Abstract
Purpose
Sequential chemotherapy with doxorubicin and gemcitabine (AG) followed by ifosfamide, paclitaxel, and cisplatin (ITP) was previously demonstrated to be well tolerated in patients with advanced transitional cell carcinoma (TCC). This study sought to evaluate the efficacy and to additionally define toxicity.
Patients and Methods
Sixty patients with advanced TCC received AG every 2 weeks for five or six cycles followed by ITP every 21 days for four cycles. Granulocyte colony-stimulating factor was given between cycles.
Results
Myelosuppression was seen with 68% of patients who experienced grades 3 to 4 neutropenia and with 25% who experienced febrile neutropenia. Grade 3 or greater nonhematologic toxicities were infrequent. Forty (73%) of 55 evaluable patients (95% CI, 59% to 84%) demonstrated a major response (complete, n = 19; partial, n = 21) and had a median response duration of 11.3 months (range, 1.7 to ≥ 105.6 months). Twenty-seven (79%) of 34 patients with locally advanced disease (ie, T4, N0, M0) or with regional lymph node involvement (ie, T3-4, N1, M0) and 10 (56%) of 18 patients with distant metastases achieved a major response. The median progression-free survival was 12.1 months (95% CI, 9.0 to 14.8 months), and the median overall survival was 16.4 months (95% CI, 14.0 to 22.5 months). At a median follow-up of 76.4 months, seven (11.7%) patients remain alive, and all were disease free.
Conclusion
AG plus ITP is an active regimen in previously untreated patients with advanced TCC; however, it is associated with toxicity and does not clearly offer a benefit compared with other nonsequential, cisplatin-based regimens.
INTRODUCTION
Transitional cell carcinoma (TCC) is a chemotherapy-sensitive malignancy, in which a survival benefit is associated with cisplatin combination chemotherapy in the metastatic setting. In two randomized trials, the regimen of methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) was compared with cisplatin alone and the combination of cisplatin, doxorubicin, and cyclophosphamide; both trials demonstrated response and survival advantages for M-VAC.1,2 Despite these results, the median survival with M-VAC is 11 to 13 months, and the 6-year progression-free survival is only 3%.3
The poor survival and substantial toxicity associated with M-VAC have led to the investigation of alternative chemotherapy. In a randomized trial in which gemcitabine plus cisplatin (GC) was compared with M-VAC, GC demonstrated similar activity and better tolerability and has become a standard of care.4,5 On the basis of phase II trials in which activity for the taxanes and ifosfamide was revealed, we performed a phase II trial of ifosfamide, paclitaxel, and cisplatin (ITP) with recombinant human granulocyte colony-stimulating factor (rhG-CSF) in patients with advanced TCC.6,7,9–12 Thirty (68%) of 44 patients (95% CI, 52% to 81%) demonstrated a response. At a median follow-up of 28 months, the median survival was 20 months, and 11 patients (25%) were disease free at last follow-up.
Theoretical models suggest that sequential administration of chemotherapy may improve targeting of different cell populations within a tumor.13,14 Additionally, administration of two- or three-drug combinations in high doses sequentially may overcome the toxicity associated with simultaneous administration of agents. To explore the hypothesis that sequential chemotherapy would improve outcome and to build on the ITP experience in advanced TCC, we evaluated therapy with doxorubicin and gemcitabine (AG) followed by ITP. In a phase I study of AG followed by ITP, fifteen patients received AG every other week for six cycles followed by ITP every 3 weeks for four cycles.15 AG was tolerated at all dose levels, and toxicity with ITP included grades 3 and 4 neutropenia in four patients and grade 3 nausea/vomiting in three patients. Eight of 14 evaluable patients experienced a major response to AG. After completion of AG plus ITP, nine of 14 evaluable patients had a response (complete response [CR], n = 3; partial response [PR], n = 6). This report details the final results of the evaluation of AG-ITP in patients with advanced TCC.
