Abstract
Purpose
Pertuzumab, a human epidermal growth factor receptor 2 (HER2) –targeted monoclonal antibody, potently inhibits HER2 dimerization and HER-mediated signaling pathways. Pertuzumab and the approved HER2-targeted monoclonal antibody trastuzumab have complementary mechanisms of action and result in enhanced antitumor activity when combined. This phase II trial assessed the efficacy and safety profile of the combination in patients with HER2-positive breast cancer whose disease had progressed during prior trastuzumab-based therapy.
Patients and Methods
This was a multicenter, open-label, single-arm, Simon two-stage study. Patients with advanced HER2-positive breast cancer in whom disease progression had occurred during prior trastuzumab-based therapy received trastuzumab weekly (4 mg/kg loading dose, then 2 mg/kg every week) or every 3 weeks (8 mg/kg loading dose, then 6 mg/kg every 3 weeks) and pertuzumab every 3 weeks (840 mg loading dose, then 420 mg every 3 weeks). Treatment continued until disease progression or excessive toxicity.
Results
All 66 patients were assessable for efficacy and safety. The objective response rate was 24.2%, and the clinical benefit rate was 50%. Five patients (7.6%) experienced a complete response, 11 patients (16.7%) experienced a partial response, and 17 patients (25.8%) experienced stable disease of ≥ 6 months. Median progression-free survival was 5.5 months. Overall, the combination of pertuzumab and trastuzumab was well tolerated, and adverse events were mild to moderate. Cardiac dysfunction was minimal, and no patients withdrew as a result of cardiac-related adverse events.
Conclusion
The combination of pertuzumab and trastuzumab is active and well tolerated in patients with metastatic HER2-positive breast cancer who had experienced progression during prior trastuzumab therapy.
INTRODUCTION
Trastuzumab, a monoclonal antibody targeting human epidermal growth factor receptor 2 (HER2), significantly improves survival in patients with HER2-positive breast cancer in both the metastatic1–3 and adjuvant settings.4–9 However, despite this notable success, there is still a need to improve HER2-directed therapy. Pertuzumab, a recombinant humanized monoclonal antibody binding to the HER2 dimerization domain, prevents dimerization of HER2 with other HER receptors (HER3, HER1, and HER4).10–12 Thus, pertuzumab is a potent inhibitor of HER-mediated signaling12,13 and has demonstrated excellent activity against several HER2-dependent breast cancer cell lines.13
Pertuzumab inhibits HER2 signaling by binding to a different HER2 epitope than trastuzumab, and the addition of pertuzumab after progression to ongoing trastuzumab in xenografts synergistically increased tumor inhibition compared with trastuzumab alone.14 This suggests that trastuzumab and pertuzumab have complementary mechanisms of action and that the addition of pertuzumab to trastuzumab may improve clinical efficacy as a result of potentially broader blockade of the HER tumor cell proliferation and survival signaling. To assess this, the current study evaluated the efficacy and safety profile of pertuzumab in combination with trastuzumab in previously treated patients with HER2-positive metastatic breast cancer (MBC) who had experienced progression during trastuzumab as most recent treatment.
PATIENTS AND METHODS
Patient Population
Women age ≥ 18 years, with histologically centrally reconfirmed HER2-positive breast cancer (as per US Food and Drug Administration guidelines),15 with at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST), who had received ≤ three prior chemotherapy regimens (prior exposure to cumulative doses of doxorubicin < 360 mg/m2, or equivalent), with a left ventricular ejection fraction (LVEF) ≥ 55% absolute value or greater than local parameter for lower limit of normal by echocardiography (ECHO) or multiple-gated acquisition (MUGA) scans, and who had experienced progression during trastuzumab-based therapy as last treatment for MBC were eligible. Study treatment had to be initiated ≥ 4 weeks after any prior radiotherapy or surgery, both with full recovery, and 4 to 9 weeks after the last dose of trastuzumab.
Signed informed consent was obtained from all patients. Patients were excluded if they had received prior treatment with any targeted agent other than trastuzumab or had a history of cardiac disease, including known symptomatic decreases in LVEF to less than 50% absolute value during prior trastuzumab therapy or congestive heart failure. Other exclusion criteria included history or clinical evidence of brain metastases; prior severe, uncontrolled, systemic disease; another malignancy within the last 5 years; and known infection with HIV, hepatitis B virus, or hepatitis C virus. Women who were pregnant, lactating, or of child-bearing age and not using adequate contraception were also excluded.
