Abstract
Purpose
To perform the first meta-analysis of the efficacy of skin-directed therapies for cutaneous metastases.
Methods
MEDLINE, EMBASE, The Cochrane Library, and ClinicalTrials.gov databases were searched for reports of prospective clinical studies published between 1960 and 2013 that assessed the response of skin-directed therapy for cutaneous metastases (47 of 2,955 unique studies were selected). Primary end points of the study were complete and objective response rates. Secondary analyses were preplanned and included subgroup analyses by skin-directed therapy, histology, and recurrence rates. Meta-analyses were performed with random-effect modeling, and extent of heterogeneity between studies was determined with the Cochran Q and I2 tests.
Results
After applying exclusion criteria, 47 prospective studies of 4,313 cutaneous metastases were assessed. Five skin-directed therapies were identified: electrochemotherapy, photodynamic therapy, radiotherapy, intralesional therapy, and topical therapy. Among all cutaneous metastases, complete response rate was 35.5% (95% CI, 27.6% to 44.3%) and objective response rate was 60.2% (95% CI, 50.6% to 69.0%). Overall recurrence rate was estimated to be 9.2% (95% CI, 3.7% to 21.2%). Melanoma and breast carcinoma comprised 96.8% of all cutaneous metastases studied and had similar objective response rates (54.5% [95% CI, 48.3% to 60.7%] and 54.0% [95% CI, 48.3% to 59.7%], respectively). Grade ≥ 3 toxicity was reported in less than 6% of patients.
Conclusion
Response to skin-directed therapy for cutaneous metastases is high but heterogeneous across treatment modalities, with low rates of recurrence post-treatment. Treatment was generally well tolerated and conferred improvements in quality of life. Standardization of response criteria for cutaneous metastases and treatment algorithms to optimally use the available skin-directed therapies are needed.
INTRODUCTION
Although less common than primary skin cancers, cutaneous metastases (CMs) are not a rare manifestation of malignancy. A meta-analysis of 22,297 patients with solid tumors estimated that 5.3% developed CMs.1 It is therefore estimated that in 2013, 77,166 of the 1,455,960 newly diagnosed cancers in the United States (excluding cancers of the integument or hematologic malignancies) will develop CMs.2 This does not include 45% of patients with metastatic melanoma who also develop CMs.3
With advances in the treatment of metastatic cancer, patients are living longer and are more likely to experience the sequelae of advanced disease, such as CMs. CMs can cause considerable morbidity, serving as a nidus for infection, bleeding, disfigurement, or pain (Appendix Fig A1, online only).4–6 Shimozuma et al7 demonstrated that, among women with advanced or recurrent breast cancer, CMs were associated with the greatest negative effect on quality of life (QOL). Systemic therapy alone often has limited efficacy with CMs, but skin-directed therapy has the potential to yield improved disease response and symptom palliation.8–14
For readers unfamiliar with the various forms of skin-directed therapy discussed herein, a brief summary is provided. Electrochemotherapy (ECT) for CMs uses short electric pulses directed at the tumor to permeabilize cell membranes to increase the absorption of either intralesional or intravenous chemotherapy. Photodynamic therapy (PDT) for CMs uses a nontoxic light to activate a topical or intravenous photosensitizer that interacts with tissue oxygen to generate toxic free radicals for its cytotoxic effects. Radiotherapy (RT) delivers ionizing radiation to the CMs and kills tumor cells by generating free hydroxyl radicals and causing direct DNA damage. Intralesional therapy (ILT) relies on the administration of an antineoplastic agent directly into or adjacent to the CM. Topical therapy (TT) is the application of an antineoplastic agent directly onto the CM.
The benefit of directed local therapy for other organ metastases, such as bone,15,16 spine,17 and brain metastases,18 has been the focus of several large randomized trials and meta-analyses designed to optimize treatment and disease control and maximize QOL. However, a limited number of prospective studies have been conducted on the treatment of CMs across a multitude of skin-directed treatment modalities. Thus, we conducted a meta-analysis on treatment efficacy of skin-directed therapies for CMs.
METHODS
Study Selection
Systematic literature searches were conducted (September 10, 2013) in four databases (MEDLINE [via PubMed], EMBASE, The Cochrane Library, and ClinicalTrials.gov) for human-only studies written in English from January 1, 1960, through September 10, 2013. Controlled vocabulary was leveraged as well as text words in the development of the search strategies. All search results were combined in a bibliographic management tool, and duplicates were eliminated both electronically and manually.
The search strategy contained two major components linked together with the AND operator: (1) skin-directed therapy: surgery, excision, topical, intralesional therapy, injection, photodynamic, photochemotherapy, electrochemotherapy, radiation, radiotherapy, brachytherapy AND (2) skin metastasis: cutaneous metastasis/metastases, dermal metastasis/metastases.
After combining the two concepts, the results were limited (by using filters) to studies in English and those regarding humans only in PubMed and EMBASE. For databases that did not have a filter (The Cochrane Library and ClinicalTrials.gov) to eliminate undesired languages and animal studies, those were excluded during the investigator's assessment of the records. For a complete list of Medical Subject Headings and keyword terms used, refer to the PubMed search strategy in the Data Supplement.
Two investigators (D.E.S. and E.A.G.S.) independently reviewed all records from the initial search strategy by using a four-stage study-selection process. During stage 1, all 2,955 record titles and abstracts (if available) were reviewed to detect potentially relevant records (details of exclusion criteria and reason for exclusion are included in the Data Supplement). During stage 2, all full-length articles and meeting abstracts that passed stage 1 were reviewed to identify studies that had extractable response data for a skin-directed local therapy for CM (Data Supplement). The definition of a CM (v primary cutaneous malignancy) was determined by the reporting author and was assumed to be valid. Importantly, studies were excluded at this stage that grouped lymph node metastases with CMs. During stage 3, studies that re-reported data from the same trials were systematically removed, yielding 107 eligible studies. Finally, during stage 4, study design was assessed, and only prospective studies were eligible for analysis.
Data Extraction
D.E.S. and E.A.G.S. independently extracted data from the 47 studies. Data extracted (Appendix Tables A1–A8, online only) included patient and CM characteristics; treatment characteristics, including the use of concurrent systemic therapy and skin-directed treatment details; response rates and criteria used for complete response (CR), partial response (PR), stable disease, progressive disease, objective response rates (ORRs) and overall recurrence rates; toxicity and QOL findings and scales used; and level of evidence and data quality.
End Point Definitions
Primary end points of the study were CR and ORR of all studies. CR was chosen rather than PR, stable disease, or progressive disease because it was deemed the least subjective assessment of response (Table 1). CR and ORRs were study defined; if not explicitly stated, ORR equaled CR plus PR. Secondary analyses were preplanned and included response rates by skin-directed treatment modality, histology, and recurrence rates. Histology subgroup analyses were performed after the study had been divided by histology when feasible. A recurrence was defined as a CM that initially underwent an objective response and subsequently recurred within the treatment field. Toxicity and QOL data were analyzed qualitatively secondary to the multiple toxicity scales used and lack of consistent reporting of specific toxicities to enable pooled analyses. In studies without formal toxicity grading scales, the words “serious” or “severe” were interpreted as grade 3 toxicities, and “life-threatening” was interpreted as grade 4 toxicity.
Table 1.
Prospective Studies of Skin-Directed Therapies for Skin Metastases
| Study | Year | No. of Patients | No. of Lesions | Local Therapy | No. of Lesions With CR | Definition of CR | No. of Lesions With Objective Response | Definition of Objective Response |
|---|---|---|---|---|---|---|---|---|
| Heller et al44* | 1996 | 4 | 12 | ECT | 5 | “Absence of any trace of tumor” | 7 | CR + PR |
| Sersa et al11 | 2000 | 9 | 27 | ECT† | 3 | WHO, 1979 | 13 | CR + PR |
| Rodriguez-Cuevas et al45* | 2001 | 6 | 29 | ECT | 11 | “Complete response” | 27 | CR + PR |
| Byrne et al28 | 2005 | 16 | 53 | ECT | 34 | “No residual disease” | 39 | CR + PR |
| Gaudy et al29 | 2006 | 12 | 24 | ECT† | 11 | “Total disappearance of the lesion” | 14 | CR + PR |
| Marty et al23 | 2006 | 41 | 171 | ECT | 126 | WHO, 1997 | 145 | CR + PR |
| Quaglino et al46 | 2008 | 14 | 233 | ECT | 136 | WHO, 1997 | 216 | CR + PR |
| Matthiessen et al30 | 2011 | 24 | 94 | ECT | 58 | RECIST, 2000 | 76 | CR + PR |
| Benevento et al47 | 2012 | 12 | 142 | ECT | 107 | RECIST, 2000 | 131 | CR + PR‡ |
| Campana et al21 | 2012 | 35 | 35 | ECT | 19 | RECIST, 2000 | 32 | CR + PR |
| Kendler et al22* | 2013 | 3 | 79 | ECT | 7 | RECIST, 2009 | 7 | CR + PR‡ |
| Sperduto et al48 | 1991 | 20 | 20 | PDT | 4 | “Clinical and pathologic regression of all tumor in the treatment field” | 13 | CR + PR‡ |
| Cairnduff et al49 | 1994 | 5 | 14 | PDT | 5 | “Absence of clinically evident tumor” | 5 | CR + PR‡ |
| Baas et al50 | 1996 | 4 | 20 | PDT† | 15 | “Complete response” | 18 | CR + PR‡ |
| Kaplan et al51 | 1998 | 3 | 13 | PDT | 13 | “Complete reduction of tumor” | 13 | CR + PR‡ |
| Mang et al52 | 1998 | 8 | 86 | PDT | 79 | “Complete response” | 86 | CR + PR‡ |
| Overgaard et al36 | 1985 | NA | 15 | RT | 10 | “Complete disappearance of the tumor in the irradiated field” | 15 | CR + PR |
| Menéndez et al35 | 2009 | 7 | 88 | RT | 52 | “Complete response” | 61 | CR + PR |
| Cohen et al53 | 1978 | 18 | 766 | ILT | NA | NA | 647 | Clinical and pathologic “regression” |
| Nathanson et al54 | 1979 | 22 | 22 | ILT | 3 | “Complete disappearance” | 10 | CR + PR |
| Cascinelli et al37 | 1993 | 16 | 47 | ILT | NA | NA | 24 | ≥ 30% reduction in tumor volume |
| Stewart et al55* | 1999 | 23 | 23 | ILT | NA | NA | 7 | “Local regression” |
| Hoeller et al56 | 2001 | 7 | 7 | ILT | 2 | “100% decrease size change of injected lesion” | 5 | CR + PR‡ |
| Stopeck et al57 | 2001 | 29 | 29 | ILT | 1 | “Disappearance of all of the clinical evidence of tumor” | 5 | ≥ 25% reduction in product of perpendicular diameter |
| Radny et al58 | 2003 | 23 | 237 | ILT | 209 | “Disappearance of all clinical evidence of the … tumor” | 230 | CR + PR‡ |
| Oratz et al59 | 2003 | 25 | 244 | ILT | 114 | 100% tumor volume regression; response must last ≥ 28 days | 130 | CR + PR; Response must last ≥ 28 days |
| Byrne et al28 | 2005 | 16 | 19 | ILT | 5 | “No residual disease” | 6 | CR + PR |
| Triozzi et al60 | 2005 | NA | 14 | ILT | 0 | “Disappearance of all the clinical evidence of tumor” | 0 | CR + PR‡ |
| Gonzalez et al61 | 2006 | 77 | 77 | ILT | 2 | WHO§ | 7 | CR + PR |
| Kimata et al62 | 2006 | 6 | 5 | ILT | 0 | “Necrosis or disappearance of all tumor cells” | 4 | CR + PR‡ |
| Gaudy et al29 | 2006 | 12 | 16 | ILT† | 2 | “Total disappearance of the lesion” | 6 | CR + PR |
| Dummer et al63 | 2008 | 25 | 25 | ILT† | 3 | “Absence of detectable residual disease maintained for a minimum of 4 weeks” | 6 | CR + PR |
| Hofmann et al19 | 2008 | 5 | 5 | ILT | 1 | “Complete response” and “complete regression” | 2 | > 25% response to tumor volume; 1 CR + 1 SD‡ |
| Thompson et al20 | 2008 | 11 | 26 | ILT | 9 | RECIST, 2000 | 11 | CR + PR |
| Bedikian et al25 | 2010 | 85 | 255 | ILT | 4 | RECIST, 2000 | 15 | CR + PR |
| Weide et al26 | 2010 | 48 | 894 | ILT | 704 | Disappearance of lesion; no regrowth for 6 months | 710 | CR + PR‡ |
| Unger et al27 | 1992 | 24 | 24 | TT | 4 | “Complete remission” | 7 | CR + PR‡ |
| Unger et al31 | 1993 | 52 | 52 | TT† | 1 | WHO§ | 11 | CR + PR |
| Terwogt et al32 | 1999 | 30 | 30 | TT | 7 | Complete disappearance of all treated lesions for ≥ 4 weeks | 13 | CR + PR |
| Smorenburg et al33 | 2000 | 18 | 18 | TT† | 0 | WHO§ | 4 | CR + PR‡ |
| Leonard et al24 | 2001 | 24 | 19 | TT† | 2 | WHO, 1979 | 8 | CR + PR |
| Eilender et al34* | 2006 | 42 | 24 | TT† | 1 | Complete disappearance of all treated lesions for ≥ 4 weeks | 7 | CR + PR‡ |
| Salazar et al40 | 2011 | 10 | 10 | TT† | 3 | “Modified WHO criteria”§ | 7 | CR + PR‡ |
| Florin et al41 | 2012 | 5 | 45 | TT | 19 | “Complete response” | 44 | CR + PR |
| Adams et al42 | 2012 | 10 | 10 | TT† | 0 | “Absence of any detectable residual disease” | 2 | CR + PR |
| Plesnicar et al43 | 1982 | 19 | 19 | ILT + RT | 14 | “Cleared completely” | 15 | CR + PR |
| Lai et al12 | 2003 | 7 | 7 | TT + RT | 3 | Disappearance of all treated skin lesions ≥ 4 weeks | 6 | CR + PR |
| Green et al38 | 2007 | 10 | 178 | TT + ILT | 74 | “Impalpable” and “disappear” | 92 | CR + PR |
| Li et al39 | 2010 | 11 | 11 | TT + PDT | 8 | RECIST§ | 11 | CR + PR |
Abbreviations: CR, complete response; ECT, electrochemotherapy; ILT, intralesional therapy; NA, not available; PDT, photodynamic therapy; PR, partial response; RT, radiotherapy; SD, stable disease; TT, topical therapy.
