1. Introduction
Preeclampsia is a pregnancy specific multisystem disorder that affects 3%-5% of pregnant women. (1) It remains a major cause of maternal and neonatal morbidities and mortality, as more than 50,000 women die annually from preeclampsia worldwide, mostly in developing countries. (1) Preeclampsia is usually diagnosed based on new onset hypertension, proteinuria or end organ damage after 20 weeks of gestation. (1) Due to lack of effective therapy for preeclampsia, and in order to prevent maternal morbidity and mortality, obstetricians tend to deliver women with preeclampsia shorty after diagnosis. However, this approach is usually associated with risk of premature delivery and its associated morbidities. Despite being a pregnancy specific condition, preeclampsia is associated with long-term maternal and neonatal adverse outcomes, as it may predispose the mother to hypertension, cardiovascular disease including stroke and ischemic heart disease, renal disease, and premature death, and the neonate to the long-term complications of preterm delivery; as well as adult metabolic, cardiovascular and neurodevelopmental disorders (1). Recent advances in understanding the pathogenesis of preeclampsia led to interest in novel therapeutic and/or preventive agents for preeclampsia. These include anti-digoxin antibodies, antithrombin, relaxin, and 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors (statins). (2) Discussion of all these therapeutics is beyond the scope of this editorial which will focus on the biological plausibility and current clinical trials using statins to treat and/or prevent preeclampsia.
2. Etiology of Preeclampsia
Preeclampsia is not a single disease but rather a syndrome with multiple subtypes, (3) and its exact pathogenesis remains unknown. Although many mechanisms have been proposed, abnormalities in the following processes are generally well accepted: angiogenic imbalance, endothelial cell dysfunction, oxidative stress, and exaggerated inflammation (1, 4, 5). It has also been proposed that uteroplacental ischemia is one plausible etiologic mechanism that underlie preeclampsia, IUGR and placental abruption, and these conditions have been classified as the syndrome of “ischemic placental disease”. (6)
Recently, preeclampsia has been considered a two-step disease. (7) The first step is asymptomatic, occurs early in pregnancy and is characterized by an abnormal trophoblastic invasion and impaired uterine spiral arteries remodeling. This leads to placenta oxidative stress and hypoxic injury. In addition, preeclampsia is associated with systemic and exaggerated inflammatory response characterized by abnormal Th1/Th2 cytokine balance with excessive release of pro-inflammatory cytokines. These ultimately lead to an angiogenic imbalance characterized by overexpression and release of anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng) which neutralize angiogenic factors such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and and transforming growth factor-b (TGF-b). The angiogenic imbalance may represent a “final common pathway” leading to endothelial dysfunction and the clinical features (second and symptomatic stage) of preeclampsia (1, 5). Although, earlier studies suggested that preeclampsia is also associated with suppression of placental heme oxygenase-1 (HO-1), (8) an inducible enzyme with cytoprotective and anti-oxidative stress properties, recent studies question that association as the ability of HO-1 to inhibit the release of sFlt-1 and sEng was not confirmed.(8, 9).
3. Can we use statins to prevent/treat preeclampsia?
Although preeclampsia is unique to pregnancy, it shares risk factors and pathophysiological similarities with adult cardiovascular disease. Both inflammation and dysfunction of the endothelium are fundamental mechanisms for the initiation and progression of both preeclampsia and atherosclerosis (1, 4, 5). Numerous attempts at primary and secondary prevention of preeclampsia, using supplements and medications, have had limited success (10). Only low-dose aspirin was found to have a modest benefit in reducing the rate of preeclampsia in an individual patient meta-analysis (11), and that benefit was only achieved if the drug was started before 16 weeks’ gestational age. On the contrary, inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase (statins) are effective in primary and secondary prevention of cardiovascular mortality and morbidity.