PATIENTS AND METHODS
Patient Population
Pathologic confirmation of advanced TCC was required. Metastatic lesions were required to be bidimensionally measurable. Examination under anesthesia, cystoscopy, and needle biopsies of pelvic nodes (when indicated) were performed to assess unresectable primary bladder tumors. All patients were ≥18 years old and had a minimum Karnofsky performance status (KPS) of 60%. Other eligibility criteria included neutrophil count ≥ 1,500 cells/mm3; platelet count ≥ 150,000 cells/mm3; serum creatinine ≤ 1.5 mg/dL or calculated creatinine clearance ≥ 60 mL/min/1.73 m2; bilirubin less than 1.5 times normal; and AST less than two times normal. Normal cardiac function, defined as a left ventricular ejection function ≥ 50%, was required. Patients may not have received systemic chemotherapy or irradiation within 3 weeks of therapy. Patients with evidence of another active cancer were excluded. Barrier method contraception was required. The institutional review boards of Memorial Sloan-Kettering Cancer Center and Weill Cornell Medical College approved this protocol; written informed consent was obtained from all patients.
Treatment Plan
Doxorubicin at 50 mg/m2 intravenous (IV) push plus gemcitabine 2,000 mg/m2 IV infusion over 2 hours were given intravenously on day 1 every 2 weeks. Each cycle was defined as one administration every 2 weeks, for a total of six administrations over 12 weeks. Because of toxicity, the protocol was amended after 16 patients were treated to provide five administrations over 10 weeks. Patients self-administered rhG-CSF 5 μg/kg/d subcutaneously on days 3 to 11 of each cycle.
Beginning on the 11th week, and no sooner than 14 days after the fifth dose of AG, ITP was administered. Irrespective of the number of cycles of AG administered, ITP commenced thirteen weeks from the first cycle of AG. Patients who required dose delays may have received less than five cycles of AG before crossover to ITP at week 13. After completion of AG, ITP was administered for four cycles every 21 days (cycles six to nine). ITP was administered as follows: paclitaxel 200 mg/m2 by 3-hour infusion followed by cisplatin 70 mg/m2 and then ifosfamide 1,500 mg/m2 by 2-hour infusion on day 1; ifosfamide was repeated on days 2 and 3. Mesna 300 mg/m2 IV was given 30 minutes before and 4 and 8 hours after ifosfamide. After completion of the originally designed study and the observed toxicity of the ITP regimen, in which all of the cisplatin and paclitaxel were given on day 1, the protocol was amended to evaluate all three drugs given on the same day. Twenty-seven additional patients received ITP on a modified schedule as follows: paclitaxel 50 mg/m2 by 1-hour infusion followed by cisplatin 20 mg/m2 IV and then ifosfamide 1,500 mg/m2 by 2-hour infusion were given on days 1, 2, and 3. Mesna 1,500 mg/m2 IV was admixed with ifosfamide. Patients received premedication with dexamethasone, diphenhydramine hydrochloride, and cimetidine before paclitaxel. Patients self-administered rhG-CSF 5 μg/kg subcutaneously daily from days 6 to 17 of each cycle.
Delays and dose attenuations were prescribed for specific toxicities by using the National Cancer Institute Common Toxicity Criteria, as previously described.15
Adjunctive surgery after AG-ITP included cystectomy for responding locally advanced bladder tumors and resection of responding metastatic disease in resectable solitary nodal or pulmonary sites.
