Abstract
Purpose
Citrus products are widely consumed foods that are rich in psoralens and furocoumarins, a group of naturally occurring chemicals with potential photocarcinogenic properties. We prospectively evaluated the risk of cutaneous malignant melanoma associated with citrus consumption.
Methods
A total of 63,810 women in the Nurses' Health Study (1984 to 2010) and 41,622 men in the Health Professionals Follow-Up Study (1986 to 2010) were included. Dietary information was repeatedly assessed every 2 to 4 years during follow-up. Incident melanoma cases were identified through self-report and confirmed by pathologic records.
Results
Over 24 to 26 years of follow-up, we documented 1,840 incident melanomas. After adjustment for other risk factors, the pooled multivariable hazard ratios for melanoma were 1.00 for overall citrus consumption < twice per week (reference), 1.10 (95% CI, 0.94 to 1.30) for two to four times per week, 1.26 (95% CI, 1.08 to 1.47) for five to six times per week, 1.27 (95% CI, 1.09 to 1.49) for once to 1.5 times per day, and 1.36 (95% CI, 1.14 to 1.63) for ≥ 1.6 times per day (Ptrend < .001). Among individual citrus products, grapefruit showed the most apparent association with risk of melanoma, which was independent of other lifestyle and dietary factors. The pooled multivariable hazard ratio for melanoma comparing the extreme consumption categories of grapefruit (≥ three times per week v never) was 1.41 (95% CI, 1.10 to 1.82; Ptrend < .001).
Conclusion
Citrus consumption was associated with an increased risk of malignant melanoma in two cohorts of women and men. Nevertheless, further investigation is needed to confirm our findings and explore related health implications.
INTRODUCTION
Malignant melanoma is a potentially lethal form of cutaneous malignancy, the incidence of which has been increasing for at least 30 years in the United States.1 Citrus products are rich sources of psoralens, a group of naturally occurring furocoumarins (furanocoumarins).2–5 Photochemotherapy using oral psoralen (methoxsalen) and ultraviolet (UV) A radiation (PUVA) is a highly effective therapy for severe psoriasis.6 PUVA takes advantage of the high UV absorbance of psoralen. Psoralen is first taken orally to sensitize the skin, and the skin is then exposed to UVA light to treat the cutaneous problem therapeutically. However, photocarcinogenic properties of psoralens and furocoumarins have been demonstrated in many experimental studies.7–12 PUVA induced melanocytic tumors in a mouse model12 and can contribute to the pathogenesis of melanoma by exerting a stimulatory effect on the outgrowth of melanoma cells.13 Epidemiologic evidence also suggests that long-term PUVA therapy increases the risk of melanoma.14,15 Psoralens had been used as tanning activators until 1996, and individuals who used psoralen tanning activators had a higher risk of melanoma.16 Psoralen-containing sunscreen users also had a substantially increased risk of melanoma as compared with regular sunscreen users.17 As a result, strict regulations have been imposed on psoalen-containing suntan lotions and cosmetic products in recent years.16,18
However, whether dietary consumption of psoralen-rich foods may increase melanoma risk is unknown, and whether the public should be advised about dietary psoralens remains a question.5 In our earlier investigation of antioxidant nutrient intake and melanoma risk in the Nurses' Health Study (NHS),19 we observed an unexpected higher risk in women who consumed more orange juice and vitamin C from foods, but not from supplements. Because vitamin C has preferential toxicity for melanoma cells,20 induces apoptosis in melanoma cells via multiple pathways,21,22 and suppresses proliferation of melanoma cells,23 we hypothesized that the increased melanoma risk associated with dietary vitamin C, but not with supplemental vitamin C, was likely the effect of other components (eg, photoactive compounds) in vitamin C–rich foods.
To address the hypothesis that the consumption of psoralen-rich foods (ie, citrus products) may be associated with an increased risk of cutaneous malignant melanoma, we expanded our previous analysis to comprehensively examine citrus consumption in relation to risk of melanoma with extended follow-up of 24 to 26 years in two large US cohorts of women and men.
METHODS
Study Population
Our study population consisted of participants from two ongoing cohorts: the NHS and Health Professionals Follow-Up Study (HPFS). The NHS was established in 1976, when 121,700 married, registered female nurses between the ages of 30 and 55 years and residing in the United States at the time of enrollment responded to a baseline questionnaire that included questions about their medical history and lifestyle risk factors. The HPFS was established in 1986, when 51,529 male health professionals between the ages of 40 and 75 years completed a baseline questionnaire. Information on medical history and lifestyle factors was collected biennially via mailed questionnaires. Dietary intake was assessed using a validated food frequency questionnaire (FFQ) at least every 4 years. The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard School of Public Health. Completion of the self-administered questionnaire was considered to imply informed consent.
We observed participants for incident melanoma starting from 1984 in the NHS and 1986 in the HPFS, when the participants completed a baseline FFQ with detailed information on citrus consumption. A total of 81,685 women and 49,617 men completed the dietary questionnaire at baseline. Study participants with a baseline history of any cancer (including nonmelanoma skin cancers) were excluded. Because of low risk and small numbers, nonwhite participants were also excluded. After exclusions, 63,810 women and 41,622 men (N = 105,432) with complete dietary data at baseline remained in our analysis.