3.1: Biological plausibility
Due to the similarity between preeclampsia and cardiovascular disease and the role of statin in primary and secondary prevention of cardiovascular morbidities and mortality, others and we focused on the potential role of statin in preeclampsia prevention (5). Using animal models, daily administration of pravastatin to rodents destined to develop preeclampsia restored angiogenic balance, lowered blood pressure, and improved their abnormal vascular reactivity. In addition, pravastatin up-regulated endothelial nitric oxide synthase and HO-1 expression, and prevented kidney injury. These benefits were observed without harmful effects to dams or increase in pup malformations, resorption, or any differences in pup birth weights (12, 13). In fact, pravastatin prevented the growth restriction associated with preeclampsia in these animal models (13). Moreover, statins are known to have anti-inflammatory properties as they have been shown to correct the imbalance in the Th1/Th2 cytokine responses that is commonly observed in preeclampsia (statins decrease Th1 proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL) -1, and IL-2, and increase Th2 anti-inflammatory cytokines such as IL-4, IL-10). Statins are also known to other pleiotropic action on reducing formation of free oxygen radicals and smooth muscle cell proliferation (5).
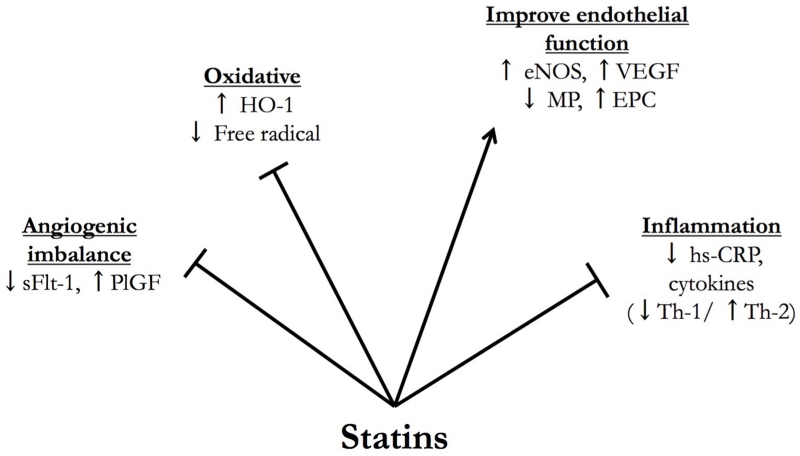
The ability of statins to revert the angiogenic imbalance, a hallmark of preeclampsia, and restore endothelial dysfunction in animal models, as well as their other pleiotropic effects make them highly promising candidates for the prevention and/or treatment of preeclampsia (Figure 1).
Figure 1.
Biological plausibility and pleiotropic actions of statin when used to treat/prevent preeclampsia. (HO-1: heme oxygenase-1; sFlt-1: soluble fms-like tyrosine kinase 1; PlGF: placental growth factor; eNOS: endothelial nitric oxide synthase; VEGF: vascular endothelial growth factor; MP: microparticles, EPC: endothelial progenitor cells, hs-CRP: high sensitivity c-reactive protein)
3.2: Pravastatin trials in pregnancy
The use of statins to prevent pregnancy complication is high-risk women, was recently reported (14). Moreover, two clinical trials have evaluated the potential role of statins in preeclampsia. The ability of pravastatin to restore the angiogenic balance was tested in a proof of concept randomized trial “StAmP trial” that enrolled subjects in the UK (Statins to Ameliorate early onset Preeclampsia; www.controlled-trials.com; ISRCTN23410175). The aim of this trial is to evaluate whether pravastatin ameliorates the angiogenic imbalance in women with severe preeclampsia before 32 weeks’ gestation. The results of the trial have not been reported.
