SUMMARY
The antipredator behavior diel vertical migration (DVM), common in aquatic keystone species Daphnia, involves daily migration from warmer surface waters before dawn to cooler deeper waters after dusk. Plasticity in Daphnia DVM behavior optimizes fitness via trade-offs between growth, reproduction, and predator avoidance. Migration behavior is affected by co-varying biotic and abiotic factors, including light, predator cues, and anthropogenic stressors making it difficult to determine each factor’s individual contribution to the variation in this behavior. This study aims to better understand this ecologically significant behavior in Daphnia by: (1) determining how Daphnia pulicaria thermal preferences vary within and among natural populations; (2) distinguishing the role of temperature verses depth in Daphnia vertical migration; and (3) defining how two anthropogenic stressors (copper and nickel) impact Daphnia migratory behavior.
Simulated natural lake stratification were constructed in 8 L (0.5 m tall, 14.5 cm wide) water columns to monitor under controlled laboratory conditions the individual effects of temperature gradients, depth, and metal stressors on Daphnia vertical migration. Three major findings are reported. First, while no difference in thermal preference was found among the four populations studied, within lake populations variability among isolates was high. Second, decoupling temperature and depth revealed that depth was a better predictor of Daphnia migratory patterns over temperature. Third, exposure to environmentally relevant concentrations of copper or nickel inhibited classic DVM behavior. These findings revealed the high variability in thermal preference found within Daphnia populations, elucidated the individual roles that depth and temperature have on migratory behavior, and showed how copper and nickel can interfere with the natural response of Daphnia to fish predator cues. Thus contributing to the body of knowledge necessary to predict how natural populations of Daphnia will be affected by climate related changes in lake temperatures and increased presence of anthropogenic stressors.
Keywords: Thermal variation, Daphnia, vertical migration, fish kairomone, metal stress
1. INTRODUCTION
The micro-crustacean zooplankton genus Daphnia are ubiquitous in the world’s lentic (e.g., lake and pond) ecosystems (Shaw et al., 2007), and are an integral component of aquatic food webs (i.e., keystone species) due to their central role in transferring the biomass of primary producer’s (e.g. algae) up the food chain by being the primary food for smaller fish (Colbourne et al., 2004; Sarnelle, 2005). Daphnia’s pervasiveness is due in part to their ability to tolerate a dynamic range of environmental conditions, in particular temperature. Daphnia migrate within the water column on a daily basis to avoid predation, a behavior called diel vertical migration (DVM), which exposes them to a wide range of temperatures (Gliwicz, 1986; Lampert, 1989, 1993; Loose, 1993; Sitch, 1989). Additionally, avoidance of inter- and intraspecific competition has also been linked to distinct patterns of Daphnia distribution within the water column (De Meester and Dumont, 1988; 1989; Dumont et al., 1985; Weider, 1984). As surface water temperatures continue to increase as a result of climate change, even large lentic systems such as the Great Lakes are predicted to become more variable, leading to changes in vertical mixing and the degree to which lakes will thermally stratify (Kling et al., 2003; McCormick, 1990; Snucins and Gunn, 2000; Thuiller, 2007; Waal et al., 2010). Thermal stratification in lakes results from seasonal climatic patterns which produce distinct thermal layers that decrease in temperature with depth (Wetzel, 2001), creating thermal niches. Thermal stratification not only influences the distribution of aquatic organisms, based on thermal optimums and ranges, but predator-prey dynamics as well (Wetzel, 2001). Therefore, changes in lake stratification are likely to impact the distribution and fitness of aquatic organisms such as Daphnia, which are heavily influenced by temperature (Heino et al., 2009; Mooij et al., 2005), thereby effecting ecosystem function due to their central role in aquatic ecosystems (Mooij et al., 2005; Thuiller, 2007; van de Waal et al., 2009).
Classic DVM behavior in Daphnia is a migration to deeper, darker, and cooler waters during the day to avoid visual predators and a migration to warmer surface waters during the night to feed when the risk of predation is reduced (Gliwicz, 1986; Lampert, 1989, 1993; Loose, 1993; Sitch, 1989). This negative photo-taxis behavior is triggered by a combination of light and predatory (i.e., kairomone) cues (Dawidowicz and Loose, 1992; Lampert, 1989, 1993; Loose, 1993). In the absence of light or predator cues, Daphnia remain in the warmer and food-rich surface waters which has been shown to lead to increased growth and reproduction rates in Daphnia (Dawidowicz and Loose, 1992; Lampert, 1989, 1993; Loose, 1993). Thus, indicating a fitness costs associated with DVM.
Lampert (1989) described three potential sources of fitness cost associated with diel vertical migration: 1) differences in food quality and quantity, 2) distance of migration (i.e., changes in depth), and 3) changes in temperature. The cost associated with food quality and quantity depends mainly on the assumption that algae is primarily found in the surface waters of the euphotic zone (Gliwicz, 1986; Lampert, 1989, 1993; Loose, 1993; Sitch, 1989); however, recent studies have shown that high levels of algal biomass can exist outside the euphotic zone (Winder et al., 2003). Thus, putting into question the validity of the assumption that food availability is a true cost associated with DVM. While the effects of migration depth and temperature on Daphnia fitness have been well documented (e.g., Dawidowicz and Loose, 1992; Lampert, 1989, 1993; Loose, 1993), how these two factors independently influence the distance Daphnia migrate, in the presence of light and predator cues, is far less understood. This is because light, temperature, and depth are all naturally coupled (Loose and Dawidowicz, 1994; Lampert, 1989, 1993; Loose, 1993).