Study divided by histology for subgroup analyses (see Appendix Table A1, online only, for information on histology details by study).
Concurrent systemic therapy used/allowed.
Definition not explicitly listed. Authors' definition used.
Year of response criteria used not stated.
Assessment of Data Quality and Reporting Risk of Bias
Level of evidence was collected by using standard definitions from the National Cancer Institute (Appendix Table A8).64 Randomized controlled trials (RCTs) were assessed by the Jadad scale (Appendix Table A7).65 Formal statistical analyses for publication bias were performed with funnel plots and Egger's test.
Statistical Analysis
For all analyses of CR, ORRs, and recurrence rates, odds ratios were calculated with 95% CIs. For meta-analysis, both a fixed-effects model and a random-effects model were considered. However, extent of heterogeneity was significant; thus, a random-effects model was reported for all analyses. Extent of heterogeneity between studies was performed with the Cochran Q test, and an I2 test. All probability values were two-tailed with P = .05. Toxicity calculations were reported as crude rates. To estimate the adjusted event rate when correcting for publication bias, the Duval and Tweedie trim-and-fill method was used.66 Statistical analyses were performed by using Comprehensive Meta-Analysis, version 2, software (Biostat, Englewood, NJ).
RESULTS
Patient and Study Characteristics
Forty-seven studies reporting on 915 patients with 4,313 CMs were included for analysis. The median age in those studies was 61 years (range, 42 to 83 years); 306 patients were males (33.4%), 565 were females (61.7%); sex could not be extracted for 44 patients (4.8%). Histologies for the CMs were 582 (13.5%) breast cancer, 3,591 (83.3%) metastatic melanoma, nine (0.2%) unspecified sarcoma, four (0.09%) Kaposi's sarcoma, three (0.07%) mucosal squamous carcinoma of the head and neck, two (0.05%) angiosarcoma, two (0.05%) unknown primary, and 120 (2.8%) other or unspecified. Of the 47 prospective studies, eight were RCTs, 38 were nonrandomized trials, and one was a prospective case series of consecutive patients (Fig 1).
Fig 1.
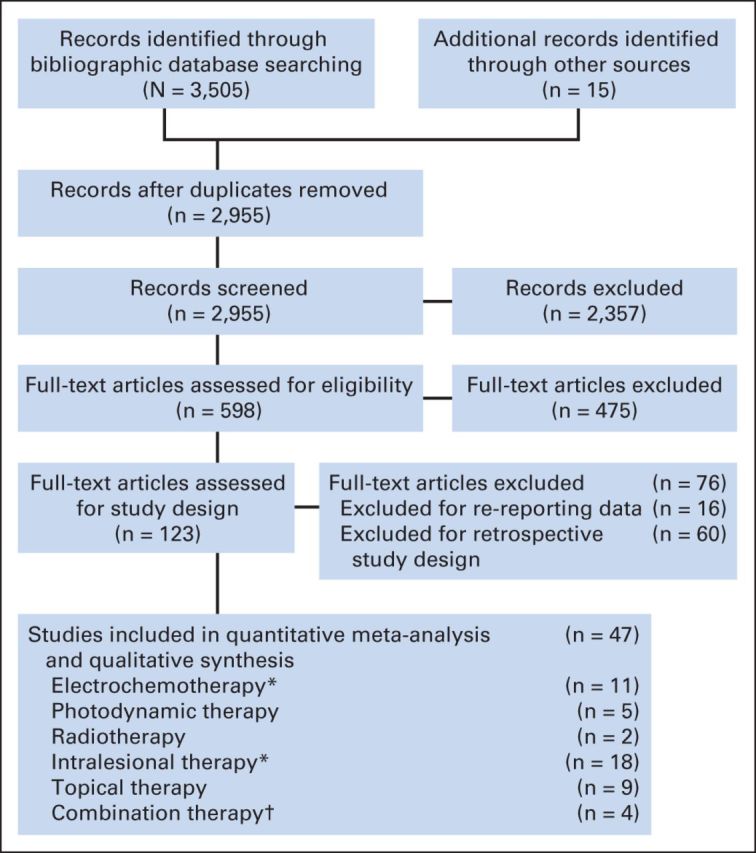
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature review process for skin-directed therapy of cutaneous metastases. (*) A randomized controlled trial comparing electrochemotherapy with intralesional therapy was divided by treatment modality. (†) More than one skin-directed therapy was used.
Primary End Points
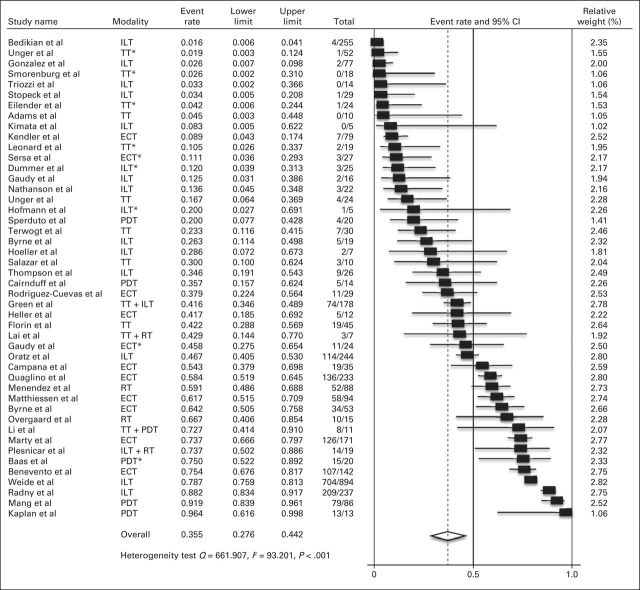
Of the 4,313 CMs, 836 (19.4%) reported ORRs but not CRs and were excluded from CR meta-analyses. Across treatment modalities, 1,890 (54.4%) of the 3,477 assessable patients with CMs had a CR. Formal criteria for defining CRs were found in eight studies (17.0%) that used WHO criteria, five (10.6%) that used criteria similar to WHO, seven (14.9%) that used RECIST, and 24 (51%) that used a variety of definitions to suggest complete clinical and/or histologic regression (Table 1). The CR rate for all included studies was 35.5% (95% CI, 27.6% to 44.2%) according to the random effects model (heterogeneity test, Q = 661.907; I2 = 93.201; P < .001; Fig 2).
Fig 2.
Meta-analysis of complete response. NOTE. Total column indicates No. of complete responses/No. of cutaneous metastases. ECT, electrochemotherapy; ILT, intralesional therapy; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy. (*) Concurrent systemic therapy was used and/or allowed.
ORR
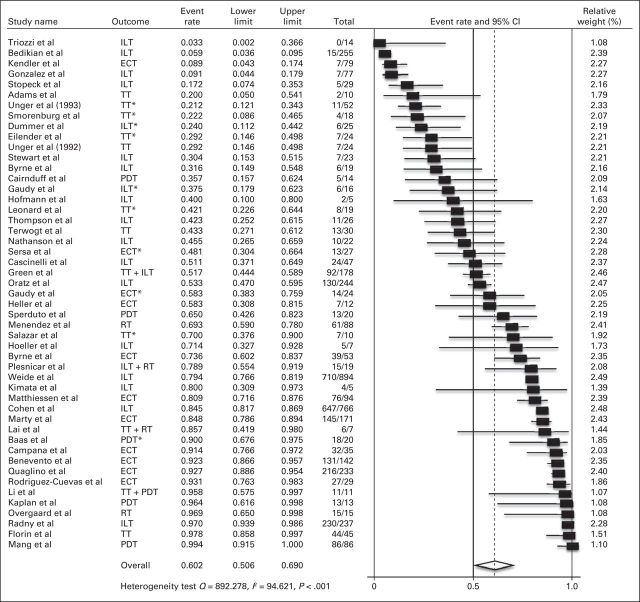
All 4,313 CMs were assessable for ORR analyses, of which 2,970 (68.9%) had ORRs. ORR was defined in 42 studies as the sum of CR plus PR, three used ≥ 25% reduction from pretreatment size, and two used other definitions (Table 1). The ORR for all studies was 60.2% (95% CI, 50.6% to 69.0%; Q = 892.278; I2 = 94.621; P < .001; Fig 3).
Fig 3.
Meta-analysis of objective response. NOTE. Total column indicates No. of objective responses/No. of cutaneous metastases. ECT, electrotherapy; ILT, intralesional therapy; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy. (*) Concurrent systemic therapy was used and/or allowed.
Secondary End Points
For response by treatment modality, CR ranged from 12.9% (95% CI, 5.4% to 27.5%) for TT to 67.8% (95% CI, 38.8% to 87.4%) for PDT (Fig 4). By treatment modality, ORR ranged from 42.1% (95% CI, 22.3% to 64.9%) for TT to 83.8% (95% CI, 37.9% to 97.8%) for RT (Fig 5). Three of the four combination studies used TT, with CR rate of 58.0% (95% CI, 27.7% to 83.3%) and ORR of 78.1% (95% CI, 44.1% to 94.1%).
Fig 4.
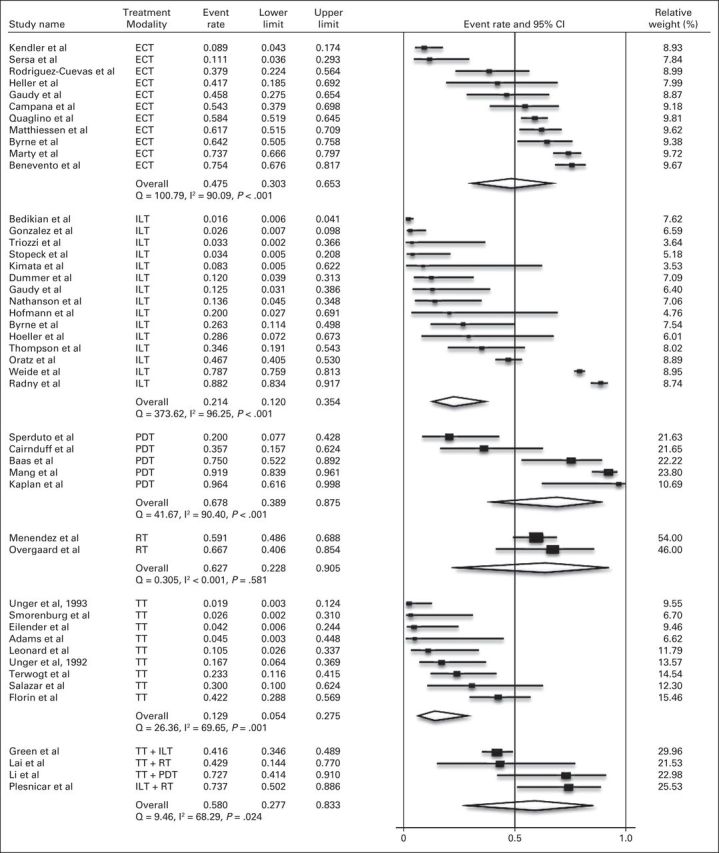
Meta-analysis of complete response by skin-directed therapy. ECT, electrochemotherapy; ILT, intralesional therapy; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Fig 5.
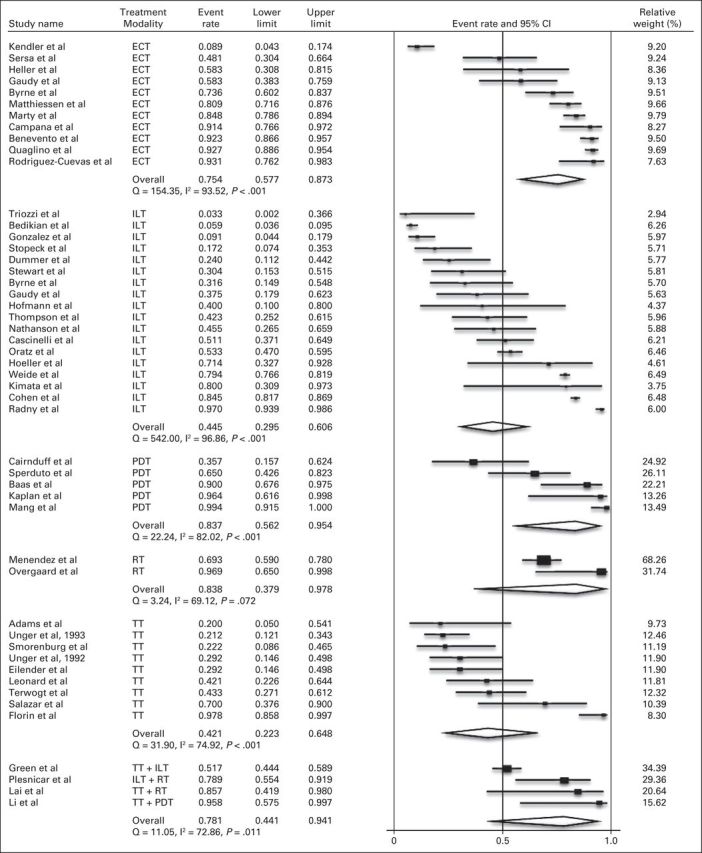
Meta-analysis of objective response by skin-directed therapy. ECT, electrochemotherapy; ILT, intralesional therapy; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Histology
Breast carcinoma and melanoma represented 96.8% of the CMs analyzed. They had nearly identical ORRs of 54.5% (95% CI, 48.3% to 60.7%) and 54.0% (95% CI, 48.3% to 59.7%), respectively. Of the remaining histologies, responses ranged from 50% for Kaposi's sarcoma and angiosarcoma to 83% for adenocarcinoma of unknown primary and mucosal squamous carcinoma of the head and neck (Appendix Fig A2, online only).
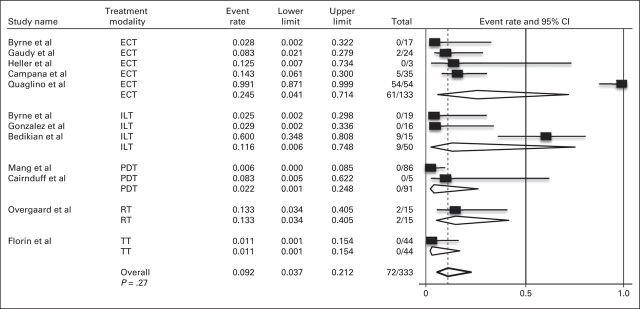
Recurrence Rates
Eleven studies had extractable recurrence information for CMs after initial ORR. From the 4,313 CMs initially evaluable, 2,970 had an ORR, of which only 333 (11.2%) had recurrence information for analysis. Seventy-two lesions experienced a recurrence at time of last follow-up, with an overall recurrence rate estimated at 9.2% (95% CI, 3.7% to 21.2%; Appendix Fig A3, online only).