Assessment of Dietary Consumption
In all FFQs, participants were asked how often on average (never to ≥ six servings per day) during the previous year they had consumed grapefruit (half), oranges (one), and grapefruit and orange juices (one small glass [6 oz]). Overall citrus consumption was calculated as the sum of these individual products. Grapefruit and grapefruit juice were asked as a single item in the 2002 and 2006 FFQs. Dietary intake collected using the FFQ has been shown to be a valid estimator of relative food intake when compared with multiple diet records.24,25 The correlation coefficients ranged from 0.75 to 0.84 for the correlations between intake of individual citrus products assessed in the baseline FFQ and intake assessed in two 1-week dietary records.24 Vitamin C content in citrus products was derived from US Department of Agriculture sources.26 Information on other dietary factors, including intake of total energy, alcohol, coffee, vegetables, and other fruits and juices, was also collected during follow-up.
Assessment of Covariates
In the biennial follow-up questionnaires, we inquired about and updated information on anthropometric and lifestyle factors for chronic diseases, including body height and weight, cigarette smoking, physical activity, vitamin C from supplements, and menopausal status and postmenopausal hormone use among women. Data on the following host and sun exposure characteristics were also collected through questionnaires27: family history of melanoma, natural hair color, number of moles on arms, skin reaction to sun exposure for ≥ 2 hours as a child or adolescent, number of lifetime blistering sunburns, cumulative UV flux exposure since baseline, and average time spent in direct sunlight since high school.
Ascertainment of Patient Cases of Melanoma
Participants reported new diagnoses of melanoma biennially. We sought permission from these participants to acquire their medical and pathologic reports, which were reviewed by study physicians to validate the diagnoses. Information on tumor stage and location was also retrieved if available. In situ melanomas were defined as early-stage tumors restricted to the epidermis, and invasive melanomas were defined as those that had grown into the dermis or surrounding tissues. Melanomas were further classified into the following two subgroups according to tumor location: melanomas on body sites with higher continuous sun exposure, including head, neck, and extremities, and melanomas on body sites with lower continuous sun exposure, including truncal sites, shoulder, back, hip, abdomen, and chest. We documented 1,840 incident patient cases of cutaneous melanoma over 2 million person-years of follow-up (NHS: 1,023 patient cases and 1,290,954 person-years; HPFS: 817 patient cases and 711,479 person-years). These included 949 invasive melanomas and 891 melanomas in situ, among which 1,154 (62.1%) occurred on the head, neck, or extremities and 614 (33.1%) occurred on truncal sites. Mucosal melanomas and acral melanomas were excluded from the analysis because of potential heterogeneous etiologies. Median tumor thickness (measured as Breslow thickness) was 0.63 mm for invasive melanomas (n = 814). Invasive melanomas occurring on the head, neck, or extremities (median thickness, 0.67 mm) were slightly thicker than those occurring on truncal sites (median thickness, 0.60 mm).
Statistical Analysis
Dietary consumption was cumulatively updated in analyses to create the best estimates of long-term intake and minimize within-person variation. That is, at the beginning of every 2-year follow-up cycle, each dietary intake was calculated as the mean of all reported intake up to that time. Because grapefruit and grapefruit juice were asked as a single item in the 2002 and 2006 FFQs, analyses for separate grapefruit and grapefruit juice used cumulative updated intake up to 1998 for the subsequent follow-up. As a sensitivity analysis, we created a new intake variable for combined intake of grapefruit and grapefruit juice. Each participant contributed person-time from the return month of the baseline questionnaire to the date of the first diagnosis of any cancer, date of death, or end of follow-up (June 2010 for women; January 2010 for men), whichever came first.
We used Cox proportional hazards models to estimate the hazard ratios (HRs), with 95% CIs, of melanoma associated with dietary consumption. Multivariable analyses were adjusted for other known melanoma risk factors and potential confounders. A detailed justification for the covariates included in the analysis is shown in the Data Supplement. Trend tests across categories of dietary consumption were performed by assigning median values for these categories and treating the new variable as a continuous term in the models.
We performed several sensitivity analyses to examine the robustness of the results. To assess the influence of common medications (statins and antiarrhythmic agents) that have been reported to interact with grapefruit,28 total vitamin C, adherence to a healthy diet (as assessed by Alternate Healthy Eating Index 2010),29 and sunscreen use, we performed separate analyses with adjustment for each of these variables. A propensity score analysis was performed to reduce the confounding effects from other covariates in the evaluation of association between exposure and outcome.30 To address the concern about reverse causality between dietary assessment and cancer diagnosis as well as to identify any potential timing effect, we performed lag analyses with different intervals from 2 to 14 years between dietary assessment and cohort follow-up. To test whether the positive association with melanoma was specific to citrus products, we examined the melanoma risk associated with consumption of other fruits and juices and vegetables (39 items). We also performed subgroup analyses stratified by potential confounders. For the sex-specific, lagged and stratified analyses, we combined the two highest categories of intake to maintain statistical power.
HRs among women and men were pooled using a random-effects model, given the similar associations. P values for heterogeneity were calculated using the Q statistic. We used SAS software (version 9.2; SAS Institute, Cary, NC) for all statistical analyses. All statistical tests were two tailed, and the significance level was set at P < .05.
RESULTS
Participants with higher citrus intake were less likely to smoke cigarettes and drink coffee, more likely to exercise, and had higher intake of individual citrus products and vitamin C (Table 1; Data Supplement). In contrast, there was no appreciable difference in sun exposure–related variables and other host risk factors over the intake categories. Citrus consumption remained relatively constant over follow-up (Data Supplement). Correlations between intake of individual citrus products were generally low (Data Supplement).
Table 1.