A cost associated with DVM not addressed in Lampert’s (1989) paper is the disruptive effect chemical stressors, such as metals, can have on Daphnia migration patterns. Globally, metals are deposited in lakes through natural processes. However, over the past century elevated levels of metals are being loaded into lakes by anthropogenic activities resulting in potentially irreversible impacts on aquatic organisms and the ecosystems as a whole (Gunn et al., 1995; Keller, 2009; Keller et al., 1992; Keller and Piblado, 1986; Pyle et al., 2005). Studies have shown that metals in aquatic environments can cause physiological stress and impair behavioral and ecological activities (Atchison et al., 1987; Scott and Sloman, 2004). In Daphnia, environmentally relevant levels of Cu and Ni have been shown to disrupt their ability to detect the kairomone cue of predators, such as the Chaoborus, an invertebrate predator, and preventing the induction of defensive neckteeth on Daphnia (Hunter and Pyle, 2004; Mirza and Pyle, 2009). However, little is known about how kairomones of vertebrate predator, such as fish, and metals interact to influence thermal preferences associated with diel vertical migration of Daphnia. Furthermore, few if any studies have characterized the individual variation in thermal preferences within and among natural populations of Daphnia, to elucidate the environmental plasticity of the DVM behavior. Given the fitness consequences of DVM, it is critical to understand how genetic variation, biotic factors and abiotic factors contribute to phenotypic variation. Therefore, the goals of this study were to: (1) determine the thermal distribution of Daphnia isolates within and among natural lake populations; (2) elucidate the influence of depth and temperature variation on vertical migration behavior of Daphnia isolates; and (3) determine the impact of Cu and Ni on the vertical migration of Daphnia isolates with different thermal ranges, in the presence and absence of classic DVM conditions.
2. MATERIALS AND METHODS
2.1 Experimental Populations
All Daphnia pulicaria isolates used in this study were collected from lakes in the Canadian Shield region of Ontario, Canada. Three lakes located in Sudbury, Ontario, Canada (Joe: 46° 44” −81° 31”, MacFarlane: 46° 25” −80° 59”, and Simon: 46° 23” −81° 11”) and one lake in Dorset, Ontario, Canada (Brandy Lake: 45° 06” −79° 31”). The lakes differ in their physiological and chemical attributes (Table 1). Daphnia from these lakes were genotyped to determine genetically distinct isolates, while being maintained for five years prior to conducting these studies under standard laboratory conditions (i.e., 20 °C, 16:8 hr light:dark cycle) as described in Shaw et al., (2006). Only adult Daphnia, defined as those with embryos in their brood chamber, were used since adult Daphnia are susceptible to predation by fish and are more likely to exhibit migratory behavior responds to avoid predation (Lampert, 1993, 2006).
Table 1.
Physical and chemical properties associated with the four study lakes (DESC, 2004 and LWQP, 2002 & 2004).
| Lake | Size (ha) |
Volume (104 |
Max Depth |
pH | Trophic Status |
|---|---|---|---|---|---|
| Brandy | 108 | 378 | 7.5 | 6.75 | Oligotrophic |
| Joe | 180 | 2012 | 34 | 6.60 | Oligotrophic |
| MacFarlane | 166 | 2990 | 18 | 7.53 | Mesotrophic |
| Simon | 102 | 1224 | 12 | 7.69 | Eutrophic |
2.2 Experimental Water Columns
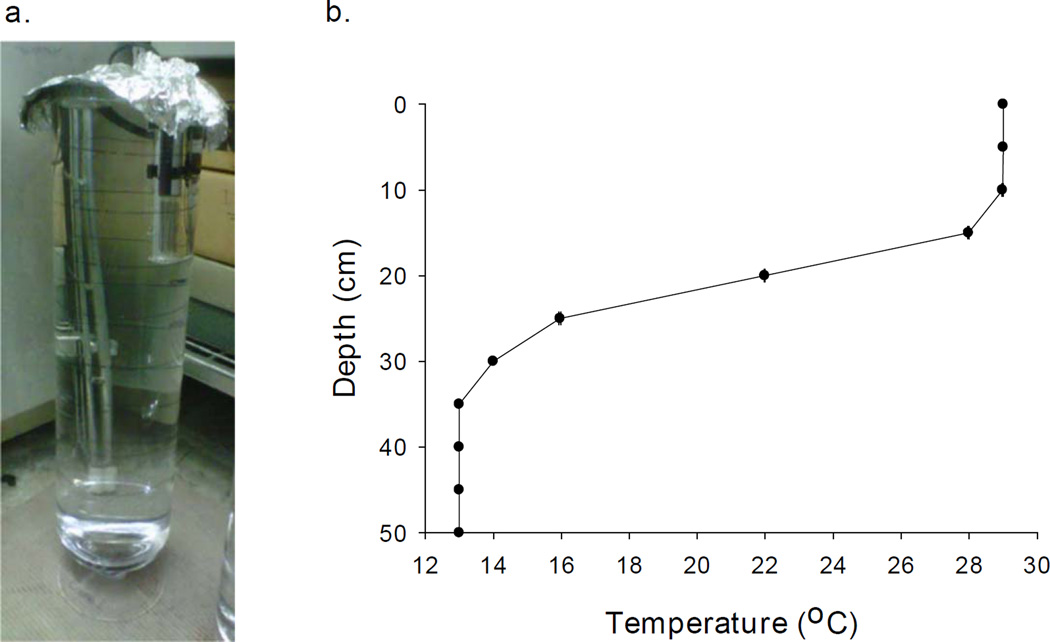
Experiments were conducted in one of eight glass columns (0.5 m tall, 14.5 cm diameter) filled with 8 L of the artificial lake media COMBO (Kilham et al., 1998; Fig. 1). To isolate the effects of depth and temperature on Daphnia vertical migration behavior we conducted laboratory experiments that decoupled these two naturally co-varying factors while controlling for other factors known to vary with depth in natural lakes to avoid confounding results. These controlled factors included light and predator cue levels, as well as food availability. The influence of food availability on diel vertical migration is unclear, due to the recent finding that algal biomass can be present outside the euphotic zone (Winder et al., 2003); therefore, we did not include food availability in our experimental design. The light sources were set-up to prevent any variation in light levels throughout the experimental water columns, with measurements taken throughout the water column on all eight columns before each experiment and showing no variation in light levels within and among water columns for any of the experiments (data not shown). The constant light levels throughout the experimental water column prevented any refuge, allowing the Daphnia to experience constant diel vertical migration behavior inducing conditions throughout the water column. The thermally stratified water column, constant level of light throughout the water column, control over the presence of kairomone, and the decoupling of temperature and depth (see Fig. 1) in our experimental design enabled us to examine the individual role temperature and depth play in determining the vertical migration response in Daphnia in a stratified system.