Response and Survival Criteria
Response evaluations were performed at the completion of AG, after two cycles of ITP, and at the completion of therapy. CR was defined as disappearance of all evidence of tumor on physical examination, radiographic studies, or both for a minimum of 4 weeks. PR was defined as 50% or greater decrease of the summed products of the perpendicular diameters of all measurable lesions for at least 4 weeks without the simultaneous increase in the size of any lesion or the appearance of any new lesion. Stable disease (SD) was defined as a less than 25% change in indicator lesions for at least 8 weeks. Progression was defined as a greater than 25% increase in tumor size or the appearance of any new lesion. Irrespective of response to five cycles of AG, patients proceeded with ITP. If response assessment after two cycles of ITP was stable, PR, or CR, therapy with the same regimen continued for two additional cycles. Patients who achieved CR at the completion of therapy were observed. Patients who did not respond after nine cycles were taken off study. The terms complete regression and partial regression, which referred to greater than 50% regression, were used to describe the activity of AG. All responses were reviewed by a reference radiologist. The preplanned sample size with a two-stage design was 30 patients. The study was designed to be promising if any response rate (CR + PR) greater than 24% was achieved, because the observed response rate for M-VAC was 33% (95% CI, 24% to 42%).1 CIs for response were calculated assuming binomial distribution. Overall survival time was calculated as the difference between the last follow-up date or date of death and the date at which chemotherapy was initiated. Progression-free survival was computed as the difference between the date of disease progression, death, or last follow-up and the date of initiation of chemotherapy. Survival distributions were calculated by using the Kaplan-Meier method.16 Any dose of chemotherapy was adequate for toxicity and survival assessment.
RESULTS
Patient Characteristics
Between July 1998 and July 2003, 60 patients (including three patients treated at the maximum-tolerated dose in the phase I trial) were enrolled and had a median KPS of 90%. Twenty (33%) of 60 patients had visceral metastases, including lung, liver, and/or bone; 15 (25%) had lymph node metastases only (regional lymph node involvement, n = 14; regional and distant lymph nodes, n = 1); and 22 patients (37%) had unresectable primaries with or without regional or distant lymph nodes. A previous analysis demonstrated the negative impact of KPS less than 80% or visceral metastases on survival of patients treated with M-VAC.17 A significant difference in survival with zero, one, or two prognostic factors was noted. In this study, 34 patients (57%) had zero risk factors, 24 patients (40%) had one risk factor, and two patients (3%) had two risk factors (Table 1).
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Characteristic | Patients (N = 60) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 62 | |
| Range | 41-78 | |
| Sex | ||
| Male | 46 | |
| Female | 14 | |
| KPS, % | ||
| Median | 90 | |
| Range | 70-90 | |
| Primary site | ||
| Bladder | 49 | 82 |
| Prostate | 1 | 2 |
| Renal pelvis | 5 | 8 |
| Urethra | 2 | 3 |
| Ureter | 3 | 5 |
| Metastatic site | ||
| Visceral disease | 20 | 33 |
| Lung | 11 | 18 |
| Liver | 6 | 10 |
| Bone | 6 | 10 |
| LN only | 15 | 25 |
| Unresectable primary ± local LN | 20 | 33 |
| Unresectable primary + distant LN | 2 | 3 |
| Other* | 3 | 5 |
| Risk factor†‡ | ||
| 0 | 34 | 57 |
| 1 | 24 | 40 |
| 2 | 2 | 3 |
Abbreviations: LN, lymph node; KPS, Karnofsky performance status.
One patient with urethral recurrence and two patients with local recurrence.
Risk factors are either Karnofsky performance status < 80% or evidence of visceral metastases.17
Risk factor groups are defined as follows: 0, KPS ≥ 80% and no visceral disease; 1, KPS < 80% or visceral disease; 2, KPS < 80% and visceral disease.
Drug Delivery
Sixteen patients received six cycles (12 weeks) of AG before the protocol was amended to administer five cycles (10 weeks). Five patients received only four cycles of AG, and three of these five patients had additional cycles held because of toxicity that led to the amendment. Two of the five patients received only four cycles of AG because of progressive disease and continued on to receive ITP. Forty-six patients received four cycles of ITP. Fourteen patients received less than four cycles of ITP; six of these experienced toxicity, eight experienced progression. A total of 304 courses of AG and 205 courses of ITP were administered to 60 patients. The median numbers of AG and ITP courses per patient were five (range, one to six courses) and four (range, zero to four courses). Two hundred ninety-one (96%) of 304 courses of AG and 176 (85%) of 205 courses of ITP were administered at full doses.