Study Design and Treatment
This phase II, single-arm, multicenter exploratory study with a Simon two-stage design was conducted at 16 centers in five countries. The primary objective was to assess the efficacy of pertuzumab combined with trastuzumab in patients who had experienced progression during trastuzumab-based therapy, as determined by the objective response rate (ORR; confirmed complete response [CR] or partial response [PR]) and/or the clinical benefit rate (CBR; total number of objective responses plus stable disease [SD] > 6 months). Efficacy was determined according to RECIST.16 Secondary objectives were to assess the safety profile of the combination, duration of response, time to response, time to progression (TTP), and progression-free survival (PFS).
Trastuzumab was administered according to the same dose schedule the patient had received before study entry; a loading dose of 4 mg/kg was administered on day –28, followed by 2 mg/kg on days 1, 8, and 15 of each cycle, or a loading dose of 8 mg/kg was administered on day –28, followed by 6 mg/kg on day 1 of each cycle. For the first cycle, trastuzumab and a loading dose of pertuzumab of 840 mg were administered as intravenous infusions on days 1 and 2, respectively, but in cycle 2 and thereafter, trastuzumab and pertuzumab 420 mg were administered as intravenous infusions on the same day, with trastuzumab administered first. The treatment period was eight cycles (24 weeks); however, patients could continue treatment if free from progressive disease (PD).
Tolerability and Safety Analysis
Adverse events (AEs) were assessed continuously until 28 days after the last dose of study medication. Clinical safety was evaluated using the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0 (NCI-CTCAE).
Cardiac Safety
ECHO was the preferred method to evaluate cardiac function. ECHO or MUGA scans were performed at screening (day –28); at the end of cycles 1, 2, 4, 6, and 8; and every four cycles thereafter for patients who continued therapy or remained in treatment-free follow-up. All patients were assessed at the final visit, 28 days after last study treatment. All ECHO scans were recorded and sent to a central laboratory for confirmation. The same method of assessment was used throughout the study for each patient, and it was recommended that the same echocardiographist perform the cardiac evaluations. Patient medical management was based on local ECHO readings.
Study-specific AEs and serious AEs (SAEs) were included to identify clinically relevant changes in LVEF. They included asymptomatic decreases in LVEF of ≥ 10 percentage points from baseline value and less than 50% absolute value and AEs of cardiac origin (related and unrelated) that were NCI-CTCAE grade ≥ 3 and had to be reported in an expedited manner (based on local ECHO or MUGA readings). For asymptomatic declines in LVEF (to ≤ 45% and/or ≥ 10 percentage points from baseline), the continuation/discontinuation of study medication algorithm was followed; if LVEF was less than 39% or 40% to 45% plus a ≥ 10 percentage point decline from baseline, study medication was held and LVEF readings were repeated in 3 weeks; if LVEF was 40% to 45% and there was a less than 10 percentage point decline from baseline, dosing was continued and LVEF readings were repeated in 3 weeks. At the next LVEF assessment, the following actions were taken: if LVEF was less than 39% or 40% to 45% plus a ≥ 10 percentage point decline from baseline, study medication was stopped; if LVEF was recovered to more than 45% or to 40% to 45% and there was a less than 10 percentage point decline from baseline, study medication was resumed.
Events not fulfilling any of the seriousness criteria (ie, the usual definition including life-threatening events, events resulting in death, or events that were medically significant if no other criterion applied) were classified as nonserious event of special interest. Congestive heart failure was reported as an SAE and was assessed according to the New York Heart Association classification system and the NCI-CTCAE.
Efficacy Assessments
Tumor response was evaluated according to RECIST using computed tomography or magnetic resonance imaging of the thorax, abdomen, and pelvis. For each patient, the same technique was used to evaluate each lesion throughout the study. Tumor assessments were performed at screening; at the end of cycles 2, 4, 6, and 8; and every four cycles or 3 months thereafter for patients who continued therapy or were on treatment-free follow-up until PD was observed. All patients were assessed at the final visit, 28 days after last treatment. Tumor response had to be confirmed a minimum of 28 days after the initial response was noted.
