Abstract
Purpose
Therapy-related myeloid neoplasms (t-MN) represent a unique clinical syndrome occurring in patients treated with chemotherapy and/or external-beam radiation (XRT) and are characterized by poorer prognosis compared with de novo disease. XRT techniques have evolved in recent years and are associated with significantly reduced bone marrow exposure. The characteristics of post-XRT t-MN in the current era have not been studied.
Patients and Methods
We analyzed patients who developed acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) after XRT alone (47 patients) or cytotoxic chemotherapy/combined-modality therapy (C/CMT, 181 patients) and compared them with patients with de novo MDS or AML (222 patients). We estimated bone marrow exposure to radiation and compared the clinical, pathologic, and cytogenetic features and outcome of the XRT patients with the C/CMT patients and with patients with de novo MDS and AML.
Results
Patients with t-MN after XRT alone had superior overall survival (P = .006) and lower incidence of high-risk karyotypes (P = .01 for AML and < .001 for MDS) compared with patients in the C/CMT group. In contrast, there were no significant differences in survival or frequency of high-risk karyotypes between the XRT and de novo groups.
Conclusion
AML and MDS diagnosed in the past decade in patients after receiving XRT alone differ from t-MN occurring after C/CMT and share genetic features and clinical behavior with de novo AML/MDS. Our results suggest that post-XRT MDS/AML may not represent a direct consequence of radiation toxicity and warrant a therapeutic approach similar to de novo disease.
INTRODUCTION
Therapy-related myelodysplastic syndromes (t-MDS) and acute myeloid leukemia (t-AML) are myeloid malignancies that develop as a complication of cytotoxic therapy (chemotherapy and/or external-beam radiation therapy [XRT]) used to treat a prior neoplasm or nonneoplastic disorder. Therapy-related myeloid neoplasms (t-MN) have been recognized since the 1970s1–4 and account for 10% to 30% of all cases of AML and approximately 27% of all cases of MDS.5–9 t-MNs have a poor prognosis, with median survival of only 8 months and 5-year survival of less than 10%, and usually exhibit high-risk cytogenetic findings that often involve losses of chromosomes 5 and/or 7.3 Bone marrow transplantation (BMT) appears to represent the only potentially curative regimen in patients diagnosed with t-MN.10,11 There is significant clinical, morphologic, and genetic overlap between t-AML and t-MDS, and the WHO Classification of Hematopoietic Neoplasms considers t-AML and t-MDS together as the unique clinicopathologic syndrome of t-MN.12,13
The carcinogenicity of chemotherapeutic agents and ionizing radiation is dependent on their ability to cause DNA damage that results in mutations and translocations through DNA double-strand breaks and loss of elements of the DNA mismatch repair system, with consequent genomic instability.14,15 t-MNs have a higher frequency of high-risk karyotypes and relatively poorer prognosis as compared with corresponding de novo diseases.16–18 Unlike de novo MDS, morphologic classification and bone marrow blast count are not correlated with prognosis in t-MDS.12 Gene expression profiling studies have demonstrated recurrent mutations in a limited number of molecular pathways in t-MN, with different mutations in postalkylating agent t-MN and topoisomerase II inhibitor–related t-MN.19 Taken together, these findings suggest that genetic alterations induced by cytotoxic therapy on a bone marrow stem cell cause an aggressive myeloid neoplasm characterized by genetic instability.
The contribution of radiation to carcinogenesis was recognized at the beginning of the twentieth century20,21 with subsequent demonstration of a dose dependence.22 XRT is frequently used in conjunction with chemotherapy, and few studies have specifically looked at the characteristics of myeloid neoplasms occurring after XRT alone.23–25 These published studies were conducted in patients treated with older XRT techniques, which often exposed large active hematopoietic marrow areas to radiation. In the past two decades, the field of radiation therapy has moved toward using more conformal treatment techniques that reduce the exposure of hematopoietic bone marrow.26–28 We hypothesized that myeloid neoplasms occurring after more modern XRT regimens, without cytotoxic chemotherapy, may differ from “classical” t-MN. We analyzed the clinical, pathologic, and cytogenetic characteristics of recently diagnosed cases of AML and MDS occurring after XRT treatment alone and compared them with a cohort of patients with t-MN occurring after cytotoxic chemotherapy or combined-modality therapy (C/CMT), as well as with a cohort of patients with de novo AML and MDS.