Toxicity
Toxicities are outlined in Table 2. Neutropenia was the most common toxicity. Forty-one patients (68%) developed grades 3 or 4 neutropenia, and febrile neutropenia was seen in 15 patients (25%). Thirteen patients (22%) underwent dose reductions for grades 3 or 4 toxicities. Three patients continued without cisplatin or ifosfamide because of renal insufficiency. Other hematologic toxicities included grades 3 or 4 anemia in 19 patients (32%) and grades 3 or 4 thrombocytopenia in 19 patients (32%). Two deaths occurred: one patient developing urosepsis after five cycles of AG and one cycle of ITP, and another patient developed nadir sepsis after self-discontinuing rhG-CSF. In addition to these two patients, three additional patients were not evaluable for response because of toxicity associated with early termination of treatment and because of early progression of disease.
Table 2.
Toxicity Assessment According to the National Cancer Institute Common Toxicity Criteria
| Toxicity | Patients by Toxicity Grade (N = 60) |
|||
|---|---|---|---|---|
| 3 |
4 |
|||
| No. | % | No. | % | |
| Neutropenia | 11 | 18 | 30 | 50 |
| Febrile neutropenia | 13 | 22 | 2 | 3 |
| Anemia | 17 | 28 | 2 | 3 |
| Thrombocytopenia | 17 | 28 | 2 | 3 |
| Bilirubin | 3 | 5 | 1 | 2 |
| Creatinine | 2 | 3 | 0 | 0 |
| Nausea | 5 | 8 | 0 | 0 |
| Vomiting | 2 | 3 | 0 | 0 |
| Diarrhea | 1 | 2 | 0 | 0 |
| Stomatitis | 3 | 5 | 2 | 3 |
| Neuropathy | 3 | 5 | 0 | 0 |
| Allergic reaction/hypersensitivity | 1 | 2 | 0 | 0 |
| Fatigue | 6 | 10 | 0 | 0 |
Nonhematologic toxicity was mild. Infusion reaction was seen in one patient (2%). Grade 3 nausea and grade 3 vomiting were seen in five patients (8%) and two patients (3%), respectively. Neurologic toxicities were mild and infrequent.
Response and Survival
There were 19 CRs and 21 PRs (73%; 95% CI, 59% to 84%) among the 55 assessable patients, and the median response duration was 11.3 months (range, 1.7 to ≥ 105.6 months; Table 3). Response assessment after AG included 13 patients with complete tumor regression and 23 with partial regression. Three patients developed progressive disease during AG. Two of the three patients with progressive disease after AG had progressive disease after ITP. Ten patients with a partial regression after AG converted to CR after ITP. Twenty-one patients with a complete or partial regression after AG had a similar response after ITP, and only two patients with complete or partial regression after AG had progressive disease after ITP.
Table 3.
Response Assessment for AG-ITP
| Response Assessment | Patients |
Response Duration (months) |
|||
|---|---|---|---|---|---|
| No. | No. Evaluable | % | Median | Range | |
| Major response | 40 | 55 | 73 | 11.3 | 1.7-105.6+ |
| CR | 19 | 55 | 35 | 12.9 | 7.2-105.6+ |
| PR | 21 | 55 | 38 | 10.2 | 1.7-68.2+ |
| Disease category* | |||||
| Locally advanced | 27 | 34 | 79 | 12.1 | 1.7-105.6+ |
| Local recurrence | 2 | 2 | 100 | 14.1† | 11.1-17.1 |
| Distant metastases | 10 | 18 | 56 | 8.2 | 3.8-63 |
Abbreviations: AG-ITP, doxorubicin plus gemcitabine followed by ifosfamide, paclitaxel, and cisplatin; CR, complete response; PR, partial response.
One patient with a local recurrence and distant metastatic disease had a PR with a response duration of 13.27 months.
Mean is 14.1 months.