TTP was defined as the interval between the day of first dose of study medication and first documentation of PD. Patients who withdrew from the study or died without documented PD were censored at the date of last tumor assessment when the patient was known to be progression free. PFS was defined as the time from the date of first dose of study medication to documented PD or death at any time, regardless of cause. Patients who did not experience progression or die while on study or during follow-up were censored at the last valid tumor assessment.
Statistical Analysis
A sample size of 58 evaluable patients was used, with a Simon-type two-stage design allowing for stopping as a result of lack of treatment activity at interim analysis among the first 24 evaluable patients (study to continue if ≥ two responders, if ≥ one responder and ≥ 12 patients with SD, or if no responders and ≥ 13 patients with SD). The probability of early termination, given an ineffective treatment, was 0.127. With critical values for the final analysis of ≥ eight patients with objective response or ≥ 14 patients with clinical benefit for 58 evaluable patients (planned recruitment of 62 patients to allow for a possible 5% inevaluable rate), the trial had a power of approximately 67% to reject the null hypothesis when the ORR was ≥ 13% or CBR was ≥ 25%, at a one-sided α level of ≤ .100. The null hypothesis was defined as follows: Ho: P01 ≥ .85, P02 ≤ .08, and P03 ≤ .07, where P01, P02, and P03 are the probability of having PD, SD for ≥ 8 cycles of therapy or ≥ 6 months, and objective response as best response, respectively.
Efficacy analyses were based on the intent-to-treat (or all-treated) population, including all patients who received any study medication. The safety analysis population included all patients who received any pertuzumab and had ≥ one postbaseline safety follow-up.
RESULTS
Patients and Treatment Characteristics
Sixty-six patients were enrolled onto the study and received treatment with the pertuzumab/trastuzumab combination (Table 1; Fig 1). All patients had previously received trastuzumab-based therapy, most recently in the metastatic setting. The mean duration of prior trastuzumab was 16.2 months (standard deviation, 14.4 months).
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | No. of Patients (N = 66) | % |
|---|---|---|
| Age, years | ||
| Median | 54.0 | |
| Range | 25-85 | |
| Received anthracyclines in previous treatment | 46 | 70 |
| Received taxanes in previous treatment | 27 | 41 |
| ECOG performance status* | ||
| 0 | 80 | |
| 1 | 19 | |
| 2 | 2 | |
| ER status | ||
| Positive | 50 | |
| Negative | 50 | |
| Organ site of target and nontarget lesions | ||
| Visceral | 52 | 78.8 |
| Lung | 25 | 37.9 |
| Liver | 35 | 53.0 |
| Adrenal | 1 | 1.5 |
| Pleura | 2 | 3.0 |
| Lymph | 26 | 39.4 |
| Bone | 19 | 28.8 |
| Soft tissue | 1 | 1.5 |
| Other† | 49 | 74.2 |
| Tumor burden of target lesions, mm | ||
| Median | 63.0 | |
| Range | 11-588 | |
| No. of metastatic sites involved | ||
| Median | 4.0 | |
| Range | 1-14 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor.
Available in 59 patients.
Includes breast, skin, and mediastinum.
Fig 1.
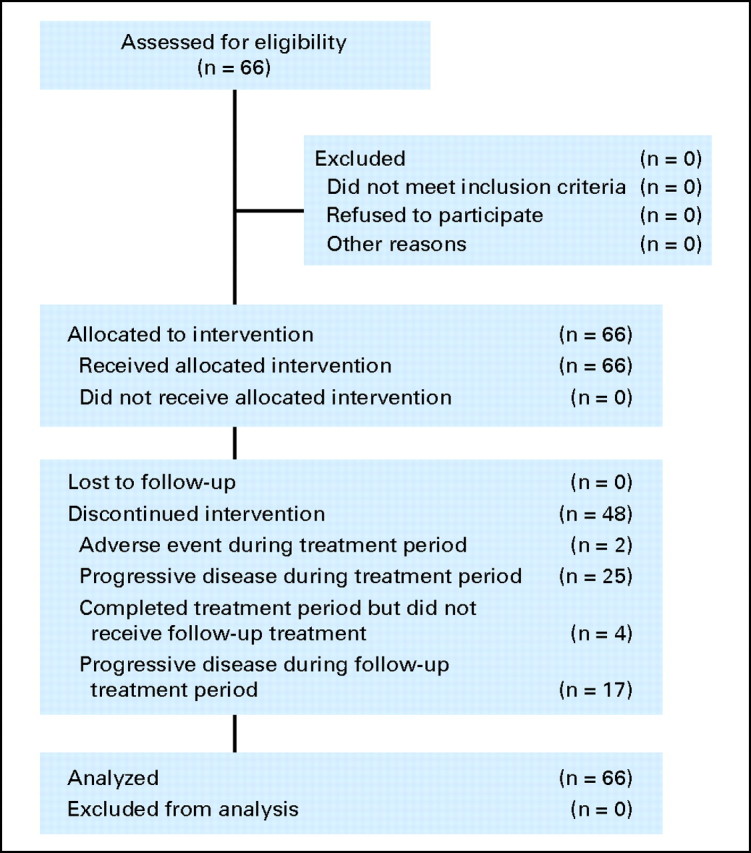
CONSORT diagram.