Qualitative Analyses
Twenty-three studies (48.9%) used a formal toxicity scale (15 used various forms of the Common Terminology Criteria for Adverse Events, seven used WHO, and one used a custom scale); an additional three studies reported toxicity grade but did not define the scale used (Appendix Table A5). Treatment was well tolerated in an estimated 862 (94.2%) of 915 patients (grade ≤ 2 toxicity or the equivalent). Thirty-nine patients (4.3%) experienced grade 3 local or systemic toxicity. Fourteen patients (1.5%) experienced grade 4 toxicities, three related to disseminated intravascular coagulation of unknown relation to the local ILT and seven related to various cytopenias or pleural effusion in a study that used concurrent systemic therapy with local ILT. The remaining four grade 4 toxicities were defined by exfoliative or ulcerative dermatitis.
Treatment site pain was highly treatment specific. In patients treated with ILT, pain was most commonly reported as injection site pain, which occurred in approximately 21% to 72% of patients and was often transient. Pain resolution after ECT varied across studies from near complete resolution to 49% of patients having mild pain 1 month post-treatment.21,22 Local pain from PDT was reported to occur in up to 95% of patients and typically resolved within 3 weeks. Multiple TT studies reported local pain but did not report duration or resolution of pain symptoms. The two RT studies did not report on pain symptoms.
QOL
Five studies used formal measures to assess QOL: three used the visual analog scale, one used a custom four-point pain scale, and one used the Rotterdam Symptom Checklist and a Body Image Scale (Appendix Table A6).12,21–24 QOL results demonstrated that treatment of CMs decreased psychological distress from baseline to last follow-up.24 ECT increased mean pain scores up to 15 minutes post-treatment and reduced pain scores below pretreatment values thereafter.22 Combined TT and RT reduced the number of daily wound dressing changes and pain scores.12
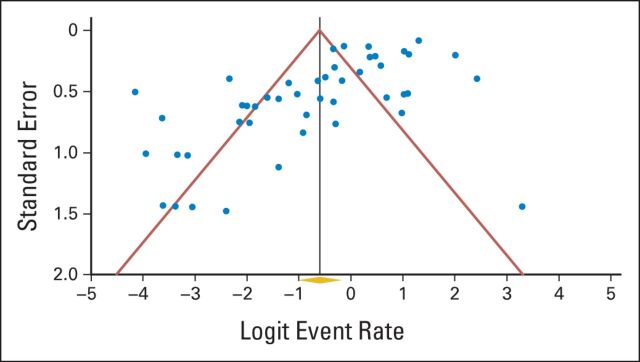
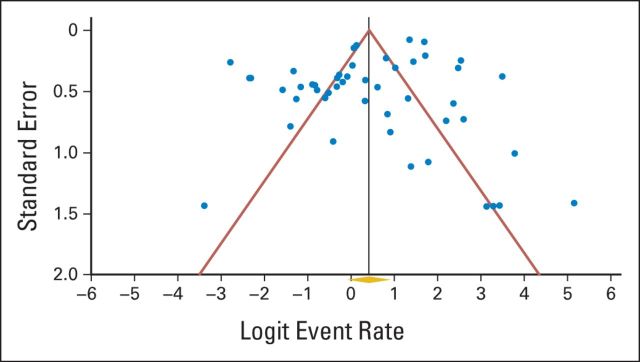
Publication Bias
A funnel plot of studies used to calculate CR rates (Appendix Fig A4, online only) demonstrated asymmetry that was confirmed with Egger's regression test (P < .001), indicating the presence of publication bias. When adjusting for this bias by using the trim-and-fill method, the original observed CR rate of 35.5% increased to 61.7% (95% CI, 52.6% to 70.1%). ORRs did not appear to be subject to significant publication bias, with relative symmetry present in the funnel plot (Appendix Fig A5, online only), confirmed with Egger's regression test (P = .06).
DISCUSSION
Decreasing symptom burden through palliative treatment can improve QOL, a goal often secondary only to improving survival in patients with cancer.67,68 CMs are increasingly prevalent and occur in approximately 10% of patients with metastatic cancer.3 Some cancers have a predilection for CMs, such as breast carcinoma and melanoma, in which the rate of CMs is nearly the same as that for brain metastasis (25% and 45%, respectively).1 Despite the prevalence of CMs, there are no guidelines for managing CMs with skin-directed therapy, and most textbooks reviewing CMs have little information on treatment.69
This meta-analysis was designed to ascertain the efficacy of a variety of skin-directed therapies commonly used to treat CMs. The data suggest that a majority of patients will respond to skin-directed therapy, and recurrence is infrequent. Moreover, toxicity appears minimal, and data suggest an improvement in QOL.10,21,24 Systemic therapy alone often has limited efficacy in CMs, with several series reporting ORRs of approximately 25%.8,11 The summary 60.2% ORR observed in this study clearly demonstrates the value of treating CMs with skin-directed therapy.
ECT typically involves electroporation of the cytotoxic drugs bleomycin and cisplatin. ECT has been shown to be more efficacious than ILT alone or systemic therapy alone.28,29 A meta-analysis of ECT for cutaneous and/or subcutaneous malignancy (including primary nonmetastatic disease) reported a crude CR rate of 59%.9 This is comparable with our crude CR rate of ECT for CMs of 57.5% (and the estimated summary CR rate of 47.5%; Fig 3). ECT is often performed as an inpatient procedure and most commonly requires general anesthesia; however, studies have successfully used local anesthesia alone.22 ECT is often performed in ≤ 30 minutes, but multiple treatments may be necessary. Pain is commonly reported, but general anesthesia can obviate this, and more than 90% of patients would agree to undergo another treatment if indicated.23,30 ECT use, especially in Europe, appears to be increasing since the publication of the European Standard Operating Procedures for Electrochemotherapy in 2006, a multicenter study standardizing the use of ECT for both primary and metastatic cancers.23,70,71
PDT has been extensively studied for premalignant and primary skin cancers, with more than 40 RCTs analyzed in a systematic review in 2010.72 However, there have been no RCTs of PDT for the treatment of CMs to date. Treatment times depend on whether an intravenous or topical photosensitizer is used but typically last less than 90 minutes. PDT is associated with treatment site pain that is mitigated by local anesthesia or oral analgesics.
RT is commonly used for the palliation of bone and brain metastases,17,18,73 but only two prospective trials have assessed RT for treating CMs.35,36 A unique advantage of RT is the ability to penetrate to any depth by selecting an appropriate type and energy of radiation. Treatments are typically given in several fractions over a period of weeks. Two studies used RT as part of combination therapy; high ORRs were observed, demonstrating the ability of TT or ILT to interact favorably with RT. Adverse effects primarily consist of local inflammatory symptoms.
ILT typically involves the injection of cytotoxic or immunomodulatory agents directly or perilesionally to the CMs.28,37 Despite two RCTs demonstrating superior efficacy of ECT over ILT, ILT can be a simple and effective treatment with limited adverse effects. ECT often requires general anesthesia, but ILT requires only local anesthesia. ILT often requires multiple treatment visits, with the majority of studies reporting two or more visits, and some reporting five or more visits.
TT for CMs was originally described using miltefosine, but three prospective trials with imiquimod have recently been reported. Both agents rely on enhancing the immune response against tumor cells. Most studies reported a median duration of therapy of ≥ 8 weeks, with some more than 1 year. Topical monotherapy appeared to have the lowest response rates in this meta-analysis; however, response rates were improved in the three studies that combined TT with another skin-directed therapy.12,38,39
We detected a less common form of publication bias among the studies analyzed; CR rates were significantly greater in larger studies. The reason for this is unclear but could be a result of factors associated with the ability to conduct a large study. Experienced institutions with a large volume of CMs were likely able to conduct larger studies and may have selected patients for successful treatment more effectively. These institutions may have also had more technical sophistication which led to improved outcomes. A related possibility is that treatment efficacy improved over time. To explore this possibility, an analysis was performed to determine whether year of publication was associated with response rates. There was no correlation between CR or ORR and year of publication (data not shown).
The analyses presented here had some limitations. Our study demonstrated significant study heterogeneity; hence, a conservative estimate of response by using a random-effects model was performed. Although all studies but one were prospective clinical trials, only 17% were RCTs. There is likely inherent bias in patient selection for particular skin-directed therapies (many of which have been shown to affect skin-directed therapy outcome), such as tumor size,74 number of CMs,21 and depth of invasion,41 which we were unable to standardize and integrate into our analyses. Because of these limitations, direct comparison of outcomes by treatment modality was not performed. Studies were limited to those in the English language, which may have introduced bias. Prospective data on surgical excision of CMs exclusively was not found in our literature search (metastasectomy trials often grouped resection of lymph nodes and CM resection together).75,76 Finally, response criteria were heterogeneous. However, we extensively recorded and categorized the response criteria to aid in the interpretation of the data.
In conclusion, this study was designed to elucidate the efficacy of skin-directed therapies for CMs. The results suggest that response rates were heterogeneous but high, with low recurrence rates and minimal toxicity. In addition, improvements in QOL were reported. To develop evidence-based guidelines and improve outcomes for the treatment of CM, response, criteria will need to be standardized, and RCTs will be necessary to define treatment algorithms on the basis of specific patient and CM characteristics, and an improved grasp of the potential benefits of combination or sequential skin-directed therapies is requisite. Skin-directed therapy should be considered an effective component of the cancer treatment armamentarium.
Supplementary Material
Acknowledgment
We thank Lawrence A. Herman, Memorial Sloan-Kettering Cancer Center, for providing editorial assistance.
Appendix
Table A1.
Patient and Skin Metastasis Details
| Study | Year | Local Therapy | No. of Patients With Evaluable Skin Metastases | No. of Lesions | Age (years) |
Sex |
Metastasis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology | Location | No. | Size |
||||||||||
| Median | Range | M | F | Median | Range | ||||||||
| Heller et al* | 1996 | ECT | 3 | 10 | 50 | 45-65 | 1 | 2 | Melanoma | Upper extremities | 1 | NA | 18-233.6 mm2 |
| Lower extremities | 2 | ||||||||||||
| Heller et al* | 1996 | ECT | 1 | 2 | 57 | 57 | 0 | 1 | Adenocarcinoma of unknown primary | Torso/buttock | 1 | NA | 75.6-98.6 mm2 |
| Sersa et al | 2000 | ECT† | 9 | 27 | NA | NA | 5 | 4 | Melanoma | NA | 1,010 mm3‡ | Estimated 600-1,500 μL | |
| Rodriguez-Cuevas et al* | 2001 | ECT | 2 | 13 | 59‡ | NA | NA | NA | Melanoma | NA | 10.3 mm‡ | Estimated 5-15 mm | |
| Rodriguez-Cuevas et al* | 2001 | ECT | 2 | 14 | 52.5‡ | NA | 0 | 2 | Breast | NA | 21.3 mm‡ | Estimated 8-34 mm | |
| Rodriguez-Cuevas et al* | 2001 | ECT | 2 | 2 | 62‡ | NA | 0 | 2 | SCC (upper aerodigestive tract) | NA | 27.5 mm‡ | Estimated 16-38 mm | |
| Byrne et al | 2005 | ECT | 16 | 53 | 75‡ | 45-86 | 10 | 6 | Melanoma | Neck | 1 | 36 mm2 | 9-1,400 mm2 |
| Torso/buttock | 2 | ||||||||||||
| Upper extremities | 2 | ||||||||||||
| Lower extremities | 12 | ||||||||||||
| Non-randomized lesion locations not known | |||||||||||||
| Gaudy et al | 2006 | ECT† | 12 | 24 | 62‡ | 49-77 | NA | NA | Melanoma | NA | 10 mm | 3-26 mm | |
| Marty et al | 2006 | ECT | 41 | 171 | 66 | 37-91 | 11 | 30 | Melanoma (49%) | Scalp, face, neck | 13 | <30 mm | NA |
| Carcinoma (46%) | Torso/buttock | 81 | |||||||||||
| Sarcoma (5%) | Upper extremities/lower extremities | 77 | |||||||||||
| Quaglino et al | 2008 | ECT | 14 | 233 | 61 | 49-77 | NA | NA | Melanoma | NA | 7-15 mm | 2-75 mm | |
| Matthiessen et al | 2011 | ECT | 24 | 94 | 69.6 | 38.9-94.7 | NA | NA | Melanoma (40%) | NA | 12 mm | 1-200 mm | |
| Breast (29%) | |||||||||||||
| Other (31%) | |||||||||||||
| Benevento et al | 2012 | ECT | 12 | 142 | 76 | NA | 1 | 11 | Breast | NA | 5-10 mm | 5 to > 30 mm | |
| Campana et al | 2012 | ECT | 35 | 35 | NA | 0 | 35 | Breast | Torso/buttock | 35 | 20 mm | 10-220 mm | |
| Kendler et al* | 2013 | ECT | 2 | 50 | 81.5 | 75-88 | 1 | 1 | Melanoma | Lower extremities | 40 | 145.5 cm2 | 135-156 cm2 |
| Kendler et al* | 2013 | ECT | 1 | 29 | 80 | 80 | 0 | 1 | Breast | Torso/buttock | 29 | 163 cm2 | 128-198 cm2 |
| Sperduto et al | 1991 | PDT | 20 | 20 | 55.5 | 39-76 | 0 | 20 | Breast | Torso/buttock | 20 | NA | 2 mm to > 5 cm |
| Cairnduff et al | 1994 | PDT | 5 | 14 | NA | NA | 0 | 5 | Breast | NA | 11 mm | 10-75 mm | |
| Baas et al | 1996 | PDT† | 4 | 20 | 49.5 | 45-74 | 0 | 4 | Breast | Torso/buttock | 20 | NA | NA |
| Kaplan et al | 1998 | PDT | 3 | 13 | 63 | 62-64 | 0 | 3 | Adenocarcinoma (submandibular gland, colon, breast) | Face | 1 | NA | NA |