Figure 1.
a) Picture shows one of eight experimental water columns. b) The thermal stratification pattern of the experimental water column showing temperature (°C) declining with depth (cm). The thermal stratification pattern was consistent during and throughout each experiment, as indicated by the small standard error bars.
The thermal gradient was established by placing the water columns each filled with 20 °C COMBO into a 13 °C walk-in incubator with a 50-W aquarium heater (Penn-Plax© Cat. CH850) placed just under the surface of the water (Fig. 1). The aquarium heater needed to be set to 29 °C in order to acquire a temperature gradient that extended from 10 cm below the surface down to a depth of 35 cm, where temperature became a constant 13 °C for the remaining 15 cm to the bottom of the experimental column (Fig. 1). This thermal stratified configuration was found to be stable and reproducible as indicated by the small error bars associated with Figure 1. Experiments were initiated by placing Daphnia in the middle of the water column at an approximate depth of 25 cm in the 20 °C COMBO prior to establishing the thermal gradient. After 1 hr, the experimental thermal profile was stable. Daphnia were then provided ample time (i.e., 1 hr) to establish their thermal preference within the stratified water column prior to recording their position. Other experiments were conducted in the same way with the addition of one or more stressors (e.g., kairomone alone or in combination with Cu or Ni) being added and under light or dark conditions. The experiment was design to determine isolate the individual effects of the naturally co-varying factors (i.e., depth, temperature, presence/absence of light and fish kairomone) and anthropogenic metal stressors (i.e., Cu and Ni) on vertical migration behavior of the thermally distinct Daphnia isolates.
2.3 Defining Thermal Preferences
The use of behavior in artificial temperature stratified water columns was deemed the most appropriate way to study how thermal preference varies among genetically distinct Daphnia isolates within and among lakes due to the fact that our experimental design was consistent, reproducible, highly sensitive (i.e., able to show degree differences among isolates) and enabled us to move past our basic question of how thermal preferences vary within and among Daphnia populations to determine the independent effects depth and temperature have on Daphnia vertical migration behavior. However, in order to conduct our experiments using more than one individual Daphnia per experimental water column we needed to first determine whether or not Daphnia group dynamics (e.g., schooling effects) would influence individual isolates behaviors.
To determine if Daphnia isolates behavior would be influenced by the presence of other Daphnia we temporarily stained the gut tracks of Daphnia isolates that differed in their thermal preferences using an assortment of food coloring dyes. Daphnia were stained by put 5 drops of concentrated food coloring in a 150 mL beaker filled with artificial lake water and leaving the Daphnia to swim in the colored water for 1 hr prior to experimentation. Daphnia isolates of different thermal preferences and stained different colors were put together in various combinations (e.g., 1 warm group isolate with 4 cold group isolates or 1 cold group isolate with 4 warm group isolates) in a single experimental water column and each individual’s thermal preference was determined. The results of each individual isolate of these group experiments were then compared to the results of testing individual isolates alone. No changes in thermal preference were found suggesting that group dynamics are not a variable of concern. However, the fact that the staining of Daphnia only lasted approximately 1 hour, made it difficult and error prone to differentiate the colored individuals by the 1 hour post treatment response measurement time, we conducted all experiments using 4–5 individual Daphnia isolates from a single thermal group in a water column and used all 8 water columns to increase experimental replication.
The full collection of 50 genetically distinct isolates representing the genetic diversity found within and among D. pulicaria populations of the 4 study lakes, were tested to determine each isolate’s thermal preference. Each isolate was maintained under laboratory conditions for over 100 generations to eliminate maternal effects. Each test was conducted using five adult individuals per isolate and the position (i.e., depth) and its corresponding temperature in the water column was recorded for each individual Daphnia every hour for 6 hours. However, due to the lack of change in position by Daphnia after 1 hr we only report the 1 hr post-treatment results. Differences in thermal distribution among isolates within and between each lake were determined using a one-way analysis of variance followed by a Tukey post-hoc analysis (SigmaPlot 12, 2012).
A group of 12 isolates were chosen from the 50 isolates tested to represent the extremes in thermal preference and sorted into two thermally distinct groups, referred hereafter as the warm group and the cold group (Table 2). In general, the warm group represents Daphnia isolates with thermal preferences >17 °C, while the cold group isolates preferences <15 °C (Table 2). Four isolates were selected to represent the warm group and eight isolates were chosen from the more common cold thermal preferences phenotype to represent the cold group.
Table 2.
Summary of the isolates tested to determine thermal preference within and among natural Daphnia populations.