For additional analysis, three patient groups were identified: 35 had primary bladder tumors that were unresectable and/or had metastatic disease to regional lymph nodes; two experienced disease recurrence in the surgical bed; and 22 had distant metastases, defined as lymph node metastases above the bifurcation of the greater vessels (n = 3) and/or visceral disease (n = 20). Twenty-seven (79%) of 34 evaluable patients with locally advanced disease (ie, T4, N0, M0) or regional lymph node involvement (ie, T3-4, N1, M0) had a major response. Two of two patients with recurrent disease confined to the surgical bed achieved a CR. Ten (56%) of 18 evaluable patients with distant metastases achieved a response. Responding sites of disease included liver (two of six patients), lung (four of eight patients), and lymph nodes (26 of 42 patients). Twelve of 28 patients with a major response after AG-ITP underwent either cystectomy or metastatecomy, and eight of 12 patients were without evidence of disease after surgery. This group of 12 patients had a median survival of 29.8 months.
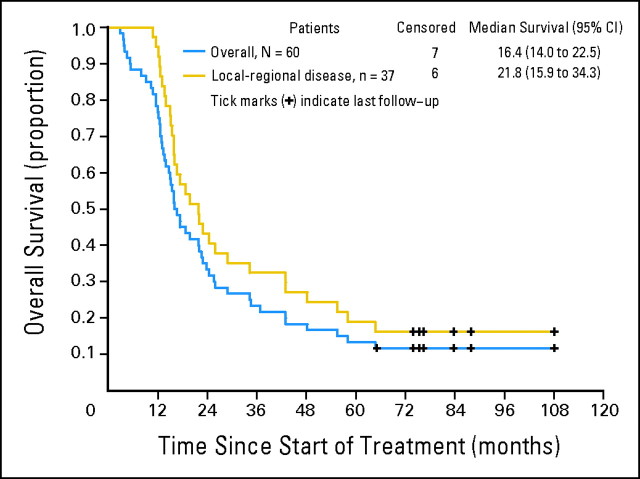
The median time to progression was 12.1 months (95% CI, 9.0 to 14.8 months). The median survival of the 60 patients was 16.4 months (95% CI, 14.0 to 22.5 months; Fig 1A). The median survival of patients with locoregional disease was 21.8 months (95% CI, 15.9 to 34.3 months; Fig 1B). During a median follow-up of 76.4 months (range, 65.1 to 108.1 months), seven patients (11.7%) remained alive and were disease free; six of these seven patients had locoregional disease.
Fig 1.
Overall survival (blue) and overall survival for local-regional disease (gold).
DISCUSSION
In this trial, the activity of AG-ITP in patients with advanced TCC was demonstrated by a 73% response rate, which included a 35% complete response rate. The median survival of 16.4 months is promising compared with the survival times associated with M-VAC (13 months) and GC (14 months); however, the median survival appears less favorable than ITP alone (20 months; Table 4). Although this type of comparison across different trials is provocative, it is severely limited by the overlapping 95% CIs. Only a randomized trial can definitively compare these different regimens with respect to survival. Despite this limitation, there appears to be a plateau of activity, for which no regimen is clearly better than another.
Table 4.