Only two patients withdrew as a result of an AE (somnolence and diplopia, not treatment related). During the eight cycles of the main treatment period, 25 patients (38%) withdrew as a result of PD; a further 17 patients (26%) withdrew as a result of PD in the follow-up period. Four patients (6%) completed eight treatment cycles but did not continue with follow-up treatment.
The median number of treatment cycles received was nine (range, one to 26 cycles) for both pertuzumab and trastuzumab. Two patients required infusion interruptions of trastuzumab, both in cycle 1 (as a result of chills in one patient who received no further treatment because of PD, and as a result of cough, dyspnea, and flushing in the other patient who continued treatment until PD was observed at the end of cycle 8). One patient required an infusion interruption of both pertuzumab and trastuzumab in cycle 2 as a result of hypersensitivity; no further treatment was administered as a result of PD observed at the end of cycle 2.
Tolerability and Safety
All 66 patients who enrolled onto the trial and received pertuzumab were assessed for toxicity. The pertuzumab/trastuzumab combination was generally well tolerated, and the most frequent AEs were mild or moderate. These grade 1 or 2 AEs included diarrhea (64%), fatigue (33%), and nausea (27%). Table 2 lists AEs observed in more than 10% of patients.
Table 2.
Adverse Events in > 10% of Patients
| Adverse Event | All Grades |
Grade 3 or 4 |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Diarrhea | 42 | 64 | 2 | 3 |
| Fatigue | 22 | 33 | 0 | 0 |
| Nausea | 18 | 27 | 0 | 0 |
| Rash | 17 | 26 | 1 | 2 |
| Headache | 13 | 20 | 0 | 0 |
| Arthralgia | 11 | 17 | 0 | 0 |
| Cough | 9 | 14 | 0 | 0 |
| Anorexia | 9 | 14 | 0 | 0 |
| Asthenia | 8 | 12 | 1 | 2 |
| Dizziness | 8 | 12 | 0 | 0 |
| Muscle spasms | 8 | 12 | 0 | 0 |
| Myalgia | 8 | 12 | 0 | 0 |
| Paresthesia | 7 | 11 | 0 | 0 |
| Pruritus | 7 | 11 | 1 | 2 |
| Vomiting | 7 | 11 | 0 | 0 |
Four patients experienced grade 3 treatment-related AEs. Two patients with grade 3 diarrhea received the antidiarrheal agent loperamide, with one patient also receiving cophenotrope. Both patients continued on active therapy without dose reductions or treatment interruptions. One patient experienced a central line infection, and another had a pruritic rash, which followed injection of contrast before receiving pertuzumab. Both of these AEs resolved, and treatment was continued.
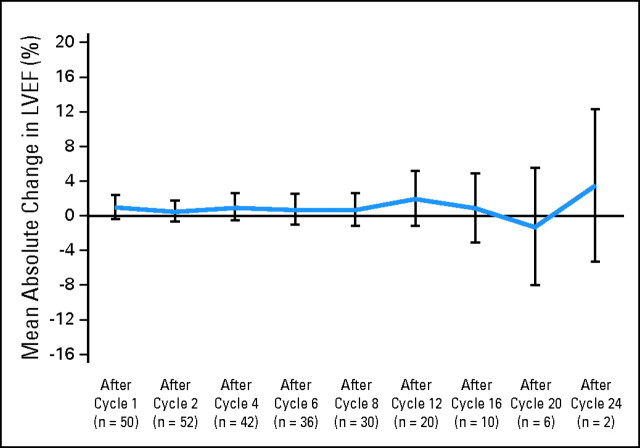
Cardiac Safety
Cardiac safety was carefully monitored during the trial, and overall, the mean LVEF remained close to the baseline level, indicating no decrease in LVEF (Fig 2). Cardiac readings were taken both locally and centrally. This led to the observation of decreases detected by central reading, which could have resulted in treatment interruptions if detected locally, that recovered despite continued pertuzumab and trastuzumab therapy. Therefore, the variability of LVEF interpretation should be taken into account when making clinical decisions regarding interruption of treatment with HER2-targeted therapy.