| Average | Standard | Thermal | Isolates | ||
|---|---|---|---|---|---|
| Lake | Isolate | Temperature | Error | Group | Selected |
| Brandy | BR1 | 13.20 | 0.20 | Cold | |
| Brandy | BR2 | 20.00 | 2.83 | Warm | * |
| Brandy | BR3 | 13.00 | 0.00 | Cold | |
| Brandy | BR4 | 13.87 | 0.62 | Cold | * |
| Brandy | BR5 | 15.50 | 2.18 | Cold | |
| Brandy | BR6 | 15.50 | 2.18 | Cold | |
| Brandy | BR8 | 13.20 | 0.20 | Cold | |
| Brandy | BR9 | 25.60 | 1.47 | Warm | * |
| Brandy | BR10 | 13.60 | 0.60 | Cold | |
| Brandy | BR11 | 13.00 | 0.00 | Cold | * |
| Brandy | BR13 | 13.25 | 0.25 | Cold | |
| Brandy | BR15 | 13.00 | 0.00 | Cold | |
| Brandy | BR16 | 15.00 | 1.76 | Cold | |
| Brandy | BR17 | 13.00 | 0.00 | Cold | |
| Brandy | Mean | 15.05 | 0.96 | -- | |
| Joe | J1 | 13.25 | 0.25 | Cold | |
| Joe | J3 | 13.25 | 0.25 | Cold | |
| Joe | J4 | 16.00 | 2.12 | Cold | |
| Joe | J6 | 13.20 | 0.20 | Cold | * |
| Joe | J7 | 13.75 | 0.75 | Cold | |
| Joe | J8 | 13.00 | 0.00 | Cold | |
| Joe | J11 | 14.20 | 0.73 | Cold | |
| Joe | J12 | 15.40 | 1.75 | Cold | |
| Joe | J13 | 15.50 | 2.18 | Cold | |
| Joe | J14 | 13.07 | 0.07 | Cold | * |
| Joe | J15 | 13.00 | 0.00 | Cold | |
| Joe | J16 | 13.00 | 0.00 | Cold | |
| Joe | J17 | 17.00 | 3.67 | Warm | |
| Joe | J18 | 13.00 | 0.00 | Cold | |
| Joe | J19 | 21.50 | 2.87 | Warm | * |
| Joe | J20 | 13.00 | 0.00 | Cold | |
| Joe | Mean | 14.45 | 0.57 | -- | |
| MacFarlane | MF1 | 13.00 | 0.00 | Cold | * |
| MacFarlane | MF2 | 13.75 | 0.75 | Cold | |
| MacFarlane | MF3 | 14.80 | 1.80 | Cold | |
| MacFarlane | MF4 | 14.67 | 0.81 | Cold | * |
| MacFarlane | MF5 | 14.80 | 1.80 | Cold | |
| MacFarlane | MF6 | 13.75 | 0.75 | Cold | |
| MacFarlane | Mean | 14.13 | 0.30 | -- | |
| Simon | S1 | 15.40 | 1.75 | Cold | |
| Simon | S2 | 13.00 | 0.00 | Cold | |
| Simon | S3 | 13.00 | 0.00 | Cold | |
| Simon | S4 | 13.00 | 0.00 | Cold | |
| Simon | S5 | 13.20 | 0.20 | Cold | |
| Simon | S6 | 13.25 | 0.25 | Cold | |
| Simon | S7 | 13.00 | 0.00 | Cold | * |
| Simon | S9 | 17.40 | 0.94 | Warm | * |
| Simon | S12 | 15.25 | 2.25 | Cold | |
| Simon | S13 | 13.25 | 0.25 | Cold | |
| Simon | S14 | 13.60 | 0.60 | Cold | |
| Simon | S16 | 13.25 | 0.25 | Cold | |
| Simon | S17 | 13.25 | 0.25 | Cold | |
| Simon | S20 | 14.73 | 0.81 | Cold | * |
| Simon | Mean | 13.90 | 0.35 | -- |
2.4 Isolates Vertical Migration Response
To determine if our selected isolates making up the two thermal groups perform classic diel vertical migration, a set of experiments were conducted in the presence and absence of both light and fish kairomone. First, fish kairomone water was created by maintaining 10 goldfish (Carassius auratus of length 3 – 4 cm) in 1.5 liters of modified COMBO at 20 °C and fed ~100 mixed age Daphnia twice during over a 24 hr period (Weetman and Atkinson, 2002, 2004). Fresh kairomone stock was created daily by filtering the fish water through 0.45 μm membrane (Brewer et al., 1999; Loose and Dawidowicz, 1994; Sakwińska, 1998). A final concentration of 0.2 fish L−1 was created by adding 0.25 L of the kairomone containing water to 7.75 L of kairomone free COMBO in the experimental columns (Brewer et al., 1999; Weetman and Atkinson, 2002, 2004). Next, the 2 hr initial set-up of the thermal stratified water column and Daphnia addition as previously described (see section 2.2) was conducted prior to any fish kairomone water slowly being added to the top of the water column using a pipette to prevent mixing of the water column, when applicable. The fish kairomone having made its way down through the stratified water column was evident by the response of Daphnia performing DVM through-out the water column, including the very bottom section. The position of the Daphnia was recorded just before any kairomone water would have been added (0 hr) and 1 hr after. A similar procedure was conducted in the absence of light and fish kairomone treatments as a control to determine if the Daphnia isolates migrated in the absence of classic diel vertical migration conditions. However, to avoid disrupting the circadian rhythm of the Daphnia these experiments were performed at night using a LED head lamp covered with a red filter (Kodak Number 25 Wratten). The red filter inhibited the transmission of light with wavelengths <160 nm, which is virtually undetectable by Daphnia (Loose and Dawidowicz, 1994; Scheffer et al., 1958). To determine whether or not each Daphnia isolate performed classic vertical migration behavior, Student t-tests were performed comparing 0 hr and 1 hr time points for each group in the presence and absence of light and kairomone (Systat Software SigmaPlot 12, 2012).
2.5 The Effects of Metal on Vertical Migration Experiment
The effects of environmentally relevant levels of Cu and Ni (5 μg Cu L−1 and 40 μg Ni L−1; LWQP, 2004) were tested in the presence and absence of fish kairomone and light conditions on the two thermally distinct Daphnia isolate groups. The experimental procedure was the same as described above (see section 2.5), except that each metal was added during the set-up of the water column to allow for proper mixing throughout the water column. Test metal solutions were made from CuCl (Matheson Coleman and Bell) or NiCl2 (J. T. Baker) dissolved in ultrapure water. To determine whether or not the addition of Cu or Ni to our experimental water columns in the presence and absence of both fish kairomone and light affected vertical migration behaviors we used two-way analyses of variance, followed by a Tukey post-hoc analysis (Systat Software SigmaPlot 12, 2012). Each analysis was done on the difference between initial starting position (0 hr) and final position (i.e., 1 hr post treatment application).
3. RESULTS
3.1 Variation in thermal preference
To determine how thermal preferences differ within and among natural Daphnia populations, the thermal distribution of 50 Daphnia isolates from four lake populations (Brandy, Joe, MacFarlane, and Simon; see method section) were assessed using the thermally stratified experimental water columns (Table 2). High variability in thermal preferences within lake populations was found, while no significant difference in thermal distribution was found among the four populations (p = 0.312; Fig. 2). Significant within lake differences in thermal preferences among Daphnia isolates were found in Brandy (n = 14; p < 0.001), Joe (n = 16; p = 0.018), and Simon (n = 14; p = 0.078) Lakes. However, no significant differences were detected among isolates from MacFarlane Lake (n = 6; p = 0.805; Fig. 2), possibly due to the low number of Daphnia pulicaria isolates collected (n = 6) being found in MacFarlane Lake, less than half the number of isolates found in the other three lakes (see Table 2). The observed variation in thermal preferences among isolates was further utilized to isolate the role temperature and depth play in vertical migration by using isolates with different thermal preferences and therefore initial starting position in the experimental water columns, to help unlock these two naturally coupled factors.