Survival Comparison for GC, M-VAC, ITP, and AG-ITP Regimens
| Variable | Treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| GC4,5 |
M-VAC27 |
ITP12 |
AG-ITP |
|||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| No. of patients | 203 | 133 | 44 | 60 | ||||
| Survival, months | ||||||||
| 6 | 82 | NR | 83 | 77 to 90 | 95 | 89 to 100 | 88 | 77 to 94 |
| 12 | 58 | 52 to 65 | 54 | 46 to 63 | 66 | 52 to 80 | 77 | 64 to 85 |
| 18 | 37 | NR | 37 | 28 to 45 | 54 | 40 to 69 | 45 | 32 to 57 |
| 24 | 25 | 19 to 31 | 30 | 22 to 37 | 44 | 29 to 59 | 33 | 22 to 45 |
| Median survival, months | 14 | 13.4 | 20 | 16.4 | ||||
| Progression-free survival, months | ||||||||
| Median | 7.7 | 9.6 | 9.6 | 12.1 | ||||
| 95% CI | 6.8 to 8.8 | 8.0 to 11.0 | 6.5 to 16.3 | 9.0 to 14.8 | ||||
| Follow-up, months | ||||||||
| Median | NR | 39.8 | 28 | 76.4 | ||||
| Range | NR | 0.3-129 | 19-43 | 65-108 | ||||
Abbreviations: M-VAC, methotrexate, vinblastine, doxorubicin, cisplatin; GC, gemcitabine, cisplatin; ITP, ifosfamide, paclitaxel, cisplatin; AG-ITP, doxorubicin, gemcitabine, ifosfamide, paclitaxel, cisplatin; NR, not reported.
Factors other than chemotherapy must be considered when comparing these trials. An evaluation of prognostic factors to predict survival of patients receiving M-VAC chemotherapy include KPS (< or ≥ 80) and the presence or absence of visceral metastases.17 The median survival times for patients with zero, one, or two risk factors treated with M-VAC were 33, 13.4, and 9.3 months, respectively. The patient characteristics are almost identical with respect to KPS less than 80% and visceral metastases in this trial and in the phase II trial of ITP alone (this trial v phase II trial data: zero risk factors, 57% v 55%; one risk factor, 41% v 40%; and two risk factors, 5% v 3%). Subset analysis confirmed our previous findings that median survival corresponds to the number of risk factors. Patients with zero risks or with one risk factor who were treated with AG-ITP had median survival times of 20.8 and 14.0 months, respectively. Two patients with two risk factors survived 5.3 and 5.4 months.
The use of postchemotherapy surgery is another potential confounding factor in comparisons across trials. Postchemotherapy surgery was similar in this trial and the trial of ITP alone, in which 12 and 14 patients, respectively, underwent consolidation surgery.12 In this trial, 12 of 28 patients with a major response after AG-ITP underwent either cystectomy or metastatecomy, and eight of 12 patients were without evidence of disease after surgery. This group had a median survival of 29.8 months. This experience is consistent with previous observations that, in properly selected patients with a major response to chemotherapy, surgical consolidation can be associated with long-term survival.18
Despite the substantial activity after AG and the absence of significant toxicity, the addition of AG does not appear to have improved upon results seen with ITP alone. This observation may suggest that, despite the activity seen after AG, it is ITP that is most important in determination of outcome. Alternatively, there may not be a benefit associated with dose-dense and/or sequential chemotherapy in metastatic TCC. The uses of dose-dense and sequential chemotherapy have been evaluated in other malignancies. In a randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive breast cancer, dose-dense treatment, but not sequential therapy, led to an improvement in disease-free survival.19 In studies of TCC, dose-intensification of M-VAC by the use of growth factor support demonstrated modest improvements in dose delivery without a substantial improvement in the CR rate, which suggests that there is little or no impact on survival.20–25 Thus, it is certainly plausible that neither sequential nor dose-dense therapy has a role in patients with metastatic TCC.
The conclusion that more chemotherapy is not necessarily better chemotherapy has been borne out in the recently reported randomized trial in which GC was compared with paclitaxel, cisplatin, and gemcitabine; a higher overall response rate for paclitaxel, cisplatin, and gemcitabine did not translate into improved progression-free or overall survivals.26 In exploratory subgroup analyses, a benefit was seen for patients with bladder primaries who received triplet therapy, and the benefit of triplet therapy appeared to be limited to patients with zero or one risk factor. Thus, more intensive chemotherapy does not appear to be associated with an improvement in survival for the entire patient population; however, subgroups who do derive a benefit may exist.