Characteristics of Person-Years According to Frequency of Overall Citrus Consumption*
| Characteristic | Frequency (mean ± SD) |
||||
|---|---|---|---|---|---|
| < Twice per Week | Two to Four Times per Week | Five to Six Times per Week | Once to 1.5 Times per Day | ≥ 1.6 Times per Day | |
| Age, years | 58.2 ± 9.8† | 60.0 ± 9.9 | 61.2 ± 10.0 | 61.0 ± 10.2 | 61.2 ± 10.1 |
| Family history of melanoma, % | 5.6 | 5.9 | 6.2 | 6.2 | 5.7 |
| Red or blonde hair, % | 14.0 | 14.2 | 14.2 | 14.4 | 14.8 |
| Arm with moles, % | 33.9 | 35.3 | 35.7 | 35.8 | 35.4 |
| Painful burn or blistering sun reaction as child or adolescent, % | 17.5 | 16.7 | 16.7 | 16.2 | 16.7 |
| No. of lifetime blistering sunburns | 10.0 ± 9.2 | 10.0 ± 9.0 | 10.0 ± 9.1 | 9.8 ± 9.0 | 10.0 ± 9.5 |
| Annual UV flux at residence (× 10−4 Robertson-Berger units)† | 128 ± 27 | 126 ± 27 | 125 ± 26 | 123 ± 25 | 123 ± 25 |
| Average time spent in direct sunlight since high school, hours per week | 6.1 ± 4.2 | 6.2 ± 4.2 | 6.2 ± 4.2 | 6.2 ± 4.2 | 6.5 ± 4.4 |
| Body mass index, kg/m2 | 26.2 ± 5.0 | 26.5 ± 5.0 | 26.4 ± 4.9 | 25.9 ± 4.7 | 25.7 ± 4.6 |
| Physical activity, metabolic equivalents per week | 19.0 ± 28.7 | 21.3 ± 28.9 | 23.1 ± 30.4 | 24.0 ± 31.0 | 29.0 ± 36.6 |
| Current smoker, % | 17.0 | 11.9 | 9.4 | 8.8 | 8.5 |
| Dietary intake | |||||
| Grapefruit, servings per day | 0.03 ± 0.04 | 0.07 ± 0.09 | 0.13 ± 0.14 | 0.16 ± 0.20 | 0.33 ± 0.37 |
| Grapefruit juice, servings per day | 0.01 ± 0.03 | 0.03 ± 0.07 | 0.06 ± 0.12 | 0.09 ± 0.18 | 0.22 ± 0.45 |
| Oranges, servings per day | 0.06 ± 0.05 | 0.14 ± 0.12 | 0.21 ± 0.18 | 0.25 ± 0.24 | 0.48 ± 0.48 |
| Orange juice, servings per day | 0.06 ± 0.06 | 0.20 ± 0.14 | 0.39 ± 0.24 | 0.71 ± 0.33 | 1.12 ± 0.78 |
| Total energy, kcal per day | 1,661 ± 495 | 1,754 ± 482 | 1,838 ± 487 | 1,913 ± 495 | 2,094 ± 529 |
| Alcohol, g per day | 8.0 ± 14.1 | 7.4 ± 12.4 | 7.3 ± 11.8 | 7.8 ± 12.0 | 8.0 ± 12.4 |
| Coffee, cups per day | 1.6 ± 1.6 | 1.5 ± 1.5 | 1.4 ± 1.4 | 1.3 ± 1.4 | 1.2 ± 1.4 |
| Total vitamin C intake, mg per day | 295 ± 355 | 332 ± 343 | 367 ± 338 | 407 ± 354 | 513 ± 390 |
| From diet | 91 ± 45 | 121 ± 41 | 152 ± 43 | 182 ± 47 | 258 ± 79 |
| From supplements | 204 ± 349 | 211 ± 338 | 216 ± 333 | 225 ± 348 | 255 ± 377 |
Abbreviations: SD, standard deviation; UV, ultraviolet.
All variables other than age have been standardized to age distribution of cohort. Separate characteristics for women and men are shown in Data Supplement.
Estimate of amount of UV radiation reaching Earth's surface of residence within 1 year.
The association between overall citrus consumption and melanoma risk appeared in an exposure-dependent manner in the pooled analysis (Ptrend < .001; Table 2) and was generally consistent among women and men (Data Supplement). Separate analyses for individual citrus products found that the positive association between grapefruit consumption and melanoma risk was most apparent. Orange juice consumption also showed a significant but less apparent association with melanoma risk. Consumption of grapefruit juice and oranges was generally not associated with melanoma risk.
Table 2.
Pooled HRs for Incident Melanoma According to Frequency of Citrus Consumption
| Citrus Type | Serving Category |
Ptrend | ||||
|---|---|---|---|---|---|---|
| < Twice per Week | Two to Four Times per Week | Five to Six Times per Week | Once to 1.5 Times per Day | ≥ 1.6 Times per Day | ||
| Overall citrus | ||||||
| No. of person-years | 404,850 | 376,476 | 471,647 | 491,559 | 257,901 | |
| No. of cases | 260 | 323 | 496 | 488 | 273 | |
| Age-adjusted HR (95% CI) | 1.00 | 1.19 (1.01 to 1.40) | 1.38 (1.19 to 1.61) | 1.40 (1.20 to 1.63) | 1.47 (1.24 to 1.75) | < .001 |
| Multivariable-adjusted HR (95% CI)* | 1.00 | 1.10 (0.94 to 1.30) | 1.26 (1.08 to 1.47) | 1.27 (1.09 to 1.49) | 1.36 (1.14 to 1.63) | < .001 |
NOTE. Bold font indicates significance.
Abbreviation: HR, hazard ratio.