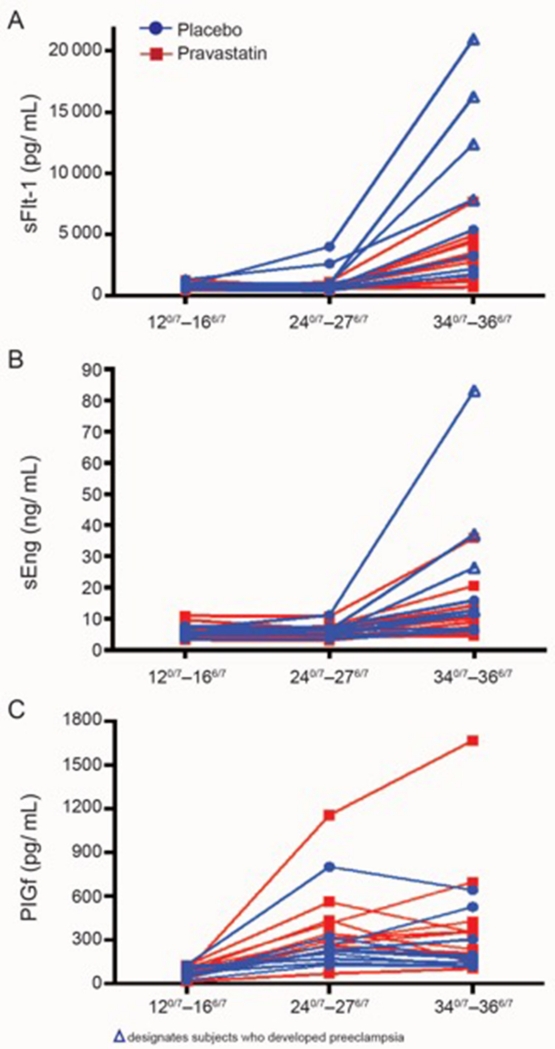
The second trial is a pilot, multicenter, double-blind, placebo-controlled, randomized trial of women at high risk for preeclampsia (prior pregnancy requiring delivery for severe preeclampsia at less than 34 weeks’ gestation). Women are randomized between 120/7 and 166/7 weeks’ gestation to daily pravastatin or placebo orally until delivery (www.clinicaltrials.gov Identifier NCT01717586) (15). The results of the first cohort of this trial showed that women who received pravastatin had lower rates of preeclampsia and indicated preterm delivery (although statistically not significant due to small sample size) compared to those who received placebo. There were no differences in rates of study drug side effects, congenital anomalies, or other adverse or serious adverse events between the two groups. The maternal and cord blood concentrations of liver (alanine and aspartate transaminases) and muscle (creatine kinase) enzymes were not increased with pravastatin therapy. The concentrations of PlGF were increased in subjects receiving pravastatin, and those of sFlt-1 and sEng were decreased. However the differences for these markers did not reach statistical significance (Figure 2). Although pravastatin reduced maternal cholesterol concentrations, umbilical cord cholesterol concentrations and infant birthweight were not different between the groups (15). The findings of this pilot trial, while reassuring, are still preliminary; as the investigators are continuing the trial at a higher pravastatin dose.
Figure 2.
Longitudinal plots of serum concentrations of soluble fms-like tyrosine kinase (Panel A; sFlt-1), soluble endoglin (Panel B; sEng), and placental growth factor (Panel C; PlGF) within individual subjects who received pravastatin (n=10, red) or placebo (n=10, blue) according to the gestational age window at time of collection: 120/7–166/7 weeks (baseline and before treatment), 24 0/7–276/7, and 340/7–366/7. (Reproduced from [15] with permission of the publisher, Elsevier, Inc.)
Δ designates the subjects who developed preeclampsia.
4. Expert opinion
Preeclampsia is a serious pregnancy complication with significant short- and long-term adverse outcomes for the mother and fetus/neonate. Multiple attempts for preeclampsia prevention using various medications and supplements had no or limited success and delivery remains the main option to prevent maternal complications (10). However this is usually at the expense of delivering a premature fetus. The similarity between preeclampsia and adult cardiovascular disease as well as the encouraging data from animal studies are driving the interest in evaluating the role of statins in preventing or treating preeclampsia. Attempts to prevent preeclampsia and other pregnancy complications based on pathophysiological pathways have previously failed. Therefore, before such therapy becomes widely used, studies are needed to determine its safety and efficacy, as animal models of preeclampsia do not represent the entire phenotype of the disease. Pravastatin is one of the older statins currently available. Its efficacy in preventing cardiovascular mortality and morbidity has been demonstrated. Pravastatin has unique physiochemical properties (16). It is one of the most hydrophilic and hepatoselective statin. Its ability to inhibit HMG-CoA reductase is limited compared to other statins and relatively minimal in non-hepatocytes (1/1000 in fibroblasts compared to hepatocytes). It is a substrate of efflux transporters such as P-glycoprotein (16). These characteristics limit the drug’s ability to cross the placenta, and this was confirmed in recent placental transfer studies (17). In addition, data from pregnancy exposure cohorts do not support the teratogenicity claims of statins (5, 18). Its current classification as Federal Drug Administration Category X is based mainly on the lack of indications to use statins in pregnancy to outweigh any potential risk. In fact, were pravastatin to have significant benefit in pregnancy, then the category X designation would have to be critically reevaluated. However, until data are available from large clinical trials to evaluate pravastatin’s effectiveness to prevent and/or treat preeclampsia, the use of pravastatin remains investigational.