| Torso/buttock | 2 | ||||||||||||
| Mang et al | 1998 | PDT | 8 | 86 | 65 | 40-71 | 0 | 8 | Breast | Torso/buttock | 86 | 3-45 mm | |
| Overgaard et al | 1985 | RT | NA | 15 | NA | NA | NA | Melanoma | Torso/buttock | 5 | 2 cm2 | 1-12 cm2 | |
| Upper extremities | 3 | ||||||||||||
| Lower extremities | 7 | ||||||||||||
| Menendez et al | 2009 | RT | 7 | 88 | 64 | 51-74 | 1 | 6 | Melanoma | Lower extremities | 88 | NA | NA |
| Cohen§ | 1978 | ILT | 9 | 199 | 48 | 27-58 | 4 | 5 | Melanoma | Scalp | 1 | NA | NA |
| Torso/buttock | 1 | ||||||||||||
| Upper extremities | 2 | ||||||||||||
| Lower extremities | 5 | ||||||||||||
| Cohen§ | 1978 | ILT | 9 | 567 | 42 | 24-68 | 5 | 4 | Melanoma | Scalp | 1 | NA | NA |
| Upper extremities | 1 | ||||||||||||
| Lower extremities | 7 | ||||||||||||
| Nathanson et al | 1979 | ILT | 22 | 22 | 51-60 (range) | 40 to > 70 | 13 | 9 | Melanoma | Scalp, face, neck | 5 | <20 mm | NA |
| Torso/buttock | 3 | ||||||||||||
| Upper extremities | 2 | ||||||||||||
| Lower extremities | 10 | ||||||||||||
| Unknown | 2 | ||||||||||||
| Cascinelli et al | 1993 | ILT | 16 | 47 | NA | NA | NA | NA | Melanoma | NA | NA | NA | |
| Stewart et al* | 1999 | ILT | 8 | 8 | NA | NA | 0 | 8 | Breast | NA | NA | NA | |
| Stewart et al* | 1999 | ILT | 15 | 15 | NA | NA | NA | NA | Melanoma | NA | NA | NA | |
| Hoeller et al | 2001 | ILT | 29 | 29 | 49 | 29-84 | 25 | 27 | Melanoma | NA | 6.1 cm2 | 0.7-24 cm2 | |
| Stopeck et al | 2001 | ILT | 7 | 7 | 76.4‡ | 45-90 | 5 | 2 | Melanoma | NA | NA | NA | |
| Radny et al | 2003 | ILT | 25 | 244 | 61 | 39-82 | 13 | 12 | Melanoma | Scalp | 9 | 0.3 cm2 | 0.01-100 cm2 |
| Face | 8 | ||||||||||||
| Neck | 7 | ||||||||||||
| Torso/buttock | 52 | ||||||||||||
| Upper extremities | 17 | ||||||||||||
| Lower extremities | 153 | ||||||||||||
| Oratz et al | 2003 | ILT | 23 | 237 | 59.2 | 19-83 | 10 | 14 | Melanoma | NA | NA | < 5 mm to > 20 mm | |
| Byrne et al | 2005 | ILT | 16 | 19 | 75‡ | 45-86 | 10 | 6 | Melanoma | Neck | 1 | 30 mm2 | 20-2,500 mm2 |
| Torso/buttock | 2 | ||||||||||||
| Upper extremities | 1 | ||||||||||||
| Lower extremities | 15 | ||||||||||||
| Triozzi et al | 2005 | ILT | NA | 14 | 63 | 34-83 | 8 | 6 | Melanoma | NA | NA | NA | |
| Gonzalez et al | 2006 | ILT | 12 | 16 | 62‡ | 49-77 | NA | NA | Melanoma | NA | 10 mm | 4-18 mm | |
| Kimata et al | 2006 | ILT | 77 | 77 | 57.7‡ | 33-82 | 46 | 31 | Melanoma | NA | <25 cm2 | NA | |
| Gaudy et al | 2006 | ILT† | 6 | 5 | 64 | 48-76 | 0 | 6 | Breast | NA | NA | NA | |
| Dummer et al | 2008 | ILT† | 25 | 25 | 59 | 22-86 | 14 | 11 | Melanoma | NA | NA | NA | |
| Hofmann et al | 2008 | ILT | 5 | 5 | 64 | 27-70 | 4 | 1 | Melanoma | Scalp | 1 | 0.82 cm2 | 0.12-16.65 cm2 |
| Torso/buttock | 4 | ||||||||||||
| Thompson et al | 2008 | ILT | 11 | 26 | 83 | 75-86 | 4 | 7 | Melanoma | Face | 1 | 0.29 cm3 | 0.02-12.8 cm3 |
| Lower extremities | 25 | ||||||||||||
| Bedikian et al | 2010 | ILT | 85 | 255 | 60 | 26-98 | 69 | 58 | Melanoma | NA | NA | ≥ 1 to ≤ 25 cm2 | |
| Weide et al | 2010 | ILT | 48 | 894 | 69 | 37-88 | 21 | 27 | Melanoma | NA | NA | NA | |
| Unger et al | 1992 | TT | 24 | 24 | 55 | 39-85 | 0 | 24 | Breast | Torso/buttock | 24 | NA | NA |
| Unger et al | 1993 | TT† | 52 | 52 | 59 | NA | 0 | 52 | Breast | Torso/buttock | 52 | NA | NA |
| Terwogt et al | 1999 | TT | 30 | 30 | 57 | 30-90 | 0 | 30 | Breast | Torso/buttock | 30 | NA | NA |
| Smorenburg et al | 2000 | TT† | 18 | 18 | 61 | 43-79 | 0 | 18 | Breast | Torso/buttock | 18 | NA | NA |
| Leonard et al | 2001 | TT† | 24 | 19 | 68‡ | 39-86 | 0 | 19 | Breast | Torso/buttock | 19 | NA | NA |
| Eilender et al* | 2006 | TT† | 12 | 12 | NA | NA | NA | NA | Breast | Scalp | 1 | 47.35 cm2 | 1.4-1,596 cm2 |
| Neck | 1 | ||||||||||||
| Torso/buttock | 9 | ||||||||||||
| Upper extremities | 1 | ||||||||||||
| Eilender et al* | 2006 | TT | 5 | 5 | NA | NA | NA | NA | Melanoma | Scalp | 1 | 30.16 cm2 | 5.75-2,574 cm2 |
| Torso/buttock | 1 | ||||||||||||
| Lower extremities | 3 | ||||||||||||
| Eilender et al* | 2006 | TT | 2 | 2 | NA | NA | NA | NA | Angiosarcoma | Scalp | 2 | 89.75 cm2 | 32.5-147 cm2 |
| Eilender et al* | 2006 | TT | 4 | 4 | NA | NA | NA | NA | Kaposi's Sarcoma | Face | 1 | 8.48 cm2 | 0.5-14.7 cm2 |
| Upper extremities | 1 | ||||||||||||
| Lower extremities | 2 | ||||||||||||
| Eilender et al* | 2006 | TT | 1 | 1 | NA | NA | NA | NA | Head and neck SCC | Neck | 1 | 42 cm2 | 42 cm2 |
| Salazar et al | 2011 | TT† | 10 | 10 | 54 | 48-92 | 0 | 10 | Breast | NA | NA | NA | |
| Florin et al | 2012 | TT | 5 | 45 | 82 | 72-88 | 0 | 5 | Melanoma | Lower extremities | 45 | NA | NA |
| Adams et al | 2012 | TT† | 10 | 10 | 50 | 44-71 | 0 | 10 | Breast | Torso/buttock | 10 | NA | NA |
| Plesnicar et al | 1982 | ILT + RT | 19 | 19 | NA | 33-80 | 11 | 8 | Melanoma | Scalp | 2 | 7-35 mm | < 7 to > 35 mm |
| Neck | 2 | ||||||||||||
| Torso/buttock | 9 | ||||||||||||
| Lower extremities | 8 | ||||||||||||
| Multiple sites involved for some patients | |||||||||||||
| Lai et al | 2003 | TT + RT | 7 | 7 | 53 | 33-71 | 0 | 7 | Breast | Torso/buttock | 7 | NA | |
| Green et al | 2007 | TT + ILT | 10 | 178 | 58.5 | 46-80 | 7 | 3 | Melanoma | Scalp | 2 | NA | NA |
| Face | 1 | ||||||||||||
| Neck | 1 | ||||||||||||
| Torso/buttock | 5 | ||||||||||||
| Upper extremities | 3 | ||||||||||||
| Lower extremities | 3 | ||||||||||||
| Li et al | 2010 | TT + PDT | 11 | 11 | 69 | 46-87 | 7 | 4 | Melanoma | NA | NA | NA | |
Abbreviations: ECT, electrochemotherapy; ILT, intralesional therapy; NA, not available; PDT, photodynamic therapy; RT, radiotherapy; SCC, squamous cell cancer; TT, topical therapy.
Study split up by histology.
Systemic therapy allowed.
Mean value rather than median was used.
Study split up by drug used for ILT injections.
Table A2.
Treatment Details
| Study | Year | Local Therapy | Local Treatment-Specific Details | Systemic Therapy Details |
||||
|---|---|---|---|---|---|---|---|---|
| Concurrent Systemic Therapy | Concurrent Therapy Used | |||||||
| ECT Drug | Route | No. of Treatments | Anesthesia | |||||
| Heller et al* | 1996 | ECT | Bleomycin | IV | 1 | Local | ||
| Heller et al* | 1996 | ECT | Bleomycin | IV | 1 | Local | ||
| Sersa et al | 2000 | ECT† | Cisplatin | IV | 4 | Local | 100% | Vinblastine, lomustine, and interferon-2β |
| Rodriguez-Cuevas et al* | 2001 | ECT | Bleomycin | IL | 1-4 | Local | ||
| Rodriguez-Cuevas et al* | 2001 | ECT | Bleomycin | IL | 1 | Local | ||
| Rodriguez-Cuevas et al* | 2001 | ECT | Bleomycin | IL | 1-4 | Local | ||
| Byrne et al | 2005 | ECT | Bleomycin | IL | 1 | Local plus oral sedative needed | ||
| Gaudy et al | 2006 | ECT | Bleomycin | IL | 1 | Local | 80% | Dacarbazine (n = 3), fotemustine (n = 4), vindesine (n = 1) |
| Marty et al | 2006 | ECT | Bleomycin or cisplatin | Bleomycin, IV or IL; cisplatin, IL | 1 | General or local | ||
| Quaglino et al | 2008 | ECT | Bleomycin | IV | Median, 1; range, 1-3 | General | ||
| Matthiessen et al | 2011 | ECT | Bleomycin | IL or IV | Median, 1; range, 1-2 | 55% general, 45% local | ||
| Benevento et al | 2012 | ECT | Bleomycin | IV | Median, 1; range, 1-3 | General | ||
| Campana et al | 2012 | ECT | Bleomycin | IV | 1 (outcomes reported after first treatment) | General | ||
| Kendler et al* | 2013 | ECT | Bleomycin | IL | 1 | Local | ||
| Kendler et al* | 2013 | ECT | Bleomycin | IL | 1 | Local | ||
| PDT Laser Used | Photosensitizer | No. of Applications | Anesthesia | |||||
| Sperduto et al | 1991 | PDT | Argon dye laser | Dihematoporphyrin ether | Median, 3; range, 1-10 | |||
| Cairnduff et al | 1994 | PDT | Copper vapor/dye laser | ALA | 1 | Local | ||
| Baas et al | 1996 | PDT† | Argon dye laser | Porfimer sodium | 1 | 35% | Mitomycin | |
| Kaplan et al | 1998 | PDT | Dye laser powered by potassium titanyl phosphate laser | Tin ethyl etiopurpurin | 1 | Local | ||
| Mang et al | 1998 | PDT | Diode laser or potassium titanyl phosphate laser: yttrium argon garnet dye laser | Tin ethyl etiopurpurin | 1 | |||
| Type of RT | No. of Fractions | Fractions/Week | ||||||
| Overgaard et al | 1985 | RT | MV electrons or photons | Median, 3; range, 3-5 | 2 | |||
| Menendez et al | 2009 | RT | Boron-neutron capture therapy | Median, 1; range, 1-3 | ||||
| IL Drug | No. of Treatments | Frequency of Dosing | Anesthesia | |||||
| Cohen et al‡ | 1978 | ILT | Bacille Calmette-Guérin | 1-2 | Every 4-6 weeks | |||
| Cohen et al‡ | 1978 | ILT | Dinitrochlorobenzene | 1-2 | Every 4-6 weeks | |||
| Nathanson et al | 1979 | ILT | Bacille Calmette-Guérin | 6 | Once per week | |||
| Cascinelli et al | 1993 | ILT | Thymopoietin pentapeptide | 6 | 3 times per week | |||
| Stewart et al* | 1999 | ILT | Interleukin-2 adenovirus | 1-2 | NA | |||
| Stewart et al* | 1999 | ILT | Interleukin-2 adenovirus | 1-5 | NA | |||
| Hoeller et al | 2001 | ILT | Granulocyte-macrophage colony-stimulating factor | 10 | Daily for 5 days, then cycle repeated after 21 days | |||
| Stopeck et al | 2001 | ILT | Allovectin-7 | 6 | Once per week | |||
| Radny et al | 2003 | ILT | Interleukin-2 | Mean of 10 per lesion | 2-3 times per week for 1-12 weeks | |||
| Oratz et al | 2003 | ILT | Cisplatin + adrenaline | Median, 5; maximum, 43 | Once per week | |||
| Byrne et al | 2005 | ILT | Bleomycin | 1 | Once | Local | ||
| Triozzi et al | 2005 | ILT | B7.1 (ALVAC) | 4 | Twice per week | |||
| Gonzalez et al | 2006 | ILT | Alovectin-7 | 6-18 | Once per week | |||
| Kimata et al | 2006 | ILT | HF10 (oncolytic herpes simplex virus-1 mutant) | 1-3 | Once only or once per day × 3 days | |||
| Gaudy et al | 2006 | ILT† | Bleomycin | 1 | Once | Local | 80% | NA |
| Dummer et al | 2008 | ILT† | Interleukin-2 adenovirus | 2-20 | Every 1-3 weeks | 24% | NA | |
| Hofmann et al | 2008 | ILT | Toll-like receptor 9 agonist | 5 | Every 2 weeks | |||
| Thompson et al | 2008 | ILT | Rose Bengal | |||||
| Bedikian et al | 2010 | ILT | Allovectin-7 | 6 | Once per week | |||
| Weide et al | 2010 | ILT | Interleukin-2 | 6-12 | 3 times per week | |||
| TT Drug | Frequency | Duration of Treatment | ||||||
| Unger et al | 1992 | TT | Miltefosine | 1-2 times per day | > 8 weeks | |||
| Unger et al | 1993 | TT† | Miltefosine | 1-2 times per day | > 8 weeks | 58% | Hormonal or chemotherapy | |
| Terwogt et al | 1999 | TT | Miltefosine | 1-2 times per day | Median, 10 weeks; range, 1-68 weeks | |||
| Smorenburg et al | 2000 | TT† | Miltefosine | 1-2 times per day | Median, 10.5 weeks; range, 3-46 weeks | 89% | Hormonal or chemotherapy | |
| Leonard et al | 2001 | TT† | Miltefosine | 1-2 times per day | Median, 8.5 weeks; range, 2-33 weeks | 10% | Hormone therapy | |
| Eilender et al* | 2006 | TT† | 4,4′-Dihydroxybenzophenone-2, 4-dinitrophenylhydrazone | Twice per day | Median, 11 weeks; range, 2-17 weeks | 100% | Hormone therapy | |
| Eilender et al* | 2006 | TT | 4,4′-Dihydroxybenzophenone-2, 4-dinitrophenylhydrazone | Twice per day | Median, 6 weeks; range, 4-20 weeks | |||
| Eilender et al* | 2006 | TT | 4,4′-Dihydroxybenzophenone-2, 4-dinitrophenylhydrazone | Twice per day | 3 weeks | |||
| Eilender et al* | 2006 | TT | 4,4′-Dihydroxybenzophenone-2, 4-dinitrophenylhydrazone | Twice per day | Median, 13 weeks; range, 2-17 weeks | |||
| Eilender et al* | 2006 | TT | 4,4′-Dihydroxybenzophenone-2, 4-dinitrophenylhydrazone | Twice per day | 4 weeks | |||
| Salazar et al | 2011 | TT† | Imiquimod | 4 days/week | 4-12 weeks | 100% | Abraxane | |
| Florin et al | 2012 | TT | Imiquimod and fluorouracil | 5 days per week | Median, 21 months; range, 3-27 months | |||
| Adams et al | 2012 | TT† | Imiquimod | 5 days per week | 8 weeks | 70% | Hormonal or chemotherapy | |
| Combination Therapy 1 | Combination Therapy 2 | |||||||
| Plesnicar et al | 1982 | ILT + RT | ILT: one treatment with Bacille Calmette-Guérin | RT: 13-39 Gy in 4.3-8.6 Gy fractions for 3-9 fractions 1-2 days per week | ||||
| Lai et al | 2003 | TT + RT | TT: arsenic gel, 5 days/week for 2-5 weeks | RT: 30-50 Gy in 2-3 Gy fractions for 10-25 fractions 5 days per week | ||||
| Green et al | 2007 | TT + ILT | TT: imiquimod nightly for first 8 weeks for > 2 months | ILT: interleukin-2 every 2 weeks for > 6 months | ||||
| Li et al | 2010 | TT + PDT | TT: imiquimod twice per day for 6 weeks (84 treatments) | PDT: 805-nm diode laser with indocyanine green every 2 weeks for two sessions | ||||
Abbreviations: ECT, electrochemotherapy; IL, intralesional; ILT, intralesional therapy; IV, intravenous; NA, not available; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Study split by histology if toxicity information was extractable by histology.