PATIENTS AND METHODS
Cases
The cases were identified by a search of the pathology databases at two institutions, Massachusetts General Hospital (MGH) and the MD Anderson Cancer Center (MDACC). These included 47 consecutive cases of MDS/chronic myelomonocytic leukemia (CMML) (26 patients, 12 at MGH and 14 at MDACC) or AML (21 patients, 12 at MGH and nine at MDACC) after XRT for a primary malignancy, but without any exposure to cytotoxic chemotherapy (XRT group) diagnosed between January 1, 2001, and March 1, 2011 (MGH) and January 1, 2004, and July 31, 2010 (MDACC). According to the 2008 WHO Classification, we excluded patients treated with brachytherapy only (n = 5, including three patients with prostate cancer and two patients with uterine cancer), patients treated with radioisotopes (n = 1, radioactive iodine for hyperthyroidism), and those whose treatment field did not include hematopoietic bone marrow (n = 1, proton beam therapy for a pituitary tumor).13 Control groups included 181 consecutive or randomly selected cases of MDS/CMML (120 patients, 78 at MDACC and 42 at MGH) or AML (61 patients, 30 at MDACC and 31 at MGH) after prior cytotoxic chemotherapy with or without XRT (C/CMT group) and 222 consecutive cases of MDS/CMML (103 patients from MGH) or AML (119 patients from MGH) without any known exposure to XRT or cytotoxic chemotherapy (de novo group). Patients younger than 18 years were excluded from all groups. All cases were classified according to the 2008 WHO classification criteria for de novo MDS and AML.13 Clinical and follow-up data were obtained from the electronic medical record. The most intensive therapy administered was recorded as supportive care (transfusion support and anti-infectives), low-intensity therapy (low-dose cytotoxic chemotherapy or clinical trial therapies not involving cytotoxic chemotherapy), induction chemotherapy, or allogeneic stem-cell transplantation at any time point in the treatment course. The study was performed with approval by the institutional review boards of both MGH and MDACC.
Cytogenetic Analysis
Cytogenetic analysis was performed with trypsin-Giemsa banding techniques on bone marrow cells from aspirates obtained at the time of diagnosis. Chromosomal abnormalities were described according to the International System for Human Cytogenetic Nomenclature. The karyotype risk strata were determined using the International Prognostic Scoring System (IPSS)29 and the 2010 United Kingdom Medical Research Council30 scoring system.
Bone Marrow Exposure to Radiation Therapy
The volume of active bone marrow exposed to radiation was estimated for each patient on the basis of the XRT treatment fields and the percentage of hematopoietic bone marrow in the skeleton.31
Statistical Analysis
All statistical analysis was performed using the STATA Statistical Software (version 11.2; STATA, College Station, TX). Patient clinical characteristics were summarized as numbers and percentages for categorical variables and median and range for continuous variables. Progression-free survival (PFS) for patients presenting with MDS/CMML was defined as time from first pathologic diagnosis of MDS/CMML to the date of transformation to AML or date of death from any cause. Comparisons and associations between categorical variables were analyzed by Fisher's exact test or Pearson's test or a nonparametric K-sample test on the equality of medians.
Overall survival (OS), PFS, and median survival rates were calculated using the Kaplan-Meier product-limit method. CIs were estimated using log(-log) transformation of the survivor function. The Cox proportional hazards method was used for assessing the univariate associations of patient and treatment variables with OS. Proportional hazards assumption was tested using Schoenfeld residuals. The log-rank test was used to assess the equality of survivor functions. P values of .05 or less were regarded as statistically significant. Multivariate Cox proportional hazards analysis was performed for OS.
RESULTS
Clinical Characteristics
The demographic and treatment information of the patient cohorts is summarized in Table 1. The male to female ratio was higher in the XRT group compared with the C/CMT group, and patients were older in the XRT group versus the C/CMT and de novo groups (Table 1). The 222 patients in the de novo group included 25 patients with a history of a primary malignancy who did not receive cytotoxic chemotherapy or XRT. The C/CMT cohort included 103 patients (57%) who were treated with chemotherapy only and 78 patients (43%) who were treated with CMT. The latency period between therapy and the development of the myeloid neoplasm was similar in the XRT (median, 60 months) and C/CMT (median, 57 months) patients (Table 1).
Table 1.