Acknowledgements
The manuscript does not necessarily represent the official views of the NICHD or the National Institutes of Health.
This work is supported in part by a grant from The Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54 HD047891).
Abbreviations
- CO
carbon monoxide
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme-A
- HO-1
heme oxygenase-1
- Il
interleukin
- PlGF
placental growth factor
- sFlt-1
soluble fms-like tyrosine kinase 1
- sEng
soluble endoglin
- TGF-b
transforming growth factor-b
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Maged M. Costantine, Department of Obstetrics and Gynecology, University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555-0587.
Cande Annath, Department of Obstetrics and Gynecology, Columbia University Medical Center, 622 W. 168th St, New York, NY 10032.
References
- 1.American College of Obstetricians and Gynecologists Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Ornaghi S, Paidas MJ. Upcoming drugs for the treatment of preeclampsia in pregnant women. Expert Rev Clin Pharmacol. 2014;7:599–603. doi: 10.1586/17512433.2014.944501. [DOI] [PubMed] [Google Scholar]
- 3.Staff AC, Benton SJ, von Dadelszen P, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–42. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 4.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- * 5.Costantine MM, Cleary K, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network Pravastatin for the Prevention of Preeclampsia in High Risk Pregnant Women. Obstet Gynecol. 2013;121:349–53. doi: 10.1097/aog.0b013e31827d8ad5. Paper summarizes the rationale and biological plausibility for statins as agents to prevent preeclampsia in high risk women.
- 6.Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol. 2014;38:131–2. doi: 10.1053/j.semperi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009 Mar;30(Suppl A):S32–7. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 8.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–97. doi: 10.1161/CIRCULATIONAHA.106.660134. This study demonstrated the role of HO-1 in inhibiting the release of sFlt-1 and sEng. This provides a strong rationale to use statins in preeclampsia
- 9.Tong S, Kaitu’u-Lino TJ, Onda K, et al. Heme Oxygenase-1 Is Not Decreased in Preeclamptic Placenta and Does Not Negatively Regulate Placental Soluble fms-Like Tyrosine Kinase-1 or Soluble Endoglin Secretion. Hypertension. 2015;66:1073–81. doi: 10.1161/HYPERTENSIONAHA.115.05847. [DOI] [PubMed] [Google Scholar]
- 10.Barton JR, Sibai B. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112:359. doi: 10.1097/AOG.0b013e3181801d56. [DOI] [PubMed] [Google Scholar]
- 11.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative Group Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 12.Costantine M, Tamayo E, Bytautiene E, et al. Using pravastatin to improve the vascular reactivity in a mouse model of soluble Fms-like tyrosine kinase-1-induced preeclampsia. Obstet Gynecol. 2010;116(116):114–120. doi: 10.1097/AOG.0b013e3181e10ebd. [DOI] [PubMed] [Google Scholar]
- *13.Kumasawa K, Ikawa M, Kidoya H, et al. Pravastatin induces placental growth factor and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108. The authors tested pravastatin ability to reverse various features of preeclampsia using a novel mouse model.
- 14.Brownfoot FC, Tong S, Hannan NJ, et al. Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension. 2015;66:687–97. doi: 10.1161/HYPERTENSIONAHA.115.05445. [DOI] [PubMed] [Google Scholar]
- **15.Costantine MM, Cleary K, Hebert M, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network Safety and Pharmacokinetics of Pravastatin Used for the Prevention of Preeclampsia in High-Risk Pregnant Women: A Pilot Randomized Controlled Trial. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.12.038. In press. This paper reports the results of a pilot clinical trial using pravastatin to prevent preeclampsia in high-risk women. In addition to clinical outcomes, the authors looked at safety and pharmacokinetic parameters of pravastatin use in pregnancy.
- 16.Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397–412. doi: 10.2165/00003088-200039060-00002. [DOI] [PubMed] [Google Scholar]
- 17.Nanovskaya TN, Patrikeeva SL, Paul J, Costantine M, Hankins GDV, Ahmed MS. Transplacental transfer and distribution of pravastatin. Am J Obstet Gynecol. 2013;209:373.e1–5. doi: 10.1016/j.ajog.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazmin A, Garcia-Bournissen F, Koren G. Risk of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):9. doi: 10.1016/S1701-2163(16)32656-1. [DOI] [PubMed] [Google Scholar]