Systemic therapy allowed.
Study split up by drug used for IL injections.
Table A3.
Additional Response Details
| Study | Year | Local Therapy | No. of Lesions | PR |
SD |
PD |
|||
|---|---|---|---|---|---|---|---|---|---|
| No. | Definition Used | No. | Definition Used | No. | Definition Used | ||||
| Heller et al* | 1996 | ECT | 10 | 2 | 50% reduction in tumor volume | 0 | NA | 5 | “No effect” |
| Heller et al* | 1996 | ECT | 2 | 0 | 50% reduction in tumor volume | 0 | NA | 0 | “No effect” |
| Sersa et al | 2000 | ECT† | 27 | 10 | > 50% reduction in tumor volume | 11 | < 25% increase or < 50% reduction in tumor volume | 3 | > 25% increase in tumor volume |
| Rodriguez-Cuevas et al* | 2001 | ECT | 13 | 8 | As listed | 0 | NA | 2 | “No response” |
| Rodriguez-Cuevas et al* | 2001 | ECT | 14 | 6 | As listed | 0 | NA | 0 | “No response” |
| Rodriguez-Cuevas et al* | 2001 | ECT | 2 | 2 | As listed | 0 | NA | 0 | “No response” |
| Byrne et al | 2005 | ECT | 53 | 5 | > 50% reduction in tumor area | 9 | Did not meet criteria for CR, PR, or PD | 5 | > 25% increase in lesion size |
| Gaudy et al | 2006 | ECT† | 24 | 3 | > 50% reduction in tumor area | 3 | Not meeting criteria for CR, PR, or PD | 1 | > 25% increase in tumor volume |
| Marty et al | 2006 | ECT | 171 | 19 | WHO 1997; > 50% reduction in diameter for ≥ 4 weeks | 18 | WHO 1997; ≤ 25% increase or < 50% reduction in tumor diameter | 8 | WHO 1997; > 25% increase in tumor diameter |
| Quaglino et al | 2008 | ECT | 233 | 80 | WHO 1997; > 50% reduction in tumor area for at least 4 weeks | 17 | WHO 1997; < 25% increase or < 50% reduction in tumor area | 0 | > 25% increase in tumor area |
| Matthiessen et al | 2011 | ECT | 94 | 18 | RECIST v1 2000; ≥ 30% decrease in target lesion | 11 | RECIST v1 2000; < 20% increase or < 30% decrease in target lesion | 7 | RECIST v1 2009; ≥ 20% increase in target lesion |
| Benevento et al | 2012 | ECT | 142 | 24 | RECIST v1 2000 | 11 | RECIST v1 2000; SD + NC | 0 | RECIST v1 2000 |
| Campana et al | 2012 | ECT | 35 | 13 | RECIST v1 2000 | 3 | RECIST v1 2000 | 0 | RECIST v1 2000 |
| Kendler et al* | 2013 | ECT | 50 | 0 | RECIST 2009; ≥ 30% decrease in tumor area | 23 | < 20% increase or < 30% decrease in tumor area | 22 | ≥ 20% increase in tumor area or an absolute increase of 5 mm |
| Kendler et al* | 2013 | ECT | 29 | 0 | RECIST 2009; ≥ 30% decrease in tumor area | 22 | < 20% increase or < 30% decrease in tumor area | 0 | ≥ 20% increase in tumor area or an absolute increase of 5 mm |
| Sperduto et al | 1991 | PDT | 20 | 9 | > 50% reduction in measurable nodules or a complete clinical regression with residual microscopic disease | 0 | NA | 7 | “No response: < 50% response, no change, or progression of disease” |
| Cairnduff et al | 1994 | PDT | 14 | 0 | 50% reduction in tumor size | 0 | NA | 9 | “No other responses were seen” |
| Baas et al | 1996 | PDT† | 20 | 3 | As listed | 0 | NA | 2 | “No change”‡ |
| Kaplan et al | 1998 | PDT | 13 | 0 | NA | 0 | NA | 0 | NA |
| Mang et al | 1998 | PDT | 86 | 7 | As listed | 0 | NA | 0 | NA |
| Overgaard et al | 1985 | RT | 15 | 5 | > 50% reduction in tumor area | 0 | < 25% progression or < 50% reduction in tumor area | 0 | > 25% progression of tumor area |
| Menendez et al | 2009 | RT | 88 | 9 | “Partial response” | 27 | “No change” | 0 | All lesions had either CR, PR, or SD‡ |
| Cohen et al | 1978 | ILT | 766 | NA | NA | NA | NA | 119 | Did not regress |
| Nathanson et al | 1979 | ILT | 22 | 7 | ≥ 50% decrease in diameters for minimum of 2 weeks | 1 | Listed as no change | 11 | ≥ 50% increase in diameters |
| Cascinelli et al | 1993 | ILT | 47 | NA | NA | NA | NA | 23 | Did not have an “objective response”‡ |
| Stewart et al* | 1999 | ILT | 8 | NA | NA | NA | NA | 6 | “No response” |
| Stewart et al* | 1999 | ILT | 15 | NA | NA | NA | NA | 10 | “No response” |
| Hoeller et al | 2001 | ILT | 29 | 2 | ≥ 50% reduction in size with no new lesions | 10 | < 25% increase in size or < 25% decrease in size | 9 | |
| Stopeck et al | 2001 | ILT | 7 | 3 | > 50% regression of tumor size‡ | 1 | ≤ 25% increase or ≤ 50% decrease in tumor size‡ | 1 | > 25% increase in tumor size‡ |
| Radny et al | 2003 | ILT | 244 | 16 | ≥ 50% tumor volume regression | NA | ≤ 25% increase or < 50% decrease in volume | NA | NA |
| Oratz et al | 2003 | ILT | 237 | 21 | > 50% decrease in sum of diameters | 0 | NA | 7 | “Progression” |
| Byrne et al | 2005 | ILT | 19 | 1 | > 50% reduction in tumor area | 3 | Did not meet criteria for CR, PR, or PD | 10 | > 25% increase in lesion size |
| Triozzi et al | 2005 | ILT | 14 | 0 | ≥ 50% reduction in volume | 2 | < 25% increase or < 25% decrease in volume | 12 | ≥ 25% increase in volume |
| Gonzalez et al | 2006 | ILT | 16 | 4 | > 50% reduction in tumor area | 3 | Not meeting criteria for CR, PR, or PD | 3 | > 25% increase in tumor volume |
| Kimata et al | 2006 | ILT | 77 | 5 | WHO; ≥ 50% reduction in area | 18 | WHO classification; < 25% increase or < 50% decrease in tumor area | 52 | WHO; > 25% increase in size or new lesions |
| Gaudy et al | 2006 | ILT† | 5 | 4 | Moderate to marked response: marked changes in one third or more of tumor cells | 1 | Mild response, mild changes in cancer cells or marked changes in less than one third of cancer cells | 0 | All had CR,PR, or SD‡ |
| Dummer et al | 2008 | ILT† | 25 | 2 | WHO; ≥ 50% reduction in size for ≥ 4 weeks | 3 | WHO; does not meet definition of CR, PR or PD | 16 | WHO; > 25% increase in size or new lesions |
| Hofmann et al | 2008 | ILT | 5 | 0 | NA | 1 | < 50% reduction in tumor area | 3 | > 20% increase in tumor area |
| Thompson et al | 2008 | ILT | 26 | 3 | RECIST JNCI 2000 | 7 | RECIST JNCI 2000 | 6 | NA |
| Bedikian et al | 2010 | ILT | 255 | 11 | RECIST JNCI 2000 | 32 | RECIST JNCI 2000 | 80 | NA |
| Weide et al | 2010 | ILT | 894 | 6 | ≥30% decrease in greatest single dimension | 146 | < 20% increase or < 30% decrease in greatest single dimension | 38 | ≥ 20% increase in greatest single dimension |
| Unger et al | 1992 | TT | 24 | 3 | “Partial remission” | 8 | “No change” | 9 | “Progressive disease” |
| Unger et al | 1993 | TT† | 52 | 10 | WHO criteria | 28 | WHO criteria | 13 | WHO criteria |
| Terwogt et al | 1999 | TT | 30 | 6 | ≥ 50% reduction in tumor size for ≥ 4 weeks | 10 | < 25% increase or < 50% decrease in tumor size | 7 | ≥ 25% increase in tumor size |
| Smorenburg et al | 2000 | TT† | 18 | 4 | WHO | 7 | 7 | WHO | |
| Leonard et al | 2001 | TT† | 19 | 6 | WHO 1979; ≥ 50% reduction in tumor area | 7 | < 25% increase or < 50% decrease in tumor area | 4 | ≥ 25% increase in tumor area |
| Eilender et al* | 2006 | TT† | 12 | 1 | ≥ 50% decrease in tumor area | 4 | < 25% increase or < 50% decrease in tumor area | 6 | ≥ 25 increase in tumor area |
| Eilender et al* | 2006 | TT | 5 | 2 | ≥ 50% decrease in tumor area | 0 | < 25% increase or < 50% decrease in tumor area | 3 | ≥ 25 increase in tumor area |
| Eilender et al* | 2006 | TT | 2 | 1 | ≥ 50% decrease in tumor area | 0 | < 25% increase or < 50% decrease in tumor area | 1 | ≥ 25 increase in tumor area |
| Eilender et al* | 2006 | TT | 4 | 2 | ≥ 50% decrease in tumor area | 0 | < 25% increase or < 50% decrease in tumor area | 2 | ≥ 25 increase in tumor area |
| Eilender et al* | 2006 | TT | 1 | 0 | ≥ 50% decrease in tumor area | 0 | < 25% increase or < 50% decrease in tumor area | 1 | ≥ 25 increase in tumor area |
| Salazar et al | 2011 | TT† | 10 | 4 | “Modified WHO criteria” | 2 | “Modified WHO criteria” | 1 | “Modified WHO criteria” |
| Florin et al | 2012 | TT | 45 | 25 | As listed: “partial response” | 1 | As listed: “stable disease” | 0 | As listed: “progressive disease” |
| Adams et al | 2012 | TT† | 10 | 2 | > 50% reduction in tumor area | 6 | < 25% increase or ≤ 50 reduction in tumor area‡ | 2 | ≥ 25% increase in tumor size |
| Plesnicar et al | 1982 | ILT + RT | 19 | 1 | “Incomplete reduction in volume of metastases” | 0 | NA | 4 | “Minimal or no response”‡ |
| Lai et al | 2003 | TT + RT | 7 | 3 | > 50% reduction in tumor area ≥ 4 weeks | 1 | < 25% increase or < 50% decrease in tumor area | 0 | ≥ 25% increase in tumor area or new lesions in treatment field |
| Green et al | 2007 | TT + ILT | 178 | 18 | ≥ 50% reduction in largest diameter | 53 | Did not meet criteria for CR, PR, or PD | 33 | Subcutaneous: ≥ 20% increase in largest diameter Cutaneous: any increase in size or pigmentation |
| Li et al | 2010 | TT + PDT | 11 | 3 | RECIST; ≥ 30% decrease in tumor area | 0 | < 20% increase or < 30% decrease in tumor area | 0 | ≥ 20% increase in tumor area or appearance of new lesions |
Abbreviations: CR, complete response; ECT, electrochemotherapy; ILT, intralesional therapy; JNCI, Journal of the National Cancer Institute; NA, not available; NC, no change; PD, progressive disease; PDT, photodynamic therapy; PR, partial response; RT, radiotherapy; SD, stable disease; TT, topical therapy.