Multivariable analyses were further adjusted for family history of melanoma (yes v no); natural hair color (red, blonde, light brown, dark brown, black, or missing); No. of arm moles (zero, one to two, three to nine, ≥ 10, or missing); sunburn susceptibility as child or adolescent (none or some redness, burn, painful burn or blisters, or missing); No. of lifetime blistering sunburns (zero, one to four, five to nine, ≥ 10, or missing); cumulative ultraviolet flux since baseline (quintiles or missing); average time spent in direct sunlight since high school (< 2, 2 to 5, 6 to 9, or ≥ 10 hours per week or missing); body mass index (< 25.0, 25.0 to 29.9, 30.0 to 34.9, or ≥ 35.0 kg/m2); physical activity (quintiles or missing); smoking status (never, past, current with one to 14, 15 to 24, or ≥ 25 cigarettes per day or missing); and intake of total energy (quintiles or missing), alcohol (0, 0.1 to 4.9, 5.0 to 9.9, 10.0 to 19.9, or ≥ 20.0 g per day or missing), coffee (zero, < one, one to two, or ≥ three cups per day or missing), and vitamin C from supplements (0, 1 to 99, 100 to 299, 300 to 599, or ≥ 600 mg per day or missing). Analyses for women were also adjusted for menopausal status and postmenopausal hormone use (premenopausal, postmenopausal never, past, or current use, or missing). Results among women and men were pooled using random-effects model. Separate results in women and men are shown in Data Supplement.
Multivariable analyses were additionally adjusted for consumption of other individual citrus products listed in table.
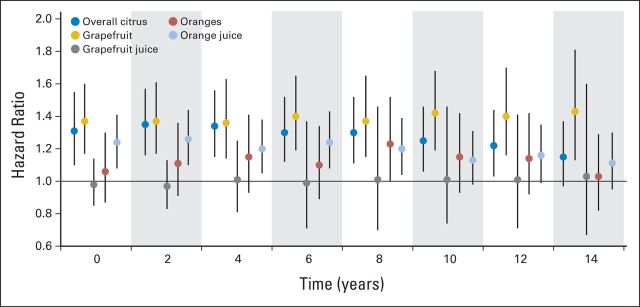
The association between citrus consumption and melanoma risk remained essentially unchanged when we adjusted for other potential confounders (Data Supplement). Lag analyses suggested associations between citrus consumption and subsequent melanoma generally similar to those from the main analyses (Fig 1; Data Supplement). The significant positive association between citrus consumption and subsequent melanoma persisted for 12 years after intake for overall citrus and 8 years after intake for orange juice; it remained constant for grapefruit over different lags (Fig 1). However, we did not find any consistent significant association between consumption of other fruits and juices and vegetables and melanoma risk (data not shown).
Fig 1.
Hazard ratios (HRs) for incident melanomas comparing extreme citrus consumption categories at lags from 0 to 14 years. HRs were computed for overall citrus consumption comparing ≥ once per day versus < twice per week, for grapefruit and grapefruit juice consumption comparing ≥ twice per week versus never, for orange consumption comparing ≥ three times per week versus never, and for orange juice consumption comparing ≥ five times per week versus < once per week. In lagged analyses, we added different intervals from 2 to 14 years between dietary assessment and cohort follow-up (eg, we used citrus consumption from 1984 questionnaire for follow-up from 1986 to 1988 for 2-year lag analysis and for follow-up from 1988 to 1990 for 4-year lag analysis). Multivariable HRs were adjusted for age, family history of melanoma, natural hair color, No. of arm moles, sunburn susceptibility as child or adolescent, No. of lifetime blistering sunburns, cumulative ultraviolet flux since baseline, average time spent in direct sunlight since high school, body mass index, physical activity, smoking status, and intake of total energy, alcohol, coffee, and vitamin C from supplements. Analyses for consumption of each individual citrus product were also adjusted for consumption of other individual citrus products. Analyses for women were also adjusted for menopausal status and postmenopausal hormone use (Table 2). Results among women and men were pooled using random-effects model. HRs for other categories of citrus consumption in lagged analyses are shown in Data Supplement. Vertical lines represent 95% CIs; x-axis indicates years of lag.
Subgroup analyses suggested that the association between grapefruit consumption and melanoma risk was generally independent of age, lifestyle, and dietary confounders (Table 3) and known melanoma risk factors (P > .10 for all interaction tests; Data Supplement). Nevertheless, the positive association seemed to be more apparent among those who had higher sunburn susceptibility as a child or adolescent, those with a higher number of blistering sunburns, those who spent more time in direct sunlight, and those with higher annual UV flux at residence. Additional subgroup analyses revealed that the association between grapefruit and melanoma was also independent of consumption of other fruits and juices and vegetables and was not affected by physical screening status during the previous 2 years (indicator of health awareness; Data Supplement).
Table 3.