Fig 2.
Mean change in left ventricular ejection fraction (LVEF) from baseline over time (local reading).
Three patients had a decrease in LVEF of ≥ 10 percentage points and less than 50% absolute value, although no patient experienced any clinical symptoms related to cardiac toxicity. In one patient, LVEF decreased by 25 percentage points (central reading); in another patient, LVEF decreased by 13 percentage points (central reading; thus not meeting the protocol definition of a study-specific AE). Both LVEFs recovered without treatment interruption, and both patients continued to receive pertuzumab and trastuzumab. In the third patient, a study-specific AE was reported as a result of a decline of 14 percentage points on two occasions by local reading (NCI-CTCAE grade 1) and was considered possibly treatment related. This was not centrally confirmed, and the patient remained asymptomatic but withdrew from the study as a result of PD. No patients withdrew from the study as a result of cardiac-related AEs.
Efficacy
The study exceeded efficacy cutoff values for stage 1 at an interim analysis. All 66 patients enrolled were eligible for efficacy analysis, with an ORR of 24.2% (five CRs, 7.6%; 11 PRs 11, 16.7%); the CBR was 50%. Improvement in tumor response was observed during treatment, with no CRs observed before cycle 6. Furthermore, the median time to response was 2.6 months (range, 1.1 to 8.6 months), and several patients experienced SD before a PR or CR. The best overall responses for all patients are listed in Table 3.
Table 3.
Best Response Rates in All Treated Patients (N = 66)
| Best Overall Response | No. of Patients | % | 80% CI (%)* |
|---|---|---|---|
| Complete response | 5 | 7.6 | 3.7 to 13.6% |
| Partial response | 11 | 16.7 | 10.9 to 24.1 |
| Stable disease ≥ 6 months | 17 | 25.8 | 18.8 to 33.9 |
| Progressive disease | 33 | 50 | 41.5 to 58.5 |
| At cycle 2 | 11 | 16.7 | |
| At cycles 4-6 (without prior response) | 15 | 22.7 |
As a result of the limited sample size, a one-sided significance level of P = .1 was specified in the protocol to provide an estimation of the activity of the treatment combination, particularly with a focus on the lower bound for this activity. Therefore, two-sided 80% CIs are presented.
It is important to note that the combination is effective in both soft tissue and visceral lesions. There did not seem to be any common characteristics in terms of hormone receptor expression, disease history, or treatment background among the five patients who experienced a CR (Table 4). Tumor response and prolonged SD were achieved in patients with several prior therapies, visceral metastases (and lymph node metastases), and a relatively high tumor burden. However, there may be a trend to higher activity in patients with lymph node metastases, low tumor burden, and longer duration of trastuzumab therapy.
Table 4.