Study split up by histology.
Systemic therapy allowed.
Response definition was not clearly stated, and we implemented the listed definition of response.
Table A4.
Recurrence Details
| Study | Year | Local Therapy | Recurrence Rate |
|---|---|---|---|
| Heller et al* | 1996 | ECT | 0 of 3 |
| Heller et al* | 1996 | ECT | 0 of 2 |
| Sersa et al | 2000 | ECT† | NA |
| Rodriguez-Cuevas et al* | 2001 | ECT | NA |
| Rodriguez-Cuevas et al* | 2001 | ECT | NA |
| Rodriguez-Cuevas et al* | 2001 | ECT | NA |
| Byrne et al | 2005 | ECT | 0 of 17 |
| Gaudy et al | 2006 | ECT† | 2 of 24 |
| Marty et al | 2006 | ECT | NA |
| Quaglino et al | 2008 | ECT | 54 of 216 |
| Matthiessen et al | 2011 | ECT | NA |
| Benevento et al | 2012 | ECT | NA |
| Campana et al | 2012 | ECT | 5 of 35 (additional ECT sessions allowed) |
| Kendler et al* | 2013 | ECT | NA |
| Kendler et al* | 2013 | ECT | NA |
| Sperduto et al | 1991 | PDT | NA |
| Cairnduff et al | 1994 | PDT | 0 of 5 |
| Baas et al | 1996 | PDT† | NA |
| Kaplan et al | 1998 | PDT | 0 of 13 |
| Mang et al | 1998 | PDT | 0 of 86 |
| Overgaard et al | 1985 | RT | 2 of 15 |
| Menendez et al | 2009 | RT | NA |
| Cohen et al | 1978 | ILT | NA |
| Nathanson et al | 1979 | ILT | NA |
| Cascinelli et al | 1993 | ILT | NA |
| Stewart et al* | 1999 | ILT | NA |
| Stewart et al* | 1999 | ILT | NA |
| Hoeller et al | 2001 | ILT | |
| Stopeck et al | 2001 | ILT | NA |
| Radny et al | 2003 | ILT | NA |
| Oratz et al | 2003 | ILT | NA |
| Byrne et al | 2005 | ILT | 0 of 19 |
| Triozzi et al | 2005 | ILT | NA |
| Gonzalez et al | 2006 | ILT | 0 of 16 |
| Kimata et al | 2006 | ILT | NA |
| Gaudy et al | 2006 | ILT† | NA |
| Dummer et al | 2008 | ILT† | NA |
| Hofmann et al | 2008 | ILT | NA |
| Thompson et al | 2008 | ILT | NA |
| Bedikian et al | 2010 | ILT | 9 of 15 |
| Weide et al | 2010 | ILT | NA |
| Unger et al | 1992 | TT | NA |
| Unger et al | 1993 | TT† | NA |
| Terwogt et al | 1999 | TT | NA |
| Smorenburg et al | 2000 | TT† | NA |
| Leonard et al | 2001 | TT† | NA |
| Eilender et al* | 2006 | TT† | NA |
| Eilender et al* | 2006 | TT | NA |
| Eilender et al* | 2006 | TT | NA |
| Eilender et al* | 2006 | TT | NA |
| Eilender et al* | 2006 | TT | NA |
| Salazar et al | 2011 | TT† | NA |
| Florin et al | 2012 | TT | 0 of 44 |
| Adams et al | 2012 | TT† | NA |
| Plesnicar et al | 1982 | ILT + RT | NA |
| Lai et al | 2003 | TT + RT | NA |
| Green et al | 2007 | TT + ILT | NA |
| Li et al | 2010 | TT + PDT | NA |
Abbreviations: ECT, electrochemotherapy; ILT, intralesional therapy; NA, not available; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Study split up by histology.
Systemic therapy allowed.
Table A5.
Toxicity Details
| Study | Year | Local Therapy | Toxicity Grading Scale Listed | Details of Toxicity by Grade | Grade 3 or Higher or Severe Toxicity | Toxicities Reported | Toxicity Resolution |
|---|---|---|---|---|---|---|---|
| Heller et al | 1996 | ECT | NA | NA | 0 | Muscle contractions during each pulse, mild pain at treatment site during each pulse, slight burning of skin, muscle fatigue, fever, chills, nausea, general malaise by 24 to 48 hours after treatment | Pain resolved by 24 to 48 hours; burning of skin resolved by 2 to 4 weeks |
| Sersa et al | 2000 | ECT* | NA | NA | 0 | Muscle contractions, slight erythema, scab, minimal scarring, slight depigmentation | NA |
| Rodriguez-Cuevas et al | 2001 | ECT | NA | NA | 0 | Muscle contractions (well tolerated), fibrosis | NA |
| Byrne et al | 2005 | ECT | CALGB CTC, 1989 | NA | 0 | During ECT: electric shock sensation, muscle spasm, pain. Treated lesions: inflammatory reaction, superficial necrosis, eschar | All healed by 16 weeks post-treatment |
| Gaudy et al | 2006 | ECT* | NA | NA | NA | ECT causes discomfort and local pain in nine of 12 patients, and three of 12 myoclonus. Hematoma in two of 12. No systemic toxicity. | “No residual pain after treatment.” Complete healing median time, 2 weeks, one patient took 8 months to heal |
| Marty et al | 2006 | ECT | NA | NA | None related to treatment | Local pain, muscle contraction (> 78% of patients) | Pain reduced significantly by 2 days post-treatment |
| Quaglino et al | 2008 | ECT | NA | NA | 0 | Erythema, slight edema at treatment site in three patient; marks from electrodes, erosion in all cases | Local erythema resolved within a “few days,” and scars healed within 1 month |
| Matthiessen et al | 2011 | ECT | CTC v3 | NA | “No serious adverse events”; “no CTC grade 3 or 4 toxicity” | Flu-like symptoms (10%), pain for 1 to 2 days post-treatment (10%), ulceration (4%), cough (2%), allergic skin reaction (2%), anxiety (2%) | NA |
| Benevento et al | 2012 | ECT | NA | NA | NA | NA | NA |
| Campana et al | 2012 | ECT | CTCAE v3.0 | Local: grade 1, 20%; grade 2, 23%; grade 3, 14% | Grade 3 ulceration in five of 35 (many had ulcerative metastases at presentation) | Fever (16.1%), uncontrolled pain (5.7%), nausea/vomiting (n = 4), syncope (n = 1), urticaria (n = 1) | 77% had pain 7 days after ECT; 49% had pain 1 month after ECT |
| Kendler et al | 2013 | ECT | NA | NA | No serious adverse events | Pain requiring medication, cutaneous infection 7 days post-treatment (n = 1), superficial ulceration at 2 weeks (n = 1), burning sensation (n = 1) | Infection resolved within 3 days with antibiotics |
| Sperduto et al | 1991 | PDT | NA | NA | One patient needed skin flap | 100% had erythema, 95% had pain, 25% had blistering, 50% had necrosis, 20% had ulceration | NA |
| Cairnduff et al | 1994 | PDT | NA | NA | 0 | Sensations and discomfort during treatment including burning, prickling, or boring sensation. Edema, erythema, weeping for 1 to 2 weeks | All healed by 2 to 3 months |
| Baas et al | 1996 | PDT | NA | NA | 0 | Bluish/brown discoloration for first 24 hours, turned black with scab over next 10 days and remained for 8 weeks to 20 months. Rare local infection treated with topical or oral antibiotics, one burning sensation. | Most scabs resolved by 20 months |
| Kaplan et al | 1998 | PDT | NA | NA | NA | Transient facial swelling, deep eschar, erythema, necrotic lesions requiring debridement | Local swelling and eschar resolved by 1 month |
| Mang et al | 1998 | PDT | NA | NA | 0 | Chest wall pain ranged from 2 days to 3 weeks, one localized infection. Pain managed by oral medication. One patient had photosensitivity. All at 1 month had scab and larger lesions formed an eschar. Cosmetic results were excellent. | Local pain resolved by 3 weeks at the latest |
| Overgaard et al | 1985 | RT | Overgaard† | Moderate erythema (n = 7); severe erythema (n = 8) | Eight had severe erythema, but none had moist desquamation | Moderate and severe erythema, fibrosis | NA |
| Menendez et al | 2009 | RT | Listed grade 1 to 3 with no definitions | Grade 1, five of seven; grade 3, three of seven | Three of seven had ulceration | Ulceration | NA |
| Cohen et al‡ | 1978 | ILT | NA | NA | Three grade 4 “near fatality” from DIC | Fever 88%, chills 84%, nausea 40%, major ulceration 44%, cellulitis 16%, distant infection 8%, DIC 12% | NA |
| Cohen et al‡ | 1978 | ILT | NA | NA | 0 | Fever 0%, chills 0%, nausea 0%, major ulceration 4%, cellulitis 2%, distant infection 0%, DIC 0% | NA |
| Nathanson et al | 1979 | ILT | NA | NA | NA | Vomiting/diarrhea (n = 4), fever (n = 16), skin symptoms (n = 5), moderate leukopenia or thrombocytopenia (n = 4), moderate change in LFTs (n = 2), severe change in LFTs (n = 1) | NA |
| Cascinelli et al | 1993 | ILT | NA | NA | NA | NA | NA |
| Stewart et al§ | 1999 | ILT | Simply listed toxicity by grade 1, 2, 3 but no definition | Grade 2, one patient (pain and fever) | 0 | Local inflammation, injection site pain, fever, tissue necrosis | Inflammation resolved after 5 to 7 days |
| Stewart et al§ | 1999 | ILT | Simply listed toxicity by grade 1, 2, 3 but no definition | Grade 2, four patients (pain and fever) | 0 | Local inflammation, injection site pain, fever, cellulitis, joint pain, nausea, myalgia, hiccups | Inflammation resolved after 5 to 7 days |
| Hoeller et al | 2001 | ILT | WHO | Of 51 evaluable patients, 46 had grade 1 toxicity, six grade 2, one grade 3 | One had pain at injection site | Pruritus, erythema at injection site, ecchymoses, pain at injection site | NA |
| Stopeck et al | 2001 | ILT | NA | NA | NA | Mild drowsiness, local erythema, increase in WBC, increase in eosinophils | NA |
| Radny et al | 2003 | ILT | NCI CTC v2.0, 1999 | Overall: erythema 100%, swelling, necrosis 89%, erosion 75%, ulceration 75%, eschar 71%, bleeding 64%, pain 50% | “Severe”: erythema 46%, swelling 36%, necrosis 61%, erosion 21%, ulceration 43%, eschar 43%, bleeding 4%, pain 21% | Erythema, swelling, necrosis, erosion, ulceration, eschar, bleeding, pain | 6 to 31 weeks for resolution of local symptoms |
| Oratz et al | 2003 | ILT | WHO Criteria | Grade 1, 100%; grade 2, 54%; grade 3, 4% | Grade 3, 4% (n = 1) severe headache | Local erythema, fever, flu-like symptoms, pain, fatigue, nausea/vomiting, stomach pain, diarrhea, headache, muscle cramps, tachycardia | All had local erythema that resolved within days of treatment |
| Byrne et al | 2005 | ILT | CALGB CTC 1989 | NA | NA | Treated lesions: inflammatory reaction, superficial necrosis, eschar | All healed by 16 weeks post-treatment |
| Triozzi et al | 2005 | ILT | NCI CTC v2.0 | 23 grade 1; 19 grade 2; zero grade 3 to 4 | 0 | Inflammatory reactions at injection site, fever, chills, myalgia, fatigue, superficial vesicles/bullae | NA |
| Gonzalez et al | 2006 | ILT | NA | NA | NA | NA | NA |
| Kimata et al | 2006 | ILT | WHO 1979 | 95% grade 1 or 2 | One grade 3 event linked to treatment: abdominal pain | Injection site pain, fatigue, pyrexia, arthralgia, dizziness, headache, abdominal pain, vomiting, hemorrhage, hypotension, exacerbated dyspnea, erythema, skin ulcer, injection site edema/hypersensitivity, vasodilation, flatulence, ecchymosis, bone pain, increased cough, pneumonia, rhinitis, pruritus, rash, skin discoloration | NA |
| Gaudy et al | 2006 | ILT | NA | NA | 0 | No adverse effects from treatment occurred | NA |
| Dummer et al | 2008 | ILT* | NCI CTC v2.0 | “Most mild to moderate adverse events” | Seven “serious” events (thrombocytopenia, pleural effusion, lymphocytopenia, anemia | Injection site pain, increase in tumor pain, chills, fatigue, fever, nausea, vomiting, constipation, stomach pain, headache, asthenia, lymphocytopenia/thrombocytopenia, diarrhea | NA |
| Hofmann et al | 2008 | ILT | NCI CTC v2.0 | Grade 1, 16 events; grade 2, 12 events; grade 3, one event | One lymphopenia | Local swelling and erythema, fever, fatigue, rigors, headache, pain, increase in blood pressure, lymphocytopenia | Lymphopenia resolved in 14 days |
| Thompson et al | 2008 | ILT | NA | “No serious adverse events” | Mild to moderate injection site pain (n = 8), local inflammation (n = 4), pruritus (n = 3) | NA | |
| Bedikian et al | 2010 | ILT | NCI CTC v2.0 | Grade 1, 199 events; grade 2, 19 events; grade 3 to 4, zero events | 0 | Myalgia (n = 23), pyrexia (n = 22), arthralgia (n = 19), headache (n = 19), injection site pain (n = 43), injection site erythema (n = 28), rigors (n = 33), fatigue, nonspecific arthritis (n = 1) | NA |
| Weide et al | 2010 | ILT | CTC v3 | Grade 1, < 70% of patients; grade 2, < 60% of patients | 0 | Inflammatory injection site reaction (swelling, erythema locally), necrosis, injection pain, fever 58%, fatigue 36%, nausea 34%, stomach pain (n = 4), myalgia (n = 4), headache (n = 4), itching exanthem (n = 3), dry oral mucosa (n = 2), pruritus (n = 2), hair loss (n = 1), diarrhea (n = 1), urticaria (n = 1), atopic dermatitis worsening (n = 1), single episode mild cardiac arrhythmia (n = 1), vitiligo-like depigmentation around treated metastases (n = 1) | NA |
| Unger et al | 1992 | TT | WHO | Grade 1 erythema, four of 24; grade 2 erythema, one of 24 | 0 | Itching, slight erythema, scaling, dryness | NA |
| Unger et al | 1993 | TT | WHO | NA | 0 | Skin pruritus (two of 74), rash, dry skin, bleeding, and skin atrophy | NA |
| Terwogt et al | 1999 | TT | WHO | Grade 1, five; grade 2, 15; grade 3, one; unknown, one | One of 33 had “severe” skin reaction | 22 of 33 adverse skin reactions including dryness, erythema, itch, pain, desquamation. Nausea in two patients. | NA |
| Smorenburg et al | 2000 | TT* | WHO | Local: grade 1, nine; grade 2, two; systemic: grade 1, three; grade 2, one | No grade 3 or 4 local or systemic events | Skin atrophy (20%), exfoliation (15%), rash (10%), pruritus (10%), pain (15%), dry skin (10%), telangiectasis (5%), nausea/vomiting (5%), anorexia (5%), fatigue (5%) | NA |
| Leonard et al | 2001 | TT* | NCI CTC 1986 | Grade 1, three of 24; grade 2, 11 of 24; grade 3, four of 24; grade 4, four of 24 | Eight of 24 had “significant” to “severe” local skin reaction | Dryness, erythema, desquamation, local pain, burning, itching. Rare ulcerating dermatitis. | NA |
| Eilender et al | 2006 | TT* | CTC v2.0 | Total cohort (n = 27); grade 1 to 2, 10; grade 3 to 4, zero | 0 | Anemia, itching, burning, rash (most patients had no toxicity) | NA |
| Salazar et al | 2011 | TT* | CTCAE v3.0 | “Primarily grade 1 to 2 neutropenia, anemia, grade 1 skin toxicity” | NA | NA | NA |
| Florin et al | 2012 | TT | NA | NA | 0 | Local inflammation, ulceration, erythema | |
| Adams et al | 2012 | TT* | CTCAE v 3.0 | Local: grade 1, five of 10; grade 2, two of 10; systemic: grade 1, two; grade 2, two | No grade 3 or 4 local or systemic events | Local pain (n = 3), infection (n = 1), itching (n = 3), burning (n = 1), desquamation (n = 3), flu-like symptoms | NA |
| Plesnicar et al | 1982 | ILT + RT | NA | NA | NA | Marked erythema, ulceration, crusting, short flu-like syndrome | NA |
| Lai et al | 2003 | TT + RT | CTC v2.0, 1999 | Grade 1, 11; grade 2, eight; grade 3, two | Two of seven had grade 3 acute radiation dermatitis | Nausea, anorexia, acute/chronic radiation dermatitis, fatigue | All grade 3 toxicity resolved 2 to 3 weeks post-radiotherapy |
| Green et al | 2007 | TT + ILT | Simply said “Grade 3” | NA | One patient had rigors | Erythema, discharge, mild influenza like symptoms, rigors (n = 1), local infections (n = 2), nausea/dyspepsia (n = 2) | Most symptoms resolved within the first “few weeks” |
| Li et al | 2010 | TT + PDT | NCI CTC v3.0, 2009 | NA | Grade 3 occurred in 25% of patients (fatigue, pain requiring narcotics, dyspnea, cellulitis) | Rash (90%), pruritus (82%), pain (55%), fatigue (55%), anorexia (55%), nausea (36%), weight loss (36%), fever (18%), chills (9%), vomiting (9%), cellulitis (9%) | NA |
Abbreviations: CALGB, Cancer and Leukemia Group B; CTC, Common Toxicity Criteria; CTCAE, Common Terminology Criteria for Adverse Events; DIC, disseminated intravascular coagulation; ECT, electrochemotherapy; ILT, intralesional therapy; LFT, liver function test; NA, not available; NCI, National Cancer Institute; NIH, National Institutes of Health; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Systemic therapy allowed.