Pooled Multivariable HRs for Incident Melanoma According to Frequency of Grapefruit Consumption in Subgroups of Potential Confounders*
| Potential Confounder | Grapefruit Serving Category |
Ptrend | Pinteraction† | |||
|---|---|---|---|---|---|---|
| Never | < Once per Week | Once per Wek | ≥ Twice per Week | |||
| Age group, years | ||||||
| < 65 | ||||||
| No. of person-years | 386,342 | 443,482 | 237,215 | 272,430 | ||
| No. of cases | 180 | 314 | 167 | 219 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.23 (1.01 to 1.50) | 1.28 (1.02 to 1.62) | 1.38 (1.10 to 1.73) | .01 | .82 |
| ≥ 65 | ||||||
| No. of person-years | 128,820 | 203,543 | 117,517 | 213,084 | ||
| No. of cases | 137 | 275 | 186 | 362 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.10 (0.89 to 1.37) | 1.31 (1.03 to 1.66) | 1.33 (1.07 to 1.66) | .002 | |
| Body mass index, kg/m2 | ||||||
| < 30 | ||||||
| No. of person-years | 423,239 | 529,285 | 293,553 | 402,710 | ||
| No. of cases | 276 | 487 | 300 | 490 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.10 (0.94 to 1.29) | 1.23 (1.03 to 1.47) | 1.28 (1.08 to 1.52) | .001 | .39 |
| ≥ 30 | ||||||
| No. of person-years | 87,025 | 114,189 | 59,136 | 80,721 | ||
| No. of cases | 41 | 100 | 53 | 89 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.64 (1.11 to 2.43) | 1.71 (1.08 to 2.69) | 1.88 (1.22 to 2.88) | .02 | |
| Physical activity | ||||||
| < Median | ||||||
| No. of person-years | 283,031 | 317,106 | 156,235 | 195,401 | ||
| No. of cases | 155 | 259 | 123 | 213 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.20 (0.74 to 1.94) | 1.16 (0.68 to 1.98) | 1.46 (1.03 to 2.05) | .008 | .90 |
| > Median | ||||||
| No. of person-years | 206,457 | 313,570 | 189,110 | 281,741 | ||
| No. of cases | 157 | 329 | 228 | 363 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.17 (0.83 to 1.65) | 1.39 (0.86 to 2.24) | 1.33 (1.07 to 1.64) | .003 | |
| Smoking status | ||||||
| Never | ||||||
| No. of person-years | 205,283 | 293,329 | 166,494 | 221,425 | ||
| No. of cases | 132 | 279 | 166 | 267 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.22 (0.91 to 1.62) | 1.31 (1.02 to 1.68) | 1.39 (1.07 to 1.82) | .01 | .72 |
| Ever | ||||||
| No. of person-years | 269,419 | 323,488 | 172,901 | 243,694 | ||
| No. of cases | 172 | 289 | 178 | 304 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.11 (0.91 to 1.36) | 1.29 (1.03 to 1.62) | 1.38 (1.11 to 1.71) | .001 | |
| Alcohol intake | ||||||
| < Median | ||||||
| No. of person-years | 275,932 | 321,995 | 166,834 | 228,432 | ||
| No. of cases | 155 | 268 | 136 | 239 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.22 (0.99 to 1.50) | 1.20 (0.93 to 1.54) | 1.37 (1.09 to 1.73) | .02 | .64 |
| > Median | ||||||
| No. of person-years | 239,231 | 325,029 | 187,898 | 257,081 | ||
| No. of cases | 162 | 321 | 217 | 342 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.14 (0.94 to 1.40) | 1.38 (1.11 to 1.73) | 1.37 (1.11 to 1.70) | .001 | |
| Coffee intake, cups per day | ||||||
| Low (< 1.0) | ||||||
| No. of person-years | 227,043 | 282,630 | 157,097 | 225,217 | ||
| No. of cases | 144 | 257 | 173 | 285 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.13 (0.91 to 1.40) | 1.40 (1.10 to 1.78) | 1.37 (1.09 to 1.72) | .002 | .64 |
| High (≥ 1.0) | ||||||
| No. of person-years | 288,120 | 364,394 | 197,635 | 260,298 | ||
| No. of cases | 173 | 332 | 180 | 296 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.20 (0.99 to 1.46) | 1.23 (0.98 to 1.54) | 1.38 (1.12 to 1.71) | .008 | |
| Vitamin C from supplements | ||||||
| < Median | ||||||
| No. of person-years | 283,462 | 324,903 | 169,728 | 214,162 | ||
| No. of cases | 152 | 253 | 135 | 223 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.16 (0.94 to 1.44) | 1.21 (0.94 to 1.56) | 1.40 (1.11 to 1.77) | .01 | .97 |
| > Median | ||||||
| No. of person-years | 231,702 | 322,120 | 185,005 | 271,353 | ||
| No. of cases | 165 | 336 | 218 | 358 | ||
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.19 (0.97 to 1.44) | 1.36 (1.10 to 1.70) | 1.36 (1.11 to 1.68) | .002 | |
Abbreviation: HR, hazard ratio.
Multivariable analyses were adjusted for age, family history of melanoma, natural hair color, No. of arm moles, sunburn susceptibility as child or adolescent, No. of lifetime blistering sunburns, cumulative ultraviolet flux since baseline, average time spent in direct sunlight since high school, body mass index, physical activity, smoking status, and intake of total energy, alcohol, coffee, vitamin C from supplements, and other individual citrus products (grapefruit juice, oranges, and orange juice). Analyses for women were also adjusted for menopausal status and postmenopausal hormone use. For each stratified analysis, stratifying variable was omitted from model, except for age. Results among women and men were pooled using random-effects model.
For pooled analysis, P values for interactions were calculated using Q statistic comparing subgroup-specific pooled multivariable-adjusted HRs for grapefruit consumption as median trend variable.
The positive association of melanoma with grapefruit consumption differed between site-specific melanomas (Table 4). There were significant associations between grapefruit consumption and melanomas on the body sites with higher continuous sun exposure (Ptrend < .001 for overall melanoma). In contrast, associations with melanomas on the body sites with lower continuous sun exposure were not apparent (Ptrend = .22 for overall melanoma). Further analysis stratified by Breslow thickness for invasive melanoma revealed an essentially similar association for tumors with thickness > and < the median of 0.63 mm (data not shown).
Table 4.
