgment
We thank Eleanor Steele for medical writing assistance in revising the first draft manuscript and collation of review comments.
Footnotes
Supported by F. Hoffmann-La Roche (Roche).
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Veronica Hersberger, F. Hoffmann La Roche (C) Consultant or Advisory Role: Luca Gianni, F. Hoffmann La Roche (C), Wyeth (C), Genentech (C), GlaxoSmithKline (C), Novartis (C); Javier Cortes, F. Hoffmann La Roche (C); Pirkko-Liisa Kellokumpu-Lehtinen, sanofi-aventis (C); David A. Cameron, F. Hoffmann La Roche (C); David Miles, F. Hoffmann La Roche (C); Andrew Wardley, F. Hoffmann La Roche (C); José Baselga, F. Hoffmann La Roche (C), Exelixis (C), Merck (C), Novartis (C) Stock Ownership: None Honoraria: Javier Cortes, F. Hoffman La Roche; Pirkko-Liisa Kellokumpu-Lehtinen, F. Hoffmann La Roche, sanofi-aventis, Pfizer; David A. Cameron, F. Hoffmann La Roche; David Miles, F. Hoffmann La Roche; Andrew Wardley, F. Hoffmann La Roche Research Funding: Pirkko-Liisa Kellokumpu-Lehtinen, sanofi-aventis, F. Hoffmann La Roche, Pfizer; David A. Cameron, F. Hoffmann La Roche; Andrew Wardley, F. Hoffmann La Roche Expert Testimony: None Other Remuneration: Andrew Wardley, F. Hoffmann La Roche
AUTHOR CONTRIBUTIONS
Conception and design: Luca Gianni, David Miles, Andrew Wardley, Veronica Hersberger, José Baselga
Provision of study materials or patients: Luca Gianni, Anna Lladó, Giulia Bianchi, Javier Cortes, Pirkko-Liisa Kellokumpu-Lehtinen, David A. Cameron, David Miles, Stefania Salvagni, Andrew Wardley, Jean-Charles Goeminne, José Baselga
Collection and assembly of data: Luca Gianni, Giulia Bianchi, Javier Cortes, Pirkko-Liisa Kellokumpu-Lehtinen, David A. Cameron, David Miles, Stefania Salvagni, Andrew Wardley, Jean-Charles Goeminne, Veronica Hersberger
Data analysis and interpretation: Luca Gianni, Giulia Bianchi, Javier Cortes, David A. Cameron, David Miles, Andrew Wardley, Veronica Hersberger, José Baselga
Manuscript writing: Luca Gianni, Giulia Bianchi, Pirkko-Liisa Kellokumpu-Lehtinen, David A. Cameron, David Miles, Andrew Wardley, José Baselga
Final approval of manuscript: Luca Gianni, Anna Lladó, Giulia Bianchi, Javier Cortes, Pirkko-Liisa Kellokumpu-Lehtinen, David A. Cameron, David Miles, Stefania Salvagni, Andrew Wardley, Jean-Charles Goeminne, Veronica Hersberger, José Baselga
REFERENCES
- 1.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 4.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 5.Olayioye MA. Update on HER-2 as a target for cancer therapy: Intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3:385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 10.Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 11.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 12.Fan Z, Mendelsohn J. Therapeutic application of anti-growth factor receptor antibodies. Curr Opin Oncol. 1998;10:67–73. doi: 10.1097/00001622-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diermeier-Daucher S, Hasmann M, Brockhoff G. Flow cytometric FRET analysis of erbB receptor interaction on a cell-by-cell basis. Ann N Y Acad Sci. 2008;1130:280–286. doi: 10.1196/annals.1430.003. [DOI] [PubMed] [Google Scholar]
- 15.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 16.Badache A, Hynes NE. A new therapeutic antibody masks ErbB2 to its partners. Cancer Cell. 2004;5:299–301. doi: 10.1016/s1535-6108(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 17.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 18.Agus DB, Akita RW, Fox WD, et al. A potential role for activated HER-2 in prostate cancer. Semin Oncol. 2000;27:76–83. [PubMed] [Google Scholar]
- 19.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 20.Malik M, Totpal K, Balter I, et al. Dose-response studies of recombinant humanized monoclonal antibody 2C4 (pertuzumab) in tumor xenograft models. Presented at the 94th American Association for Cancer Research Annual Meeting; July 11-14, 2003; Washington, DC. [Google Scholar]
- 21.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 22.Allison DE, Malik M, Qureshi F, et al. Pharmacokinetics of HER2-targeted rhuMAb 2C4 (pertuzumab) in patients with advanced solid malignancies: Phase Ia results. Proc Am Soc Clin Oncol. 2003;22:197. abstr 790. [Google Scholar]
- 23.Allison DE, Ng CM, Jumbe NL, et al. Pharmacokinetics (PK) of pertuzumab (rhuMAb 2C4) in Phase II studies of ovarian, breast, prostate, and lung cancer. J Clin Oncol. 2005;23:173s. abstr 2532. [Google Scholar]