This trial and its predecessors still fail to answer the dilemma facing those clinicians who treat patients with advanced TCC. Although encouraging activity has been seen with various permutations of two- and three-drug combinations, the optimal regimen for the management of all patients remains uncertain. This trial does not support the use of sequential chemotherapy with AG-ITP compared with standard doublet therapy, triplet combinations, or M-VAC. However, it is unlikely that there will be a one-regimen-fits-all approach, so a clearer understanding of the biology of the disease and the identification of additional patient-related factors that predict response are needed. Although the higher CR rate with regimens such as ITP and AG-ITP may improve the possibility of postchemotherapy surgery, the majority of patients with metastatic disease will experience relapse and will die as a result of their disease.
We, like others, have focused our attention on the investigation of novel therapies, including those that target the angiogenic pathway. On the basis of significant activity of sunitinib, sorafenib, and bevacizumab in other tumor types and on the basis of multiple lines of evidence that support a role for angiogenesis in bladder cancer, we are exploring these novel agents in patients with metastatic TCC. At this time, there is no clear role for the use of sequential and/or dose-dense chemotherapy for patients with advanced TCC, and the standard therapy for patients with normal renal function should continue to be a cisplatin-based regimen. Research on newer targeted agents is clearly warranted to improve outcomes.
Footnotes
Supported in part by Eli Lilly and Bristol-Myers Squibb.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Dean F. Bajorin, Eli Lilly (C), Bristol-Myers Squibb (C), GPC Biotech (C) Stock Ownership: David M. Nanus, Amgen Honoraria: None Research Funding: Matthew I. Milowsky, Bristol-Myers Squibb; Dean F. Bajorin, Eli Lilly, Bristol-Myers Squibb, GPC Biotech, Genentech, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Dean F. Bajorin
Provision of study materials or patients: Matthew I. Milowsky, David M. Nanus, Fernando C. Maluf, Dean F. Bajorin
Collection and assembly of data: Matthew I. Milowsky, David M. Nanus, Fernando C. Maluf, Weiji Shi, Alexia Iasonos, Jamie Riches, Ashley Regazzi, Dean F. Bajorin
Data analysis and interpretation: Matthew I. Milowsky, Fernando C. Maluf, Svetlana Mironov, Weiji Shi, Alexia Iasonos, Jamie Riches, Ashley Regazzi, Dean F. Bajorin
Manuscript writing: Matthew I. Milowsky, Weiji Shi, Jamie Riches, Dean F. Bajorin
Final approval of manuscript: Matthew I. Milowsky, David M. Nanus, Fernando C. Maluf, Svetlana Mironov, Weiji Shi, Alexia Iasonos, Jamie Riches, Ashley Regazzi, Dean F. Bajorin
REFERENCES
- 1.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 2.Logothetis CJ, Dexeus F, Sella A, et al. A prospective randomized trial comparing CISCA to MVAC chemotherapy in advanced metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–1055. doi: 10.1200/JCO.1990.8.6.1050. [DOI] [PubMed] [Google Scholar]
- 3.Saxman S, Propert K, Einhorn L, et al. Long term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine and doxorubicin in patients with metastatic urothelial cancer: A cooperative group study. J Clin Oncol. 1997;15:2564–2569. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 4.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 5.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 6.Roth BJ, Dreicer R, Einhorn LH, et al. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: A phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 1994;12:2264–2270. doi: 10.1200/JCO.1994.12.11.2264. [DOI] [PubMed] [Google Scholar]
- 7.Gad-el-Mawla N, Hamza MR, Zikri ZK, et al. Chemotherapy in invasive carcinoma of the bladder: A review of phase II trials in Egypt. Acta Oncol. 1989;28:73–76. doi: 10.3109/02841868909111185. [DOI] [PubMed] [Google Scholar]
- 8. Reference deleted.