Pooled Multivariable HRs for Incident Melanoma by Subgroup According to Frequency of Grapefruit Consumption*
| Risk | Grapefruit Serving Category |
Ptrend | |||
|---|---|---|---|---|---|
| Never | < Once per Week | Once per Week | ≥ Twice per Week | ||
| No. of person-years | 515,163 | 647,023 | 354,732 | 485,515 | |
| Risk of overall melanoma on body sites with higher continuous sun exposure (head, neck, extremities) | |||||
| No. of cases | 175 | 374 | 224 | 381 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.36 (1.12 to 1.65) | 1.45 (1.16 to 1.81) | 1.58 (1.28 to 1.95) | < .001 |
| Risk of overall melanoma on body sites with lower continuous sun exposure (truncal sites) | |||||
| No. of cases | 121 | 198 | 118 | 177 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.04 (0.78 to 1.38) | 1.13 (0.76 to 1.69) | 1.14 (0.88 to 1.49) | .22 |
| Risk of melanoma in situ on body sites with higher continuous sun exposure (head, neck, extremities) | |||||
| No. of cases | 87 | 187 | 117 | 206 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.32 (1.00 to 1.75) | 1.50 (1.07 to 2.11) | 1.61 (1.13 to 2.30) | .03 |
| Risk of melanoma in situ on body sites with lower continuous sun exposure (truncal sites) | |||||
| No. of cases | 46 | 93 | 47 | 79 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.13 (0.78 to 1.63) | 1.14 (0.74 to 1.75) | 1.16 (0.78 to 1.74) | .59 |
| Risk of invasive melanoma on body sites with higher continuous sun exposure (head, neck, extremities) | |||||
| No. of cases | 88 | 187 | 107 | 175 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 1.40 (1.06 to 1.84) | 1.37 (1.00 to 1.88) | 1.53 (1.13 to 2.06) | .03 |
| Risk of invasive melanoma on body sites with lower continuous sun exposure (truncal sites) | |||||
| No. of cases | 75 | 105 | 71 | 98 | |
| Multivariable-adjusted HR (95% CI) | 1.00 | 0.96 (0.52 to 1.77) | 1.15 (0.69 to 1.91) | 1.10 (0.62 to 1.95) | .33 |
Abbreviation: HR, hazard ratio.
Multivariable analyses were adjusted for age, family history of melanoma, natural hair color, No. of arm moles, sunburn susceptibility as child or adolescent, No. of lifetime blistering sunburns, cumulative ultraviolet flux since baseline, average time spent in direct sunlight since high school, body mass index, physical activity, smoking status, and intake of total energy, alcohol, coffee, vitamin C from supplements, and other individual citrus products (grapefruit juice, oranges, and orange juice). Analyses for women were also adjusted for menopausal status and postmenopausal hormone use. Table 2 footnote provides additional details on these variables. Results among women and men were pooled using random-effects model.
Finally, we also investigated the risks of other major nonskin cancers (eg, breast cancer and prostate cancer) associated with citrus consumption and did not find any similar positive associations (data not shown).
DISCUSSION
In these two large prospective cohorts, we found that citrus consumption was associated with an increased risk of cutaneous malignant melanoma among women and men after adjusting for other known melanoma risk factors and potential confounders. Those who consumed overall citrus items ≥ 1.6 times per day had a 36% higher risk compared with those who consumed < twice per week. Among individual citrus products, grapefruit showed the most apparent positive association with melanoma risk, followed by orange juice.
Citrus products are dietary sources rich in psoralens,2–5 which have been identified as a group of carcinogens for decades.7,9 Photocarcinogenic properties of these chemicals have been well demonstrated in experimental studies.7–12 Animal experiments have found a correlation between epidermal and serum concentrations of psoralens after oral administration, and the appearance of phototoxicity is associated with serum concentrations of psoralens.31 It has been hypothesized that the actions of psoralens in the skin are a result of their ability to form DNA adducts after UVA light irradiation,32,33 whereas they may also mediate phototoxicity through binding to other sites in mammalian cells.34 A previous study demonstrated that PUVA therapy increased the number of human melanocytes as well as the size of the melanosomes, and these abnormalities were also present in PUVA-treated skin that was examined 15 months after treatment had been stopped.35
Oranges and grapefruit are the two most commonly consumed citrus fruits in the United States, accounting for > 90% of citrus market shares.36 Interestingly, fresh grapefruit showed the most apparent association with melanoma among individual citrus products, which may be explained by its higher levels of psoralens and furocoumarins when compared with oranges.2,3 The significant but less apparent association between orange juice with melanoma risk may be partly explained by its much higher consumption levels, which contributed to > 50% of overall citrus consumption, whereas the null association of grapefruit juice with melanoma risk may be a result of its much lower consumption levels and a large number of nonconsumers, as compared with the other individual citrus products (Data Supplement). In addition, industrial processing (eg, pasteurization treatment) for fresh fruits may reduce the contents of furocoumarins in processed juices because of heat.37 The industrial processing effect may help further explain the null association for grapefruit juice, given its low intake levels in the study population. In contrast to orange juice, the association between orange consumption and melanoma risk did not reach statistical significance in the main analyses, which may be explained by the much lower consumption levels when compared with those for orange juice. Nevertheless, there were also indicative positive associations between oranges and melanoma in lag analyses (Fig 1; Data Supplement).
Previous experimental studies have revealed that PUVA could induce melanocytic tumors in a mouse model12 and stimulate more apparent in vivo outgrowth of melanoma cells than psoralen or UVA alone.13 Similarly, exposure to psoralens or UVA alone was not tumorigenic in mice, whereas exposure to psoralens plus UVA substantially increased the number of mice with skin tumors.10,11 In our study, we found that the positive association between grapefruit consumption and melanoma risk seemed to be more apparent among those who had higher sunburn susceptibility and higher exposure to UV radiation and also seemed to be stronger for melanomas on body sites with higher continuous sun exposure (eg, head, neck, and extremities) than for melanomas on body sites with lower continuous sun exposure (eg, truncal sites), which may suggest a potential synergistic effect between dietary consumption and solar UV radiation. It is likely that consumption of psoralen-rich foods may have a stronger photocarcinogenic effect in the presence of higher UV radiation as compared with that in the presence of lower UV radiation.