Demographics and Clinical Characteristics of Responders
| Characteristic | CR (n = 5) |
PR (n = 11) |
SD (n = 17) |
CB (n = 33) |
No CB (n = 33) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||||||
| Median | 55 | 54 | 57 | 55 | 51 | |||||
| Range | 34-74 | 45-71 | 38-77 | 34-77 | 25-85 | |||||
| ER positive | 3 | 60 | 8 | 73 | 8 | 47 | 19 | 58 | 14 | 42 |
| PgR positive | 2 | 40 | 3/8* | 38 | 6/14 | 43 | 11/27 | 41 | 8/31 | 26 |
| ECOG performance status | ||||||||||
| 0 | 4 | 80 | 9 | 82 | 9/13 | 69 | 22/29 | 76 | 25/30 | 83 |
| 1 | 1 | 20 | 2 | 18 | 4/13 | 31 | 7/29 | 24 | 4/30 | 13 |
| 2 | — | — | — | — | — | — | — | — | 1/30 | 3 |
| Previous No. of chemotherapy regimens | ||||||||||
| 1 | 3 | 60 | 4 | 36 | 8 | 47 | 15 | 45 | 7 | 21 |
| 2 | — | — | 5 | 45 | 8 | 47 | 13 | 39 | 15 | 45 |
| 3 | 2 | 40 | 2 | 18 | 1 | 6 | 5 | 15 | 8 | 24 |
| 4 | — | — | — | — | — | — | — | — | 1 | 3 |
| 5 | — | — | — | — | — | — | — | — | 2 | 6 |
| Duration of previous trastuzumab, months | ||||||||||
| Median | 28 | 15 | 15 | 16 | 9 | |||||
| Range | 8-76 | 5-48 | 3-66 | 3-76 | 2-35 | |||||
| Site of target lesions | ||||||||||
| Lymph | 4 | 2 | 7 | 13 | 7 | |||||
| Lung | 1 | 1 | 9 | 11 | 9 | |||||
| Liver | — | 8 | 8 | 16 | 18 | |||||
| Breast | — | 2 | — | 2 | 4 | |||||
| Mediastinum | — | 1 | — | 1 | 3 | |||||
| Skin | — | — | 1 | 1 | 2 | |||||
| Other | — | — | — | — | 2 | |||||
| Sum of lesions at BL, mm | ||||||||||
| Median | 27 | 59 | 65 | 59 | 73 | |||||
| Range | 11-46 | 12-108 | 13-588 | 11-588 | 17-168 | |||||
| Response observed at cycle, No. of cycles | ||||||||||
| Median | 8 | 4 | — | — | — | |||||
| Range | 6-12 | 2-12 | — | — | — | |||||
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; CB, clinical benefit; ER, estrogen receptor; PgR, progesterone receptor; ECOG, Eastern Cooperative Oncology Group; BL, baseline.
No. of patients/total No. of patients with available data.
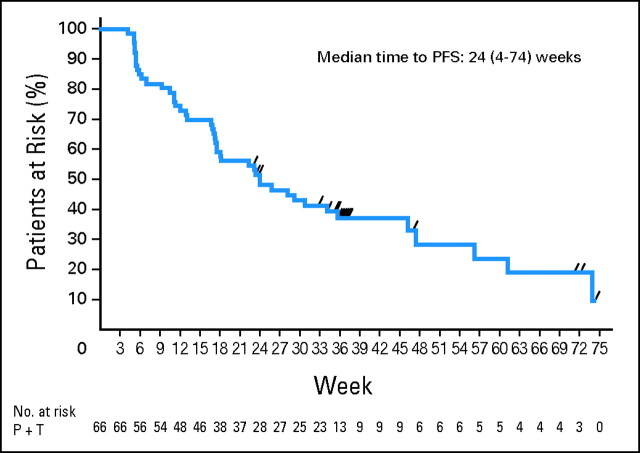
The clinical benefit afforded by the pertuzumab/trastuzumab combination was durable, with an overall median PFS time of 5.5 months (range, 0.9 to 17.0 months; 80% CI, 18 to 31 months; Fig 3). The median duration of response was 5.8 months (range, 2.9 to 15.3 months). For patients who did experience progression (n = 45), the median TTP was 3.9 months (range, 0.9 to 17.0 months).
Fig 3.
Kaplan-Meier curve of progression-free survival (PFS). P, pertuzumab; T, trastuzumab.
DISCUSSION
The combination of pertuzumab and trastuzumab is well tolerated and shows encouraging results in patients with HER2-positive breast cancer with documented progression on trastuzumab as prior therapy. The observed AEs were generally mild or moderate, with GI and skin toxicities among the most frequent. These AEs are probably caused by reduced numbers of HER1:HER2 heterodimers because similar AEs are frequently seen in agents targeting HER1 or HER1/2, such as cetuximab, erlotinib, and lapatinib.17–19 In contrast with some of these HER1- or HER1/2-targeted therapies, the GI and skin toxicities were not severe, and all patients continued therapy.