Figure 2.
Thermal preferences of the fifty isolate representing four lakes within the Sudbury and Dorset region of Ontario, Canada, tested in the presence of light and the absence of fish kairomone. For each isolate five individuals were tested and the data shown here represent the means ± standard error. The isolates that make up each lake’s Daphnia pulicaria population showed significant variation in thermal preferences. However, no difference in thermal variation was found among the four lake populations.
As a quality assurance measure, we tested whether Daphnia isolates selection behavior of initial starting position in the experimental water columns was based on temperature rather than depth. By reducing the heat applied to the water column we were able to move the stratification regime upwards and measured whether the Daphnia isolates would move to more shallow waters to stay at their selected temperatures or simply remain at their preferred depth. As expected, we found that all Daphnia isolates moved with their thermal preference (data not shown), validating our use of thermal preference behavioral response.
3.2 Effects of Light and Fish Kairomone on Vertical Migration
The influence of light and fish kairomone to stimulate diel vertical migration in Daphnia is well known (Wetzel, 2001); however, not all Daphnia exhibit this response. To determine if the Daphnia selected for the two distinct thermal groups exhibit the classic diel vertical migration behavior, we tested their response to kairomone and light in a full factorial designed experiment. Based on classical diel vertical migration behavior Daphnia should migrate downward in the presence of light and fish kairomone, but would exhibit no downward migration behavior in the absence of either light or fish kairomone. As predicted, no Daphnia exhibited any downward migration behavior in the absence of either light (-L) or kairomone (-K) (see Appendix S1 and S2) and all Daphnia isolates in warm and cold thermal groups exhibited the classic diel vertical migration behavior in the presence of kairomone and light using depth as the response variable (warm group: p = 0.008, Fig. 3b and cold group: p = 0.01, Fig. 3d). However, when using temperature as the response variable only the warm group migrated to a significantly lower temperature in the +L and +K treatment (warm group: p = 0.003, Fig. 3a and cold group: p = 0.372; Fig. 3c). This is due to the fact that the cold group started at the lowest thermal gradient (13 °C) in the experiment and therefore could not migrate to a lower temperature. This experimental design allowed for the decoupling of temperature and depth effects in determining the distance Daphnia vertical migration, and showed that response to predator cues occurred whether or not a thermal gradient was present.
Figure 3.
Migration behavior in response to kairomone and copper. Mean, trajectory and standard error of depth or preferred temperature for both Daphnia thermal groups, selected based on their thermal preference profiles to making up the warm (n = 8) or cold thermal groups (n = 12), response (in the presence of light) to the presence or absence of either fish kairomone or metal (e.g., Cu). The response was measured as either a change in temperature (a and c) or depth (b and d). -K is the absence of fish kairomone and +K is the presence of fish kairomone.
Warm group results using depth as the response variable were consistent with those obtained using temperature (see Fig. 3a and 3b), because temperature and depth were strongly correlated at temperatures above 13 °C (see Fig. 1); however, once the thermal gradient reached 13 °C depth became decoupled from temperature. Decoupling the effects of temperature and depth on Daphnia vertical migration behavior revealed three findings that would not have been found if temperature was the only response variable measured. Using depth as the response variable instead of temperature revealed that Daphnia in the cold group did in-fact perform diel vertical migration behavior under classic diel vertical migration conditions (+L, +K). The cold group Daphnia started at an average depth of 35 cm and temperature 13 °C and migrated to an average depth of 45 cm while temperature remained constant (Fig. 3d), resulting in a significant difference in final migration depth between the control (+L, -K) and +L, +K treatment (p < 0.001; Fig. 3d). In addition, depth as the response variable revealed that Daphnia from the warm group exposed to classic diel vertical migration conditions (i.e., +L, +K treatment) responded by ending their vertical migration at the onset of the lowest temperature thermal cline in the experimental water columns (i.e. 13 °C), but not the lowest depth in the experimental water column (Fig. 3a). Therefore, warm group isolates migrated only as far as temperature changes exist, stopped short of the maximum depth. Finally, average migratory distance traveled by Daphnia isolates in both warm and cold group under +L, +K treatment (12.25 cm ± 2.20 cm and 10.5 cm ± 1.22 cm, respectively) did not differ statistically (p = 0.228; Fig. 3b and 3d).
3.3 Effects of Metal on Vertical Migration
The direct effects of two metals on vertical migration of Daphnia were tested to determine if Daphnia migratory response would be impacted by the presence of either Cu or Ni in the water column. In this experiment, all warm and cold group isolates were exposed to each metal individually in the presence of light, with and without kairomone and the migration response was recorded. Due to the consistency found in the results of the two metals only the slightly clearer Cu results are presented in the main text of the paper, with Ni results provided as supplemental material (Appendix S2).
To determine if the metals Cu or Ni themselves caused Daphnia to migrate, the migration patterns with metals present but without fish kairomone were tested for all warm and cold group isolates. These experiments showed that while there was a trend of Daphnia having a lower initial starting position in the presence of metals, the presence of Cu or Ni did not result in Daphnia migration response (Fig. 3 and Appendix S2). Therefore, we then tested the effects of Cu and Ni on these Daphnia isolates natural response to light and the presence of fish predatory cues. We found that Daphnia isolates of both thermal groups predatory avoidance behavioral response (i.e., vertical migration) was inhibited in the presence of light and fish kairomone by both Cu and Ni (Fig. 3).