The strengths of this study include its prospective design, large sample size, long-term follow-up over 24 to 26 years, repeated assessment of dietary and lifestyle factors, and ability to include a number of potential confounders. However, our study also has several limitations. The two cohorts mostly comprised white, educated US health professionals, which potentially limits the generalizability of the findings. However, restricting the sample to health professionals also reduces potential residual confounding from socioeconomic status. Nevertheless, future studies are needed to confirm this association in populations of other ethnicities. The dietary data were self-reported and may be subject to misclassification. However, we used cumulatively averaged intake from multiple time points to minimize the measurement error. Furthermore, misclassification was likely to be random, given that dietary information was collected prospectively, and thus may have resulted in an underestimation of the association.
In conclusion, our analysis based on two large cohorts of health professionals showed that citrus consumption was associated with an increased risk of incident cutaneous malignant melanoma. Among the citrus products examined in the study, grapefruit showed the most apparent association with melanoma risk, independent of other lifestyle and dietary factors. These findings provide evidence for the potential photocarcinogenic effect of psoralen-rich foods. However, previous studies have also suggested that fruit intake may have potential beneficial effects for the prevention of chronic diseases, such as breast cancer and type 2 diabetes.38,39 Although our findings are consistent with evidence from animal experiments, which revealed a potential synergistic effect between psoralens and UV radiation,10–13 further investigation is needed to confirm our findings and guide sun exposure behaviors among individuals with high citrus consumption.
Supplementary Material
Acknowledgment
We thank the participants and staff of the Nurses' Health Study and Health Professionals Follow-Up Study for their valuable contributions as well as the state cancer registries in the following states for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Minnesota, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Glossary Terms
- Breslow's tumor thickness:
the microstage of malignant melanoma is defined by Breslow's and Clark's classifications. The Breslow classification defines the absolute vertical thickness in mm of the primary tumor in the skin. This microstaging more accurately predicts subsequent behavior of cutaneous melanoma.
- confounding variables:
extraneous variables in a statistical model that are associated/correlated with both the independent and dependent variables but are not on the causal pathway between independent and dependent variables. When confounding variables are present, crude (unadjusted) statistical models describing the association between independent and dependent variables are biased (ie, wrong) as the risk estimate includes the effect of the confounding variable as well (type 1 error). As a result, to properly describe the relationship between independent and dependent variables, a multivariable model that includes both the independent variable and all relevant confounding variables as predictors must be executed.
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
Footnotes
See accompanying editorial on page 2487
Supported in part by National Cancer Institute Grants No. UM1 CA186107, P01 CA87969, UM1 CA167552, and R01 CA137365.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Certain data used in this report were obtained from the Connecticut Department of Public Health, the Human Investigations Committee of which approved this study.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Shaowei Wu, Jiali Han, Eunyoung Cho, Meir J. Stampfer, Abrar A. Qureshi
Financial support: Abrar A. Qureshi
Administrative support: Jiali Han, Abrar A. Qureshi
Provision of study materials or patients: Walter C. Willett
Collection and assembly of data: Shaowei Wu, Jiali Han, Diane Feskanich, Eunyoung Cho, Meir J. Stampfer, Abrar A. Qureshi
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Citrus Consumption and Risk of Cutaneous Malignant Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Shaowei Wu
No relationship to disclose
Jiali Han
No relationship to disclose
Diane Feskanich
No relationship to disclose
Eunyoung Cho
No relationship to disclose
Meir J. Stampfer
No relationship to disclose
Walter C. Willett
No relationship to disclose
Abrar A. Qureshi
Employment: University Dermatology
Leadership: University Dermatology
Consulting or Advisory Role: Abbvie, Novartis, Janssen Pharmaceuticals, Pfizer
Research Funding: Regeneron (Inst)
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 2.Dugo P, Piperno A, Romeo R, et al. Determination of oxygen heterocyclic components in citrus products by HPLC with UV detection. J Agric Food Chem. 2009;57:6543–6551. doi: 10.1021/jf901209r. [DOI] [PubMed] [Google Scholar]
- 3.Dugrand A, Olry A, Duval T, et al. Coumarin and furanocoumarin quantitation in citrus peel via ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS) J Agric Food Chem. 2013;61:10677–10684. doi: 10.1021/jf402763t. [DOI] [PubMed] [Google Scholar]
- 4.Frérot E, Decorzant E. Quantification of total furocoumarins in citrus oils by HPLC coupled with UV, fluorescence, and mass detection. J Agric Food Chem. 2004;52:6879–6886. doi: 10.1021/jf040164p. [DOI] [PubMed] [Google Scholar]
- 5.Sayre RM, Dowdy JC. The increase in melanoma: Are dietary furocoumarins responsible? Med Hypotheses. 2008;70:855–859. doi: 10.1016/j.mehy.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Parrish JA, Fitzpatrick TB, Tanenbaum L, et al. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291:1207–1211. doi: 10.1056/NEJM197412052912301. [DOI] [PubMed] [Google Scholar]