- 9.Pronzato P, Vigani A, Pensa F, et al. Second-line chemotherapy with ifosfamide as outpatient treatment for advanced bladder cancer. Am J Clin Oncol. 1997;20:519–521. doi: 10.1097/00000421-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Witte RS, Elson P, Bono B, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol. 1997;15:589–593. doi: 10.1200/JCO.1997.15.2.589. [DOI] [PubMed] [Google Scholar]
- 11.Bajorin DF, McCaffrey JA, Hilton S, et al. Treatment of patients with transitional-cell carcinoma of the urothelial tract with ifosfamide, paclitaxel, and cisplatin: A phase II trial. J Clin Oncol. 1998;16:2722–2727. doi: 10.1200/JCO.1998.16.8.2722. [DOI] [PubMed] [Google Scholar]
- 12.Bajorin DF, McCaffrey JA, Dodd PM, et al. Ifosfamide, paclitaxel, and cisplatin for patients with advanced transitional cell carcinoma of the urothelial tract: Final report of a phase II trial evaluating two dosing schedules. Cancer. 2000;88:1671–1678. [PubMed] [Google Scholar]
- 13.Norton L, Simon R. The Norton-Simon hypothesis revisited. Cancer Treat Rep. 1986;70:163–169. [PubMed] [Google Scholar]
- 14.Norton L. Conceptual basis for advances in the systemic drug therapy of breast cancer. Semin Oncol. 1997;24:S11–S12. [PubMed] [Google Scholar]
- 15.Dodd PM, McCaffrey JA, Hilton S, et al. Phase I evaluation of sequential doxorubicin gemcitabine then ifosfamide paclitaxel cisplatin for patients with unresectable or metastatic transitional-cell carcinoma of the urothelial tract. J Clin Oncol. 2000;18:840–846. doi: 10.1200/JCO.2000.18.4.840. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 17.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 18.Donat SM, Herr HW, Bajorin DF, et al. Methotrexate, vinblastine, doxorubicin and cisplatin chemotherapy and cystectomy for unresectable bladder cancer. J Urol. 1996;156:368–371. doi: 10.1097/00005392-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 20.Logothetis CJ, Finn LD, Smith T, et al. Escalated MVAC with or without recombinant human granulocyte-macrophage colony-stimulating factor for the initial treatment of advanced malignant urothelial tumors: Results of a randomized trial. J Clin Oncol. 1995;13:2272–2277. doi: 10.1200/JCO.1995.13.9.2272. [DOI] [PubMed] [Google Scholar]
- 21.Seidman AD, Scher HI, Gabrilove JL, et al. Dose-intensification of MVAC with recombinant granulocyte colony-stimulating factor as initial therapy in advanced urothelial cancer. J Clin Oncol. 1993;11:408–414. doi: 10.1200/JCO.1993.11.3.408. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilove JL, Jakubowski A, Scher H, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988;318:1414–1422. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- 23.Moore MJ, Tannock IF, Iscoe N, et al. A phase II study of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) + GM-CSF in patients with advanced transitional cell carcinoma. Proc Am Soc Clin Oncol. 1992;11:199a. doi: 10.1016/s0022-5347(17)35706-3. [DOI] [PubMed] [Google Scholar]
- 24.Loehrer PJ, Sr, Elson P, Dreicer R, et al. Escalated dosages of methotrexate, vinblastine, doxorubicin, and cisplatin plus recombinant human granulocyte colony-stimulating factor in advanced urothelial carcinoma: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 1994;12:483–488. doi: 10.1200/JCO.1994.12.3.483. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg CN, de Mulder PH, van Oosterom AT, et al. Escalated M-VAC chemotherapy and recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF) in patients with advanced urothelial tract tumors. Ann Oncol. 1993;4:403–407. doi: 10.1093/oxfordjournals.annonc.a058520. [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial cancer without prior systemic therapy: EORTC 30987/Intergroup Study. J Clin Oncol. 2007;25(suppl):242s. doi: 10.1200/JCO.2011.38.6979. abstr LBA5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternberg C, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin and cisplatin for advanced transitional cell carcinoma of the urothelium. Cancer. 1989;64:2448–2458. doi: 10.1002/1097-0142(19891215)64:12<2448::aid-cncr2820641209>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]