4. DISCUSSION
Three major findings emerged from these studies examining the influence of temperature, co-varying abiotic and biotic factors, and two anthropogenic stressors (i.e. Cu and Ni) on vertical migration within and among Daphnia populations: (1) comparison of Daphnia thermal preferences across the four lake populations showed no differences among populations (Fig. 2); however, for Daphnia isolates thermal preference differences within lake populations were common (Fig. 2); (2) depth rather than temperature was the more deterministic variable of Daphnia migration, since vertical migration occurred even in the absence of a temperature gradient (Fig. 1 and Fig. 3). (3) Under classic diel vertical migration conditions (i.e., +L, +K), but in the presence of environmentally relevant concentrations of either Cu or Ni, Daphnia predatory avoidance behavior (i.e. diel vertical migration) was inhibited (Fig. 3 and Appendix S1).
The four lakes in this study were selected because they share similar geological characteristics, all occur within the boreal environment of the Canadian Shield, and all are lakes that contain fish and therefore their Daphnia populations likely exhibit diel vertical migration behavior. Variation among all four lakes does exist in terms of size (area, volume, and depth), pH, and trophic state (Table 1). Even though these lakes differed in their physical and chemical attributes (Table 1), the Daphnia populations found in these lakes did not differ in their overall thermal preference (p = 0.312). This was surprising, because it suggests that the thermal preferences of Daphnia populations are independent of pH, depth, volume, area, and trophic status of their respective systems. Further examination showed that the lack a variation among populations was due to the similarities in the range of different thermal preferences among isolates of the various populations.
The studied Daphnia populations all had isolates that significantly differed in their thermal preference, except in MacFarlane Lake that yielded half as many genetically distinct Daphnia isolates (n = 6) as the other study lakes and only contributed cold thermal group isolates (Table 2). This high variability in the range of temperature preferences found among isolates within each lake’s Daphnia population, suggests the existence of niche partitioning that could reduce intra-specific competition among Daphnia isolates by minimizing the resource allocation overlap in the water column as demonstrated in earlier seminal works (De Meester and Dumont, 1988; 1989; Dumont et al., 1985; Weider, 1984). Thermal partitioning through intra-specific competition among genetically distinct Daphnia isolates helps explain not only the high variability in thermal preferences found within lake Daphnia populations, but also how lakes that differ in their physical and chemical attributes can still have the same overall thermal range in their Daphnia populations.
This study also revealed that cold thermal group Daphnia in the presence of light and fish kairomone migrated to deeper waters, even in the absence of changes in temperature (Fig. 1 and Fig. 3d), suggesting that a change in temperature was not a necessary factor in determining the depth at which Daphnia migrate in response to predator cues. Furthermore, Daphnia isolates in the warm thermal group under classic diel vertical migration conditions started their migration higher in the water column (where temperatures were warmer), but migrated the same distance (~10 cm) as the cold thermal group. The average migration depth took the warm group Daphnia just inside the 13 °C, stopping short of the experimental water column’s bottom. This raises the questions: Why stop there? Why not migrate as deep as the cold group under the same +L, +K conditions? After all, migrating to these depths would not come at a cost due to changes in temperature. It is worth noting that while we report only results of Daphnia’s position in the water column after 1 hr post treatment addition, we collected hourly data for 6 hrs post treatment addition. We only report the 1 hr post treatment depths because we found that the Daphnia remained at the same position over the 6 hr period (data not shown). The fact that Daphnia persisted at this final position for 6 hours also suggests this is a regulated behavior as Daphnia had ample time to migrate elsewhere. Our findings show that both cold and warm thermal group isolates traveled the same distance on average during their vertical migration despite ample time and space available for them to move further, suggesting that distance, not temperature, determines the final depth to which Daphnia isolates migrated.
Lampert (1989) observed that there are several possible sources of costs limiting diel vertical migration. Our study suggests that distance migrated, rather than temperature, dictates the depth to which Daphnia isolates vertically migrate when light conditions are held constant. Given the scale of this study, the migration distance of 10cm, it is difficult to directly translate these findings into something meaningful in the context of a natural lake environment. For example, the known effects of hydrostatic pressure on Daphnia migration (Lincoln, 1971) were not evaluated due to the size of our experimental chambers. However, our controlled laboratory study was designed to elucidate the naturally coupled mechanisms of light, depth, migration distance, and temperature to show how their roles differ in controlling the migration depth associated with Daphnia vertical migration behavior.
The present study also demonstrates that Daphnia isolates within a population occupy tight thermal ranges, which may represent niche-partitioning in response to intra-specific competition. However, the ability of individuals and populations to occupy alternative or wider thermal ranges through acclimation or adaptation will be important in determining how Daphnia populations will respond to thermal perturbations such as climate change. A study by Palaima and Spitze (2004) examined the height and breadth of temperature tolerance curves for 29 D. pulex complex genotypes and demonstrated that some Daphnia with high fitness at their optimal temperatures also had high fitness across a wide range of temperatures, supporting the hypothesis that some individuals within a population are able to alter their thermal location and maintain a high level of fitness. Therefore, any changes in thermal conditions of a lake whether from increased intra-specific competition due to a reduction in the number of thermally distinct strata, costs associated with the need to acclimate, or the bottlenecking associated with thermal selection, will likely result in a reduction in diversity and size (at least initially) of Daphnia lake populations.
The mechanism dictating the depth at which Daphnia vertically migrate to avoid predation is an important factor determining Daphnia fitness (e.g., how much energy is being devoted to predator avoidance versus reproduction). However, this defense mechanism depends on Daphnia’s ability to perceive the predation threat by detecting kairomone cues. Any factor that interferes with this ability would prevent Daphnia from vertically migrating which would result in a dramatic increase in the rate of mortality. This study has demonstrated that environmentally relevant levels of the metals Cu and Ni inhibit the classic vertical migration behavioral response exhibited by both warm and cold thermally constrained Daphnia groups in the presence of light and vertebrate predator cue. The lack of vertical migration by all isolates suggests that even at low levels metals are interfering with Daphnia’s response to fish kairomone cues. Similar findings have been shown in other studies examining invertebrate predator kairomone and their influence on Daphnia neonate neckteeth development in the presents of these same metals (Hunter and Pyle, 2004; Mirza and Pyle, 2009), and fish kairomone influence on photo-tactic behavior of Daphnia in the present of Cu (Dang Kieu et al., 2001; Yuan et al., 2003). While our study confirms earlier findings about copper it adds nickel to the list of metals that at low concentrations inhibits Daphnia’s responds to predatory fish cues. Furthermore, our results showed a surprising direct effect of metal on Daphnia positioning in the water column that we were unable to explain and warrants more research. The constant additions of copper and nickel into aquatic ecosystems around the world through atmospheric deposition suggests that disruptions to Daphnia’s vertical migration response to predator cues will become more common and could result in an increased rate of fish predation and a dramatic decline in Daphnia populations globally.