Toxicity grading scale from Overgaard J: Cancer 48:1116-1123, 1981.
Study split up by drug used for ILT injections.
Study split by histology if toxicity information was extractable by histology.
Table A6.
QOL Details
| Study | Year | Local Therapy | QOL Scales Used | Results |
|---|---|---|---|---|
| Marty et al | 2006 | ECT | VAS | Patients treated with general anesthesia had lower pain scores than those with local anesthesia; 93% would be willing to undergo another treatment if indicated. |
| Campana et al | 2012 | ECT | Four-point pain scale | Pain scores improved from 1 week to 1 month post-ECT. Pain worsened with increased number of ECT treatments. |
| Kendler et al | 2013 | ECT | VAS and two custom QOL questions | Mean pain scores increased at time of treatment, but dropped below baseline value by 15 minutes post-treatment and remained low. QOL questions demonstrated improvement in all patients. |
| Leonard et al | 2001 | TT* | Rotterdam Symptom Checklist and a body image scale | Improved psychological distress from baseline and over placebo at last follow-up. |
| Lai et al | 2003 | TT + RT | Change in daily wound dressings and VAS | Treatment significantly reduced need for daily wound dressing changes and pain. |
Abbreviations: ECT, electrochemotherapy; QOL, quality of life; RT, radiotherapy; TT, topical therapy; VAS, visual analogue scale (to assess pain).
Systemic therapy allowed.
Table A7.
Jadad Scale for Randomized Controlled Trials
| Study | Year | Jadad Scale65 |
|||
|---|---|---|---|---|---|
| Randomization | Blinding | Withdrawal | Total | ||
| Sersa et al | 2000 | 1 | 0 | 0 | 1 |
| Byrne et al | 2005 | 1 | 0 | 1 | 2 |
| Gaudy et al | 2006 | 1 | 0 | 1 | 2 |
| Overgaard et al | 1985 | 1 | 0 | 0 | 1 |
| Cohen et al | 1978 | 2 | 0 | 0 | 2 |
| Nathanson et al | 1979 | 2 | 0 | 1 | 3 |
| Cascinelli et al | 1993 | 2 | 2 | 1 | 5 |
| Leonard et al | 2001 | 1 | 1 | 1 | 3 |
Table A8.
National Institutes of Health Level of Evidence Scale (all studies)
| Study | Year | Level of Evidence64 |
|---|---|---|
| Heller et al | 1996 | 2 |
| Sersa et al | 2000 | 1B |
| Rodriguez-Cuevas et al | 2001 | 2 |
| Byrne et al | 2005 | 1B |
| Gaudy et al | 2006 | 1B |
| Marty et al | 2006 | 2 |
| Quaglino et al | 2008 | 2 |
| Matthiessen et al | 2011 | 2 |
| Benevento et al | 2012 | 3B |
| Campana et al | 2012 | 2 |
| Kendler et al | 2013 | 2 |
| Sperduto et al | 1991 | 2 |
| Cairnduff et al | 1994 | 2 |
| Baas et al | 1996 | 2 |
| Kaplan et al | 1998 | 2 |
| Mang et al | 1998 | 2 |
| Overgaard et al | 1985 | 1B |
| Menendez et al | 2009 | 2 |
| Cohen et al | 1978 | 1B |
| Nathanson et al | 1979 | 1B |
| Cascinelli et al | 1993 | 1A |
| Stewart et al | 1999 | 2 |
| Hoeller et al | 2001 | 2 |
| Stopeck et al | 2001 | 2 |
| Radny et al | 2003 | 2 |
| Oratz et al | 2003 | 2 |
| Triozzi et al | 2005 | 2 |
| Gonzalez et al | 2006 | 1B |
| Kimata et al | 2006 | 2 |
| Dummer et al | 2008 | 2 |
| Hofmann et al | 2008 | 2 |
| Thompson et al | 2008 | 2 |
| Bedikian et al | 2010 | 2 |
| Weide et al | 2010 | 2 |
| Unger et al | 1992 | 2 |
| Unger et al | 1993 | 2 |
| Terwogt et al | 1999 | 2 |
| Smorenburg et al | 2000 | 2 |
| Leonard et al | 2001 | 1A |
| Eilender et al | 2006 | 2 |
| Salazar et al | 2011 | 2 |
| Florin et al | 2012 | 2 |
| Adams et al | 2012 | 2 |
| Plesnicar et al | 1982 | 2 |
| Lai et al | 2003 | 2 |
| Green et al | 2007 | 2 |
| Li et al | 2010 | 2 |
Fig A1.
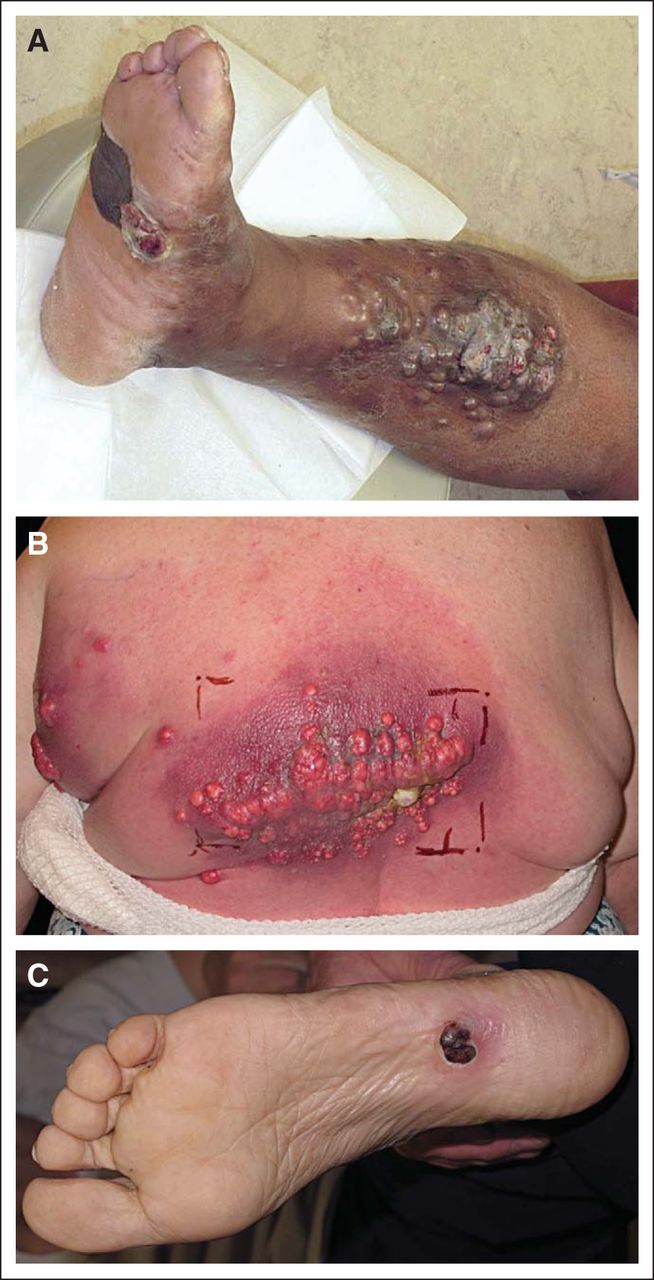
Examples of morbidity of cutaneous metastases. (A-C) Representative examples of cutaneous metastases from patients with melanoma.
Fig A2.
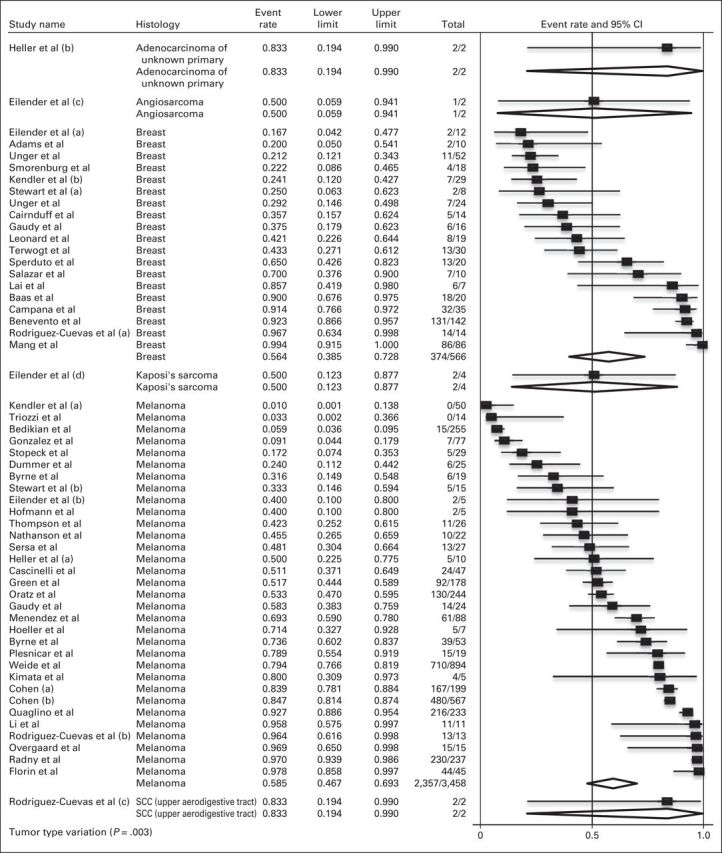
Meta-analysis of objective response by histology. (a-d) indicate unique histology from the same study. NOTE. Total column indicates No. of objective responses/No. of cutaneous metastases. SCC, squamous cell cancer.
Fig A3.
Meta-analysis of recurrence rates by skin-directed therapy. NOTE. Total column indicates No. of recurrences/No. of cutaneous metastases. ECT, electrochemotherapy; ILT, intralesional therapy; PDT, photodynamic therapy; RT, radiotherapy; TT, topical therapy.
Fig A4.
Funnel plot of standard error by logit event rate for complete response. Egger's regression P < .001.
Fig A5.