No clinically significant cardiac events were observed in this trial of 66 patients, in comparison with a recent phase II trial of trastuzumab and pertuzumab in 11 patients with HER2-positive MBC where cardiac toxicity was reported in six patients.20 In addition to the difference in patient numbers in the studies, there were variations in the patient inclusion criteria, definition of changes in cardiac function, and criteria used to evaluate decline in LVEF, which may explain the apparent differences in cardiac function observed. First, patients who had previously experienced a decrease in LVEF during trastuzumab therapy or had a history of hypertension were eligible in the small study but were excluded from the present study. Second, in the study by Portera et al,20 only two patients had decreases in LVEF of ≥ 10 percentage points from baseline and less than 50% absolute value. Furthermore, the only patient in the other study who experienced congestive heart failure had a prior history of left chest wall radiation, baseline tachycardia, and extensive chest wall disease. Taken together, pertuzumab seems to be associated with minimal cardiac dysfunction.
The observed efficacy and low rate of grade 3 or 4 AEs achieved by combining the two agents are encouraging. Both the overall response rate (24.2%) and complete response rate (7.6%), the final primary end points for this trial, exceeded study expectations. The CBR was 50%, and the durable clinical benefit is highlighted by the observed median PFS time of 5.5 months. These data compare favorably with results from a randomized trial of trastuzumab in combination with lapatinib in patients with HER2-positive MBC, where an ORR of 10.3%, CBR of 24.7%, and median PFS time of 2.8 months were observed. However, it should be noted that patients in the trastuzumab plus lapatinib trial were more heavily pretreated (median of three prior trastuzumab regimens or four to five prior chemotherapy regimens).21 Our results also suggest that pertuzumab may be more active in HER2-positive tumors because in a study of HER2-negative breast cancer, single-agent pertuzumab showed only modest antitumor activity (10% of patients had a PR or SD ≥ 6 months).22
Recent randomized trials have demonstrated the effectiveness of combining HER2-targeted therapy with chemotherapy in patients who experience progression on prior trastuzumab. A trial of second-line trastuzumab and capecitabine reported a response rate of 48% and a median TTP of 8.2 months,23 whereas a trial of lapatinib plus capecitabine, in a similar patient population as that of the present study, reported a response rate of 24% and a median TTP of 6.2 months.24 The efficacy and, in particular, the safety of the pertuzumab and trastuzumab combination compare favorably with these results because lapatinib plus capecitabine resulted in a high incidence of grade 3 or 4 diarrhea and rash (14% and 9%, respectively).24 Therefore, it is conceivable that a nonchemotherapy-containing regimen may be an option in the therapy of patients who have experienced progression on first-line anti-HER2 therapy.
Additional studies are planned or ongoing to further evaluate the use of pertuzumab in HER2-positive breast cancer. Single-agent pertuzumab activity in the same patient population as this trial is being evaluated. A phase II trial of neoadjuvant trastuzumab and pertuzumab in HER2-positive breast cancer and an international phase III randomized, double-blind, placebo-controlled trial of trastuzumab + docetaxel ± pertuzumab in first-line HER2-positive MBC are currently recruiting patients. The potential advantage of studying this combination in the first-line setting is that it may provide enhanced antitumor activity in less pretreated patients and delay acquired resistance.
Acknowledgment
We thank Eleanor Steele for medical writing support.
Footnotes
Supported by F. Hoffmann-La Roche, Basel, Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00301899.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Virginia A. McNally, Roche (C); Graham A. Ross, Roche (C) Consultant or Advisory Role: José Baselga, Roche (C), Exelixis (C), Novartis (C), Merck (C); Karen A. Gelmon, Roche (C), GlaxoSmithKline (C), Novartis (C), AstraZeneca (C), Pfizer (C); Andrew Wardley, Roche (C); David Miles, Roche (C); Javier Cortes, Roche (C); Pierre Fumoleau, Roche (C), sanofi-aventis (C), GlaxoSmithKline (C); Luca Gianni, Roche (C), Genentech (C), GlaxoSmithKline (C), Wyeth (C) Stock Ownership: Virginia A. McNally, Roche; Graham A. Ross, Roche, GlaxoSmithKline Honoraria: José Baselga, Roche, GlaxoSmithKline; Karen A. Gelmon, Roche, GlaxoSmithKline, AstraZeneca, Pfizer, Novartis, Genentech; Andrew Wardley, Roche; David Miles, Roche; Javier Cortes, Roche; Pierre Fumoleau, Roche, sanofi-aventis, GlaxoSmithKline Research Funding: Andrew Wardley, Roche Expert Testimony: None Other Remuneration: Shailendra Verma, Roche; Andrew Wardley, Roche