5. CONCLUSION
These studies demonstrate how individual Daphnia genotypes can distribute themselves among the various thermal niches that result from stratification within a lake, and how this thermal niche partitioning is similar in lakes that differ in physical and chemical properties. Experiments designed to decouple the naturally co-varying factors of temperature and depth revealed that tracking changes in depth was a better predictor of Daphnia migratory behavior than changes in temperature. Furthermore, the results of these experiments also showed that regardless of initial thermal positions in the water column cold- and warm group Daphnia migrated a similar distance, supporting the hypothesis that the cost associated with distance migrated limits vertical migration behavior. Finally, these studies revealed the disruption of natural diel vertical migration behavioral response in Daphnia exposed to environmentally relevant levels of Cu or Ni. The addition of either of these common atmospherically derived anthropogenic stressors inhibits the response of Daphnia to fish kairomone in the presence of light, putting these non-migrating Daphnia at a greater risk of predation.
Collectively, these findings suggest diversity and overall size of Daphnia populations are dependent on highly stratified lentic systems and the absence of Cu and Ni pollution. However, a reduction in the variation in thermal stratification of lakes is predicted to occur in response to climate change (Heino et al., 2009; Mooij et al., 2005) and Cu and Ni loading into aquatic ecosystems is likely to continue (Keller, 2009; Keller and Piblado, 1986; Pyle et al., 2005); therefore, our findings predict a global decline in Daphnia diversity due to increased intra-specific competition within lakes and Daphnia populations will decrease in size as a result of increased predation pressure by fish. Because of Daphnia’s global presence and its role as a keystone species in aquatic food webs, a significant decline in Daphnia diversity and population size could dramatically affect aquatic ecosystem function and stability (Lampert, 2006; Mooij et al., 2005; Thuiller, 2007; van de Waal et al., 2009).
Supplementary Material
Highlights.
No variation in thermal preference among the four Daphnia populations.
High within lake variability in thermal preference among Daphnia isolates.
Depth was the better predictor of Daphnia migratory patterns than temperature.
Exposure to low levels of Cu or Ni inhibited diel vertical migration behavior.
Acknowledgments
Funding for this research was provided by the National Institute of Environmental Health Science through the Outstanding New Environmental Science program (R01ES019354) awarded to JRS. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS. Support was also provided by The School of Public and Environmental Affairs at Indiana University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Atchison GJ, Henry MG, Sandheinrich MB. Effects of metals on fish behavior: A review. Environmental Biology of Fishes. 1987;18:11–25. [Google Scholar]
- 2.Brewer MC, Dawidowicz P, Dodson SI. Interactive effects of fish kairomone and light on Daphnia escape behavior. Journal of Plankton Research. 1999;21:1317–1335. [Google Scholar]
- 3.Colbourne JK, Robison B, Bogart K, Lynch M. Five hundred and twenty-eight microsatellite markers for ecological genomic investigations using Daphnia . Molecular Ecology Notes. 2004;4:485–490. [Google Scholar]
- 4.Dang Kieu N, Michels E, De Meester L. Phototactic behavior of Daphnia and the continuous monitoring of water quality: Interference of fish kairomones and food quality. Environmental Toxicology and Chemistry. 2001;20:1098–1103. [PubMed] [Google Scholar]
- 5.Dawidowicz P, Loose CJ. Metabolic costs during predator-induced diel vertical migration of Daphnia . Limnology & Oceanography. 1992;37:1589–1595. [Google Scholar]
- 6.De Meester L, Dumont HJ. The genetics of phototaxis in Daphnia magna: Existence of three phenotypes for vertical migration among parthenogenetic females. Hydrobiologia. 1988;162:47–55. [Google Scholar]
- 7.De Meester L, Dumont HJ. Phototaxis in Daphnia: Interaction of hunger and genotype. Limnology & Oceanography. 1989;34:1322–1325. [Google Scholar]
- 8.Dumont HJ, Guisez Y, Carels I, Verheye HM. Experimental isolation of positively and negatively phototactic phenotypes from a natural population of Daphnia magna Straus: A contribution to the genetics of vertical migration. Hydrobiologia. 1985;126:121–127. [Google Scholar]
- 9.Gliwicz MZ. Predation and the evolution of vertical migration in zooplankton. Nature. 1986;320:746–748. [Google Scholar]
- 10.Gunn J, Keller W, Negusanti J, Potvin R, Beckett P, Winterhalder K. Ecosystem recovery after emission reductions: Sudbury, Canada. Water, Air and Soil Pollution. 1995;85:1783–1788. [Google Scholar]
- 11.Heino J, Virkkala R, Toivonen H. Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biological Reviews. 2009;84:39–54. doi: 10.1111/j.1469-185X.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.Hunter K, Pyle G. Morphological responses of Daphnia pulex to Chaoborus americanus kairomone in the presence and absence of metals. Environmental Toxicology and Chemistry. 2004;23:1311–1316. doi: 10.1897/03-369. [DOI] [PubMed] [Google Scholar]
- 13.Keller W. Limnology in northeastern Ontario: from acidification to multiple stressors. Canadian Journal of Fisheries and Aquatic Sciences. 2009;66:1189–1198. [Google Scholar]
- 14.Keller W, Gunn JM, Yan ND. Evidence of biological recovery in acid-stressed lakes near Sudbury, Canada. Environmental Pollution. 1992;78:79–85. doi: 10.1016/0269-7491(92)90013-z. [DOI] [PubMed] [Google Scholar]