Funnel plot of standard error by logit event rate for objective response. Egger's regression P = .06.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Mario E. Lacouture, GlaxoSmithKline (C), Genentech (C), Roche (C), Bristol-Myers Squibb (C), Novartis (C), Reata Pharmaceuticals (C), sanofi-aventis (C), Novocure (C), BioPharm Communications (C), AVEO Pharmaceuticals (C), Bayer (C), Pfizer (C), Merck (C), EMD Serono (C), Advancell (C), Galderma (C), Helsinn Therapeutics (C), Threshold Pharmaceuticals (C) Stock Ownership: None Honoraria: Mario E. Lacouture, GlaxoSmithKline, Genentech, Roche, Bristol-Myers Squibb, Novartis, Reata Pharmaceuticals, Amgen, Sandoz, sanofi-aventis, BioPharm Communications, AVEO Pharmaceuticals, Bayer, Pfizer, Merck, EMD Serono, Advancell, Galderma, Novocure, Helsinn Therapeutics, Threshold Pharmaceuticals Research Funding: Mario E. Lacouture, BERG, Bristol-Myers Squibb Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Daniel E. Spratt, Elizabeth A. Gordon Spratt, Christopher A. Barker
Collection and assembly of data: Daniel E. Spratt, Elizabeth A. Gordon Spratt, Antonio DeRosa
Data analysis and interpretation: Daniel E. Spratt, Elizabeth A. Gordon Spratt, Shenhong Wu, Nancy Y. Lee, Mario E. Lacouture, Christopher A. Barker
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: A meta-analysis of data. South Med J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 4.Gehl J, Geertsen PF. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res. 2000;10:585–589. doi: 10.1097/00008390-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty JM, Xing M, Loh CT. Particle embolization to control life-threatening hemorrhage from a fungating locally advanced breast carcinoma: A case report. J Med Case Rep. 2012;6:186. doi: 10.1186/1752-1947-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CY, Chang CC, Chang HC. Erythematous painful nodules on the scalp and ulcerative nodules on the back of a 49-year-old woman. Dermatol Sinica. 2008;26:43–44. [Google Scholar]
- 7.Shimozuma K, Sonoo H, Ichihara K. Analysis of the factors influencing the quality of life of patients with advanced or recurrent breast cancer. Surg Today. 1995;25:874–882. doi: 10.1007/BF00311752. [DOI] [PubMed] [Google Scholar]
- 8.Richtig E, Ludwig R, Kerl H, et al. Organ- and treatment-specific local response rates to systemic and local treatment modalities in stage IV melanoma. Br J Dermatol. 2005;153:925–931. doi: 10.1111/j.1365-2133.2005.06796.x. [DOI] [PubMed] [Google Scholar]
- 9.Mali B, Jarm T, Snoj M, et al. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur J Surg Oncol. 2013;39:4–16. doi: 10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: Clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol. 2009;16:191–199. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]
- 11.Sersa G, Stabuc B, Cemazar M, et al. Electrochemotherapy with cisplatin: The systemic antitumour effectiveness of cisplatin can be potentiated locally by the application of electric pulses in the treatment of malignant melanoma skin metastases. Melanoma Res. 2000;10:381–385. doi: 10.1097/00008390-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lai YL, Chang HH, Huang MJ, et al. Combined effect of topical arsenic trioxide and radiation therapy on skin-infiltrating lesions of breast cancer: A pilot study. Anticancer Drugs. 2003;14:825–828. doi: 10.1097/00001813-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bourke M, Salwa S, Sadadcharam M, et al. Electrochemotherapy for the treatment of intractable cutaneous lesions secondary to breast cancer. Eur J Surg Oncol. 2011;37:1011. [Google Scholar]
- 14.Kis E, Oláh J, Ócsai H, et al. Electrochemotherapy of cutaneous metastases of melanoma: A case series study and systematic review of the evidence. Dermatol Surg. 2011;37:816–824. doi: 10.1111/j.1524-4725.2011.01951..x. [DOI] [PubMed] [Google Scholar]
- 15.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 16.Sze WM, Shelley M, Held I, et al. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy—A systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2:CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 18.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann MA, Kors C, Audring H, et al. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18:405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 21.Campana LG, Valpione S, Falci C, et al. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: A phase-II study. Breast Cancer Res Treat. 2012;134:1169–1178. doi: 10.1007/s10549-012-2095-4. [DOI] [PubMed] [Google Scholar]
- 22.Kendler M, Micheluzzi M, Wetzig T, et al. Electrochemotherapy under tumescent local anesthesia for the treatment of cutaneous metastases. Dermatol Surg. 2013;39:1023–1032. doi: 10.1111/dsu.12190. [DOI] [PubMed] [Google Scholar]
- 23.Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl. 2006;4:3–13. [Google Scholar]
- 24.Leonard R, Hardy J, van Tienhoven G, et al. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J Clin Oncol. 2001;19:4150–4159. doi: 10.1200/JCO.2001.19.21.4150. [DOI] [PubMed] [Google Scholar]
- 25.Bedikian AY, Richards J, Kharkevitch D, et al. A phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010;20:218–226. doi: 10.1097/CMR.0b013e3283390711. [DOI] [PubMed] [Google Scholar]
- 26.Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: Results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116:4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 27.Unger C, Sindermann H, Peukert M, et al. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Prog Exp Tumor Res. 1992;34:153–159. doi: 10.1159/000420840. [DOI] [PubMed] [Google Scholar]
- 28.Byrne CM, Thompson JF, Johnston H, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy) Melanoma Res. 2005;15:45–51. doi: 10.1097/00008390-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Gaudy C, Richard MA, Folchetti G, et al. Randomized controlled study of electrochemotherapy in the local treatment of skin metastases of melanoma. J Cutan Med Surg. 2006;10:115–121. doi: 10.2310/7750.2006.00037. [DOI] [PubMed] [Google Scholar]
- 30.Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629. doi: 10.3109/0284186X.2011.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unger C, Herrmann R, Berdel WE, et al. Topically applied miltefosine (hexadecylphosphocholine) in patients with skin-metastasized breast cancer. A phase II study. Onkologie. 1993;16:170–173. [Google Scholar]
- 32.Terwogt JM, Mandjes IA, Sindermann H, et al. Phase II trial of topically applied miltefosine solution in patients with skin-metastasized breast cancer. Br J Cancer. 1999;79:1158–1161. doi: 10.1038/sj.bjc.6690184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smorenburg CH, Seynaeve C, Bontenbal M, et al. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anticancer Drugs. 2000;11:825–828. doi: 10.1097/00001813-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Eilender D, LoRusso P, Thomas L, et al. 4,4′-Dihydroxybenzophenone-2,4-dinitrophenylhydrazone (A-007): A topical treatment for cutaneous metastases from malignant cancers. Cancer Chemother Pharmacol. 2006;57:719–726. doi: 10.1007/s00280-005-0124-2. [DOI] [PubMed] [Google Scholar]
- 35.Menéndez PR, Roth BM, Pereira MD, et al. BNCT for skin melanoma in extremities: Updated Argentine clinical results. Appl Radiat Isot. 2009;67:S50–S53. doi: 10.1016/j.apradiso.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Overgaard J, von der Maase H, Overgaard M. A randomized study comparing two high-dose per fraction radiation schedules in recurrent or metastatic malignant melanoma. Int J Radiat Oncol Biol Phys. 1985;11:1837–1839. doi: 10.1016/0360-3016(85)90042-2. [DOI] [PubMed] [Google Scholar]
- 37.Cascinelli N, Clemente C, Bufalino R, et al. Perinodular injection of thymopentin (TP5) in cutaneous and subcutaneous metastases of melanoma. Melanoma Res. 1993;3:471–476. doi: 10.1097/00008390-199311000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Green DS, Bodman-Smith MD, Dalgleish AG, et al. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156:337–345. doi: 10.1111/j.1365-2133.2006.07664.x. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: A preliminary study. Cancer Biol Ther. 2010;10:1081–1087. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salazar LG, Lu H, Gray H, et al. Phase II study of topical imiquimod and abraxane for treatment of breast cancer cutaneous metastases. Cancer Res. 2011;(suppl):71. poster P1-13-04. [Google Scholar]
- 41.Florin V, Desmedt E, Vercambre-Darras S, et al. Topical treatment of cutaneous metastases of malignant melanoma using combined imiquimod and 5-fluorouracil. Invest New Drugs. 2012;30:1641–1645. doi: 10.1007/s10637-011-9717-2. [DOI] [PubMed] [Google Scholar]
- 42.Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–6757. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plesnicar S, Rudolf Z. Combined BCG and irradiation treatment of skin metastases originating from malignant melanoma. Cancer. 1982;50:1100–1106. doi: 10.1002/1097-0142(19820915)50:6<1100::aid-cncr2820500613>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Heller R, Jaroszeski MJ, Glass LF, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77:964–971. doi: 10.1002/(sici)1097-0142(19960301)77:5<964::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Cuevas S, Barroso-Bravo S, Almanza-Estrada J, et al. Electrochemotherapy in primary and metastatic skin tumors: Phase II trial using intralesional bleomycin. Arch Med Res. 2001;32:273–276. doi: 10.1016/s0188-4409(01)00278-8. [DOI] [PubMed] [Google Scholar]
- 46.Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–2222. doi: 10.1245/s10434-008-9976-0. [DOI] [PubMed] [Google Scholar]
- 47.Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: A preliminary report. BMC Surg. 2012;12:S6. doi: 10.1186/1471-2482-12-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperduto PW, DeLaney TF, Thomas G, et al. Photodynamic therapy for chest wall recurrence in breast cancer. Int J Radiat Oncol Biol Phys. 1991;21:441–446. doi: 10.1016/0360-3016(91)90793-4. [DOI] [PubMed] [Google Scholar]
- 49.Cairnduff F, Stringer MR, Hudson EJ, et al. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer. 1994;69:605–608. doi: 10.1038/bjc.1994.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baas P, van Geel IP, Oppelaar H, et al. Enhancement of photodynamic therapy by mitomycin C: A preclinical and clinical study. Br J Cancer. 1996;73:945–951. doi: 10.1038/bjc.1996.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan MJ, Somers RG, Greenberg RH, et al. Photodynamic therapy in the management of metastatic cutaneous adenocarcinomas: Case reports from phase 1/2 studies using tin ethyl etiopurpurin (SnET2) J Surg Oncol. 1998;67:121–125. doi: 10.1002/(sici)1096-9098(199802)67:2<121::aid-jso9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Mang TS, Allison R, Hewson G, et al. A phase II/III clinical study of tin ethyl etiopurpurin (Purlytin)-induced photodynamic therapy for the treatment of recurrent cutaneous metastatic breast cancer. Cancer J Sci Am. 1998;4:378–384. [PubMed] [Google Scholar]
- 53.Cohen MH, Jessup JM, Felix EL, et al. Intralesional treatment of recurrent metastatic cutaneous malignant melanoma: A randomized prospective study of intralesional Bacillus Calmette-Guerin versus intralesional dinitrochlorobenzene. Cancer. 1978;41:2456–2463. doi: 10.1002/1097-0142(197806)41:6<2456::aid-cncr2820410654>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 54.Nathanson L, Schoenfeld D, Regelson W, et al. Prospective comparison of intralesional and multipuncture BCG in recurrent intradermal melanoma. Cancer. 1979;43:1630–1635. doi: 10.1002/1097-0142(197905)43:5<1630::aid-cncr2820430511>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Stewart AK, Lassam NJ, Quirt IC, et al. Adenovector-mediated gene delivery of interleukin-2 in metastatic breast cancer and melanoma: Results of a phase 1 clinical trial. Gene Ther. 1999;6:350–363. doi: 10.1038/sj.gt.3300833. [DOI] [PubMed] [Google Scholar]
- 56.Hoeller C, Jansen B, Heere-Ress E, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2001;117:371–374. doi: 10.1046/j.0022-202x.2001.01427.x. [DOI] [PubMed] [Google Scholar]
- 57.Stopeck AT, Jones A, Hersh EM, et al. Phase II study of direct intralesional gene transfer of allovectin-7, an HLA-B7/beta2-microglobulin DNA-liposome complex, in patients with metastatic melanoma. Clin Cancer Res. 2001;7:2285–2291. [PubMed] [Google Scholar]
- 58.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89:1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oratz R, Hauschild A, Sebastian G, et al. Intratumoral cisplatin/adrenaline injectable gel for the treatment of patients with cutaneous and soft tissue metastases of malignant melanoma. Melanoma Res. 2003;13:59–66. doi: 10.1097/00008390-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Triozzi PL, Allen KO, Carlisle RR, et al. Phase I study of the intratumoral administration of recombinant canarypox viruses expressing B7.1 and interleukin 12 in patients with metastatic melanoma. Clin Cancer Res. 2005;11:4168–4175. doi: 10.1158/1078-0432.CCR-04-2283. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez R, Hutchins L, Nemunaitis J, et al. Phase 2 trial of Allovectin-7 in advanced metastatic melanoma. Melanoma Res. 2006;16:521–526. doi: 10.1097/01.cmr.0000232299.44902.41. [DOI] [PubMed] [Google Scholar]
- 62.Kimata H, Imai T, Kikumori T, et al. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol. 2006;13:1078–1084. doi: 10.1245/ASO.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 63.Dummer R, Rochlitz C, Velu T, et al. Intralesional adenovirus-mediated interleukin-2 gene transfer for advanced solid cancers and melanoma. Mol Ther. 2008;16:985–994. doi: 10.1038/mt.2008.32. [DOI] [PubMed] [Google Scholar]
- 64.National Cancer Institute. Levels of Evidence for Adult and Pediatric Cancer Treatment Studies: Strength of Study Design. http://www.cancer.gov/cancertopics/pdq/levels-evidence-adult-treatment/HealthProfessional/page2. [PubMed]
- 65.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 66.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 67.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–760. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 68.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 69.Bolognia JL, Jorizzo JL, Schaffer JV, editors. Cutaneous metastases. Dermatology (ed 3) Philadelphia, PA: Elsevier Saunders; 2012. pp. 2049–2055. [Google Scholar]
- 70.Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator by means of invasive or non-invasive electrodes. EJC Supplement. 2006;4:14–25. [Google Scholar]
- 71.Miklavčič D, Serša G, Brecelj E, et al. Electrochemotherapy: Technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50:1213–1225. doi: 10.1007/s11517-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fayter D, Corbett M, Heirs M, et al. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol Assess. 2010;14:1–288. doi: 10.3310/hta14370. [DOI] [PubMed] [Google Scholar]
- 73.Sze WM, Sheeley M, Held I. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy—A systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2:CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mali B, Miklavcic D, Campana LG, et al. Tumor size and effectiveness of electrochemotherapy. Radiol Oncol. 2013;47:32–41. doi: 10.2478/raon-2013-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: Analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) Ann Surg Oncol. 2012;19:2547–2555. doi: 10.1245/s10434-012-2398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919–924. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.