- 7.Griffin AC, Hakim RE, Knox J. The wave length effect upon erythemal and carcinogenic response in psoralen treated mice. J Invest Dermatol. 1958;31:289–295. [PubMed] [Google Scholar]
- 8.Ashwood-Smith MJ, Poulton GA, Barker M, et al. 5-Methoxypsoralen, an ingredient in several suntan preparations, has lethal, mutagenic and clastogenic properties. Nature. 1980;285:407–409. doi: 10.1038/285407a0. [DOI] [PubMed] [Google Scholar]
- 9.Mullen MP, Pathak MA, West JD, et al. Carcinogenic effects of monofunctional and bifunctional furocoumarins. Natl Cancer Inst Monogr. 1984;66:205–210. [PubMed] [Google Scholar]
- 10.Zajdela F, Bisagni E. 5-Methoxypsoralen, the melanogenic additive in sun-tan preparations, is tumorigenic in mice exposed to 365 nm u.v. radiation. Carcinogenesis. 1981;2:121–127. doi: 10.1093/carcin/2.2.121. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright LE, Walter JF. Psoralen-containing sunscreen is tumorigenic in hairless mice. J Am Acad Dermatol. 1983;8:830–836. doi: 10.1016/s0190-9622(83)80012-7. [DOI] [PubMed] [Google Scholar]
- 12.Alcalay J, Bucana C, Kripke ML. Cutaneous pigmented melanocytic tumor in a mouse treated with psoralen plus ultraviolet A radiation. Photodermatol Photoimmunol Photomed. 1990;7:28–31. [PubMed] [Google Scholar]
- 13.Aubin F, Donawho CK, Kripke ML. Effect of psoralen plus ultraviolet A radiation on in vivo growth of melanoma cells. Cancer Res. 1991;51:5893–5897. [PubMed] [Google Scholar]
- 14.Stern RS, Nichols KT, Väkevä LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA): The PUVA Follow-Up Study. N Engl J Med. 1997;336:1041–1045. doi: 10.1056/NEJM199704103361501. [DOI] [PubMed] [Google Scholar]
- 15.Stern RS. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44:755–761. doi: 10.1067/mjd.2001.114576. [DOI] [PubMed] [Google Scholar]
- 16.Autier P, Doré JF, Césarini JP, et al. Should subjects who used psoralen suntan activators be screened for melanoma? Epidemiology and Prevention Subgroup, EORTC Melanoma Cooperative Group EORTC Prevention Research Division. Ann Oncol. 1997;8:435–437. doi: 10.1023/a:1008205513771. [DOI] [PubMed] [Google Scholar]
- 17.Autier P, Doré JF, Schifflers E, et al. Melanoma and use of sunscreens: An EORTC case-control study in Germany, Belgium and France—The EORTC Melanoma Cooperative Group. Int J Cancer. 1995;61:749–755. doi: 10.1002/ijc.2910610602. [DOI] [PubMed] [Google Scholar]
- 18.Scientific Committee on Consumer Products. SCCP/0942/05 Opinion on Furocoumarins in Cosmetic Products. Brussels, Belgium: European Commission Health and Consumer Protection Directorate General; 2005. [Google Scholar]
- 19.Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381–1387. doi: 10.1038/sj.bjc.6600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bram S, Froussard P, Guichard M, et al. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980;284:629–631. doi: 10.1038/284629a0. [DOI] [PubMed] [Google Scholar]
- 21.Kang JS, Cho D, Kim YI, et al. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003;52:693–698. doi: 10.1007/s00262-003-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang JS, Cho D, Kim YI, et al. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005;204:192–197. doi: 10.1002/jcp.20286. [DOI] [PubMed] [Google Scholar]
- 23.Lee SK, Kang JS, Jung da J, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216:180–188. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 25.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Agriculture Agricultural Research Service. USDA nutrient database for standard reference, release 10. http://www.ars.usda.gov/ba/bhnrc/ndl.
- 27.Wu S, Han J, Li WQ, et al. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178:890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? CMAJ. 2013;185:309–316. doi: 10.1503/cmaj.120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samieri C, Sun Q, Townsend MK, et al. The association between dietary patterns at midlife and health in aging: An observational study. Ann Intern Med. 2013;159:584–591. doi: 10.7326/0003-4819-159-9-201311050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Kornhauser A, Wamer WG, Giles AL., Jr Psoralen phototoxicity: Correlation with serum and epidermal 8-methoxypsoralen and 5-methoxypsoralen in the guinea pig. Science. 1982;217:733–735. doi: 10.1126/science.7100920. [DOI] [PubMed] [Google Scholar]
- 32.Dall'Acqua F, Marciani S, Ciavatta L, et al. Formation of inter-strand cross-linkings in the photoreaction between furocoumarins and DNA. Z Naturforsch B. 1971;26:561–569. doi: 10.1515/znb-1971-0613. [DOI] [PubMed] [Google Scholar]
- 33.Vos JM, Hanawalt PC. Processing of psoralen adducts in an active human gene: Repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987;50:789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- 34.Laskin JD, Lee E, Yurkow EJ, et al. A possible mechanism of psoralen phototoxicity not involving direct interaction with DNA. Proc Natl Acad Sci U S A. 1985;82:6158–6162. doi: 10.1073/pnas.82.18.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelickson AS, Mottaz JH, Muller SA. Melanocyte changes following PUVA therapy. J Am Acad Dermatol. 1979;1:422–430. doi: 10.1016/s0190-9622(79)70034-x. [DOI] [PubMed] [Google Scholar]
- 36.US Department of Agriculture Economic Research Service. Fruit and tree nut data. http://www.ers.usda.gov/data-products/fruit-and-tree-nut-data/yearbook-tables.aspx.
- 37.Uesawa Y, Mohri K. The use of heat treatment to eliminate drug interactions due to grapefruit juice. Biol Pharm Bull. 2006;29:2274–2278. doi: 10.1248/bpb.29.2274. [DOI] [PubMed] [Google Scholar]
- 38.Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105:219–236. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.