- 15.Keller W, Piblado JR. Water quality changes in Sudbury area lakes: A comparison of synoptic surveys in 1974-76 and 1981-83. Water, Air & Soil Pollution. 1986;29:285–296. [Google Scholar]
- 16.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia. 1998;377:147–159. [Google Scholar]
- 17.Kling GW, Hayhoe K, Johnson LB, Magnuson JJ, Polasky S, Robinson BJ, Shuter MW. Confronting climate change in the Great Lakes Region. Impacts on our communities and ecosystems. Union of concerned scientists, Cambridge, MA, and Ecological Society of America, Washington, DC. 2003. ( http://www.ucsusa.org/greatlakes) [Google Scholar]
- 18.Lampert W. The adaptive significance of diel vertical migration of zooplankton. Functional Ecology. 1989;3:21–27. [Google Scholar]
- 19.Lampert W. Ultimate cause of diel vertical migration of zooplankton: New evidence for the predator-avoidance hypothesis. Archiv für Hydrobiologie Beïhefte Ergebniss der Limnologie. 1993;39:79–88. [Google Scholar]
- 20.Lampert W. Daphnia: Model herbivore, predator and prey. Polish Journal of Ecology. 2006;54:607–620. [Google Scholar]
- 21.Lincoln RJ. Observations of the effects of changes in hydrostatic pressure and illumination on the behavior of some planktonic crustaceans. Journal of Experimental Biology. 1971;54:677–688. [Google Scholar]
- 22.Loose CJ. Daphnia diel vertical migration behavior: Response to vertebrate predator abundance. Archiv für Hydrobiologie Beïhefte Ergebniss der Limnologie. 1993;39:29–36. [Google Scholar]
- 23.Loose CJ, Dawidowicz P. Trade-offs in diel vertical migration by zooplankton: The costs of predator avoidance. Ecology. 1994;75:2255–2263. [Google Scholar]
- 24.McCormick MJ. Potential changes in thermal structure and cycle of Lake Michigan due to global warming. Transactions of the American Fisheries Society. 1990;119:183–194. [Google Scholar]
- 25.Mirza RS, Pyle GG. Waterborne metals impair inducible defenses in Daphnia pulex: Morphology, life-history traits and encounters with predators. Freshwater Biology. 2009;54:1016–1027. [Google Scholar]
- 26.Mooij W, Hulsmann S, De Senerpont Domis LN, Nolet BA, Bodelier PLE, Boers PCM, et al. The impact of climate change on lakes in the Netherlands: A review. Aquatic Ecology. 2005;39:381–400. [Google Scholar]
- 27.Palaima A, Spitze K. Is a jack-of-all-temperatures a master of none? An experimental test with Daphnia pulicaria (Crustacea: Cladocera) Evolutionary Ecology Research. 2004;6:215–225. [Google Scholar]
- 28.Pyle GG, Rajotte JW, Couture P. Effects of industrial metals on wild fish populations along a metal contamination gradient. Ecotoxicology and Environmental Safety. 2005;61:287–312. doi: 10.1016/j.ecoenv.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Sakwińska O. Plasticity of Daphnia magna life history traits in response to temperature and information about a predator. Freshwater Biology. 1998;39:681–687. [Google Scholar]
- 30.Sarnelle O. Daphnia as keystone predators: effects on phytoplankton diversity and grazing resistance. Journal of Plankton Research. 2005;27:1229–1238. [Google Scholar]
- 31.Scheffer D, Robert P, Medioni J. Reactions oculo-motrices de la Daphnie (Daphnia pulex De Geer) en reponseA d es lumieresm onochromatiquesd 'egalee nergie. Sensibility visuelle et sensibility dermatoptique. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales. 1958;152:1000–1003. [PubMed] [Google Scholar]
- 32.Scott GR, Sloman KA. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology. 2004;68:369–392. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Shaw JR, Dempsey TD, Chen CY, Hamilton JW, Folt CL. Comparative toxicity of cadmium, zinc, and mixtures of cadmium and zinc to daphnids. Environmental Toxicology and Chemistry. 2006;25:182–189. doi: 10.1897/05-243r.1. [DOI] [PubMed] [Google Scholar]
- 34.Shaw JR, Colbourne JK, Davey JC, Glaholt SP, Hampton TH, Chen CY, Folt CL, Hamilton JW. Gene response profiles for Daphnia pulex exposed to the environmental stressor cadmium reveals novel crustacean metallothioneins. BMC Genomics. 2007;8:477–488. doi: 10.1186/1471-2164-8-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snucins E, Gunn J. Interannual variation in the thermal structure of clear and coloured lakes. Limnology and Oceanography. 2000;45:1639–1646. [Google Scholar]
- 36.Systat Software Inc. SigmaPlot Ver. 12. San Jose, CA, USA: 2012. [Google Scholar]
- 37.Thuiller W. Biodiversity: Climate change and the ecologist. Nature. 2007;448:550–552. doi: 10.1038/448550a. [DOI] [PubMed] [Google Scholar]
- 38.van de Waal DB, Verschoor AM, Verspagen JMH, van Donk E, Huisman J. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Frontiers in Ecology and the Environment. 2009;8:145–152. [Google Scholar]
- 39.Weetman D, Atkinson D. Antipredator reaction norms for life history traits in Daphnia pulex: Dependence on temperature and food. Oikos. 2002;98:299–307. [Google Scholar]
- 40.Weetman D, Atkinson D. Evaluation of alternative hypotheses to explain temperature-induced life history shifts in Daphnia . Journal of Plankton Research. 2004;26:107–116. [Google Scholar]
- 41.Weider LJ. Spatial heterogeneity of Daphnia genotypes: Vertical migration and habitat partitioning. Limnology & Oceanography. 1984;29:225–235. [Google Scholar]
- 42.Wetzel RG. Limnology: Lake and River Ecosystems. 3rd. San Diego: Academic Press; 2001. [Google Scholar]
- 43.Winder M, Boersma M, Spaak P. On the cost of vertical migration: Are feeding conditions really worse at deeper depth? Freshwater Biology. 2003;48:383–393. [Google Scholar]
- 44.Yuan L, Michels E, De Meester L. Changes in phototactic behavior of Daphnia magna clone C1 242 in response to copper, cadmium and pentachlorophenol. Journal of Environmental Sciences. 2003;15:841–847. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.