Patient Characteristics
| Characteristic | XRT (n = 47) | C/CMT (n = 181) | P (XRT v C/CMT) | De Novo (n = 222) | P (XRT v De Novo) |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex | .03* | 0.3* | |||
| Male | 31 | 88 | 127 | ||
| Female | 16 | 93 | 95 | ||
| Age, years | < .001† | .001† | |||
| Median | 74 | 65 | 66 | ||
| Range | 40-87 | 14-88 | 18-93 | ||
| Latency, months | .9* | NA | NA | ||
| Median | 60 | 57 | |||
| Range | 8-462 | 6-360 | |||
| Reason for prior therapy‡ | < .001§ | .001§ | |||
| No prior therapy | 0 | 0 | 197 | ||
| Breast cancer | 15 | 29 | 1 | ||
| GI cancer | 0 | 9 | 3 | ||
| Genitourinary cancer | 2 | 7 | 3 | ||
| Gynecologic cancer | 0 | 12 | 2 | ||
| Head and neck cancer | 4 | 6 | 1 | ||
| Lymphoma or myeloma | 2 | 87 | 2 | ||
| Lung cancer | 0 | 5 | 1 | ||
| Prostate cancer | 24 | 5 | 11 | ||
| Melanoma | 0 | 2 | 1 | ||
| Other neoplasms | 0 | 12 | 0 | ||
| Other nonneoplastic | 0 | 7 | 0 |
Abbreviations: C/CMT, cytotoxic chemotherapy/combined-modality therapy; XRT, external-beam radiation therapy; NA, not applicable.
Two-sample test of proportions.
Pearson's test of medians with continuity correction.
In the de novo group, this denotes prior malignancies treated with surgery only or noncytotoxic therapy (such as monoclonal antibodies).
Fisher's exact test.
Radiation Therapy
Radiation therapy was administered to patients in the XRT group between 1958 and 2008. The median year at which XRT treatment occurred was the year 2000, and 75% of the patients were treated after 1995. The most common primary malignancies treated with XRT were prostate and breast cancer. The calculated amount of hematopoietic marrow exposed to radiation ranged from 1% to 25% of all hematopoietic marrow. The highest amount of calculated marrow exposure occurred in men treated for prostate cancer before the year 2000; most of the treatment fields from this era were composed of a standard “four-field box” that treated large portions of the pelvis, and in four of these patients, greater than 10% of the hematopoietic marrow was exposed. In contrast, only an estimated 4% of the marrow was exposed in patients treated for prostate cancer after the year 2000, when XRT was generally delivered to the prostate or prostate bed alone using intensity-modulated radiation therapy (Appendix Fig A1, online only).
Pathologic Findings
Twenty-six (55%) of 47 XRT patients, 120 (66%) of 181 C/CMT patients, and 103 (46%) of 222 de novo patients presented with MDS or CMML, whereas the remainder presented with AML. The clinicopathologic features of the patients with MDS/CMML are summarized in Table 2. The distribution of MDS types according to the WHO Classification for non–therapy-related (primary) MDS and the blood counts at presentation were similar among the three groups. Only 27% of the XRT patients had intermediate-2 or high IPSS scores, compared with 60% of the C/CMT patients. Patients in the XRT group had IPSS scores that were significantly lower than the C/CMT patients (P < .001), but similar to the de novo MDS/CMML patients.
Table 2.