- 24.Leyland-Jones B. Dose scheduling: Herceptin. Oncology. 2001;61(suppl 2):31–36. doi: 10.1159/000055399. [DOI] [PubMed] [Google Scholar]
- 25.Ng CM, Lum BL, Gimenez V, et al. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res. 2006;23:1275–1284. doi: 10.1007/s11095-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Gordon MS, Matei D, Aghajanian C, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: Potential predictive relationship with tumor HER2 activation status. J Clin Oncol. 2006;24:4324–4332. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 28.Agus DB, Sweeney CJ, Morris MJ, et al. Efficacy and safety of single-agent pertuzumab (rhuMAb 2C4), a human epidermal growth factor receptor dimerization inhibitor, in castration-resistant prostate cancer after progression from taxane-based therapy. J Clin Oncol. 2007;25:675–681. doi: 10.1200/JCO.2006.07.0649. [DOI] [PubMed] [Google Scholar]
- 29.Attard G, Kitzen J, Blagden SP, et al. A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumours. Br J Cancer. 2007;97:1338–1343. doi: 10.1038/sj.bjc.6604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Davies AM, Natale RB, et al. Efficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin Cancer Res. 2007;13:6175–6181. doi: 10.1158/1078-0432.CCR-07-0460. [DOI] [PubMed] [Google Scholar]
- 31.Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657–670. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Burtness B. The role of cetuximab in the treatment of squamous cell cancer of the head and neck. Expert Opin Biol Ther. 2005;5:1085–1093. doi: 10.1517/14712598.5.8.1085. [DOI] [PubMed] [Google Scholar]
- 33.Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16:1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 34.Janmaat ML, Giaccone G. The epidermal growth factor receptor pathway and its inhibition as anticancer therapy. Drugs Today (Barc) 2003;39:61–80. [PubMed] [Google Scholar]
- 35.Norman P. ZD-1839 (AstraZeneca) Curr Opin Investig Drugs. 2001;2:428–434. [PubMed] [Google Scholar]
- 36.de Bono JS, Bellmunt J, Attard G, et al. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol. 2007;25:257–262. doi: 10.1200/JCO.2006.07.0888. [DOI] [PubMed] [Google Scholar]
- 37.Muehlbauer S, Revil C, Leyland-Jones B, et al. Pooled cardiac safety analysis of patients with HER2-positive metastatic breast cancer receiving trastuzumab. Presented at the 31st Annual San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. abstr 6136. [Google Scholar]
- 38.Friess T, Bauer S, Burger AM, et al. In vivo activity of recombinant humanized monoclonal antibody 2C4 in xenografts is independent of tumor type and degree of HER2 overexpression. Presented at the 14th Annual European Organisation for Research and Treatment of Cancer-National Cancer Institute-American Association for Cancer Research Symposium; November 19-22, 2002; Frankfurt, Germany. [Google Scholar]
- 39.Friess T, Scheuer W, Hasmann M. Superior antitumour activity after combination treatment with pertuzumab and trastuzumab against NSCLC and breast cancer xenograft tumours. Presented at the 31st Annual European Society for Medical Oncology Meeting; September 29-October 3, 2006; Istanbul, Turkey. [Google Scholar]
- 40.Baselga J, Perez EA, Pienkowski T, et al. Adjuvant trastuzumab: A milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11(suppl 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 41.Hortobagyi GN. Overview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer. Semin Oncol. 2001;28:43–47. [PubMed] [Google Scholar]
- 42.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 43.Gelmon K, Fumoleau P, Verma S, et al. Results of a Phase II trial of trastuzumab (H) and pertuzumab (P) in patients (pts) with HER2-positive metastatic breast cancer (MBC) who had progressed during trastuzumab therapy. J Clin Oncol. 2008;26:47s. doi: 10.1200/JCO.2009.24.2024. abstr 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto N, Yamada Y, Fujiwara Y, et al. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2009;39:260–266. doi: 10.1093/jjco/hyp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang PA, Tsao MS, Moore MJ. A review of erlotinib and its clinical use. Expert Opin Pharmacother. 2006;7:177–193. doi: 10.1517/14656566.7.2.177. [DOI] [PubMed] [Google Scholar]