AUTHOR CONTRIBUTIONS
Conception and design: José Baselga, Karen A. Gelmon, Shailendra Verma, Andrew Wardley, David Miles, Luca Gianni
Administrative support: Graham A. Ross
Provision of study materials or patients: José Baselga, Karen A. Gelmon, Shailendra Verma, Andrew Wardley, PierFranco Conte, David Miles, Giulia Bianchi, Javier Cortes, Pierre Fumoleau, Luca Gianni
Collection and assembly of data: Karen A. Gelmon, Shailendra Verma, Andrew Wardley, David Miles, Giulia Bianchi, Graham A. Ross, Luca Gianni
Data analysis and interpretation: José Baselga, Karen A. Gelmon, Shailendra Verma, Andrew Wardley, David Miles, Giulia Bianchi, Javier Cortes, Virginia A. McNally, Graham A. Ross, Luca Gianni
Manuscript writing: José Baselga, Karen A. Gelmon, Shailendra Verma, Andrew Wardley, David Miles, Giulia Bianchi, Javier Cortes, Virginia A. McNally, Graham A. Ross, Pierre Fumoleau, Luca Gianni
Final approval of manuscript: José Baselga, Karen A. Gelmon, Shailendra Verma, Andrew Wardley, PierFranco Conte, David Miles, Giulia Bianchi, Javier Cortes, Virginia A. McNally, Graham A. Ross, Pierre Fumoleau, Luca Gianni
REFERENCES
- 1.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Smith IE. Efficacy and safety of Herceptin in women with metastatic breast cancer: Results from pivotal clinical studies. Anticancer Drugs. 2001;12(suppl 4):S3–S10. doi: 10.1097/00001813-200112004-00002. [DOI] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol. 2007;25(suppl):6s. abstr 512. [Google Scholar]
- 5.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Slamon D, Eiermann W, Robert N, et al. BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. 29th Annual San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. abstr 52. [Google Scholar]
- 8.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 9.Dawood SS, Kristine B, Hortobagyi GN, et al. Prognosis of women with stage IV breast cancer by HER2 status and trastuzumab treatment: An institutional based review. J Clin Oncol. 2008;26:45s. doi: 10.1200/JCO.2008.19.9844. abstr 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diermeier-Daucher S, Hasmann M, Brockhoff G. Flow cytometric FRET analysis of erbB receptor interaction on a cell-by-cell basis. Ann N Y Acad Sci. 2008;1130:280–286. doi: 10.1196/annals.1430.003. [DOI] [PubMed] [Google Scholar]
- 12.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 14.Friess T, Thier M, Scheuer W, et al. Combination treatment with pertuzumab and trastuzumab against Calu-3 human NSCLC xenograft tumors is superior to monotherapy. Presented at the 17th Annual Meeting of the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer; November 14-18, 2005; Philadelphia, PA. [Google Scholar]
- 15.Birner P, Oberhuber G, Stani J, et al. Evaluation of the United States Food and Drug Administration-approved scoring and test system of HER-2 protein expression in breast cancer. Clin Cancer Res. 2001;7:1669–1675. [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.GlaxoSmithKline. Highlights of prescribing information: TYKERB (Lapatinib) 2007. http://us.gsk.com/products/assets/us_tykerb.pdf.
- 18.Genetech. TARCEVA (erlotinib) package insert, 2008. http://www.gene.com/gene/products/information/pdf/tarceva-prescribing.pdf.
- 19.ImClone Systems, Bristol-Myers Squibb. ERBITUX (cetuximab) package insert, 2007. http://www.erbitux.com/erbitux/erb/home/index.jsp?BV_UseBVCookie=Yes.
- 20.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Shaughnessy J, Blackwell KL, Burstein H, et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy. J Clin Oncol. 2008;26:44s. abstr 1015. [Google Scholar]
- 22.Cortes J, Baselga J, Wardley A, et al. Open-label, randomized, phase II study of pertuzumab (Omnitarg) in patients with metastatic breast cancer (MBC) with low expression of HER2. J Clin Oncol. 2005;23:208s. abstr 3068. [Google Scholar]
- 23.von Minckwitz G, Zielinski C, Maartense E, et al. Capecitabine vs. capecitabine + trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: The TBP phase III study (GBG 26/BIG 3-05) J Clin Oncol. 2008;26:47s. abstr 1025. [Google Scholar]
- 24.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]