Clinicopathologic Features of MDS/CMML Cases
| Feature | XRT (n = 26) |
C/CMT (n = 120) |
P (XRT v C/CMT) | De Novo (n = 103) |
P (XRT v De Novo) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| WHO classification | .8* | .5* | ||||||
| RCUD | 1 | 4 | 5 | 4 | 5 | 5 | ||
| RARS | 3 | 12 | 4 | 3 | 14 | 14 | ||
| RCMD | 11 | 42 | 53 | 44 | 31 | 30 | ||
| RAEB-1 | 5 | 19 | 24 | 20 | 19 | 18 | ||
| RAEB-2 | 5 | 19 | 25 | 21 | 16 | 16 | ||
| MDS-U | 1 | 4 | 5 | 4 | 2 | 2 | ||
| MDS with isolated del (5q) | 0 | 0 | 4 | 4 | ||||
| CMML | 0 | 3 | 3 | 12 | 12 | |||
| Blood counts at presentation | ||||||||
| ANC, × 109/L | .4† | .8† | ||||||
| Median | 2.0 | 1.5 | 2.2 | |||||
| Range | 0-10.3 | 0.3-23.6 | 0-35.5 | |||||
| Hemoglobin, g/dL | .6† | .9† | ||||||
| Median | 9.5 | 9.7 | 9.5 | |||||
| Range | 7.2-12.8 | 5.6-15.3 | 3.7-15.6 | |||||
| Platelets, × 109/L | .3† | .9† | ||||||
| Median | 83 | 68 | 110 | |||||
| Range | 11-331 | 10-355 | 14-524 | |||||
| IPSS score | < .001 (excluding NA category) | .4 (excluding NA category) | ||||||
| Low (0) | 10 | 38 | 4 | 4 | 32 | 31 | ||
| Intermediate-1 (0.5-1) | 9 | 35 | 37 | 36 | 35 | 34 | ||
| Intermediate-2 (1.5-2) | 5 | 19 | 54 | 53 | 27 | 26 | ||
| High (≥ 2.5) | 2 | 8 | 7 | 7 | 2 | 2 | ||
| NA | 0 | 18 | 15 | 7 | 7 | |||
Abbreviations: ANC, absolute neutrophil count; C/CMT, cytotoxic chemotherapy/combined-modality therapy; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; MDS-U, myelodysplastic syndrome, unclassified; NA, not available; RAEB-1, refractory anemia with excess blasts-1; RAEB-2, refractory anemia with excess blasts-2; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCUD, refractory cytopenia with unilineage dysplasia; XRT, external-beam radiation therapy.
Fisher's exact test.
Pearson's test of medians with continuity correction.
Cytogenetic Analysis
A summary of the cytogenetic findings is shown in Table 3. Although approximately half of the XRT and de novo patients had abnormal bone marrow karyotypes, 83% of patients in the C/CMT group had abnormal karyotypes (P < .001 compared with the XRT group). The most common abnormalities in the C/CMT cohort involved loss of the long arm or entire chromosome 5 or 7, which were far less frequent in the XRT cohort (P < .001), where they occurred with similar frequency to the de novo cohort.
Table 3.
Cytogenetic Features and Risk Groups of Patients With MDS/CMML and AML
| Cytogenetic Finding | XRT (n = 47) |
C/CMT (n = 181) |
P (XRT v C/CMT) | De Novo (n = 222) |
P (XRT v De Novo) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Karyotype | ||||||||
| Normal | 20 | 43 | 26 | 14 | < .001 | 99 | 45 | 1.0 |
| Abnormal | 24 | 51 | 150 | 83 | 113 | 51 | ||
| NA | 3 | 6 | 5 | 3 | 10 | 5 | ||
| Abnormalities of chromosomes 5 and 7 | ||||||||
| Deletion/loss of 5 | 9 | 19 | 95 | 52 | < .001 | 38 | 17 | .8 |
| Deletion/loss of 7 | 9 | 19 | 92 | 51 | < .001 | 35 | 16 | .5 |
| Deletion/loss of 5 or 7 | 12 | 26 | 115 | 63 | < .001 | 50 | 23 | .7 |
| AML cytogenetic risk group (UKMRC, AML only) | 21 | 61 | .03 (fav/int v adverse) | 119 | 1.0 (fav/int v adverse) | |||
| Favorable | 2 | 10 | 1 | 2 | .01 (fav/int v adv/11q23) | 17 | 14 | .8 (fav/int v adv/11q23) |
| Intermediate* | 10 | 48 | 16 | 26 | 58 | 49 | ||
| Adverse* | 6 | 29 | 33 | 54 | 37 | 31 | ||
| 11q23 rearrangement | 1 | 5 | 11 | 18 | 2 | 2 | ||
| Unknown | 2 | 10 | 0 | 5 | 4 | |||
| MDS cytogenetic risk group (IPSS, MDS/CMML only) | 26 | 120 | < .001 | 103 | .8 | |||
| Good | 17 | 65 | 14 | 12 | 67 | 65 | ||
| Intermediate | 4 | 15 | 15 | 12 | 11 | 11 | ||
| Poor | 4 | 15 | 86 | 72 | 20 | 19 | ||
| Unknown | 1 | 4 | 5 | 4 | 5 | 5 | ||
NOTE. All are two-sided Fisher's exact test.
Abbreviations: AML, acute myeloid leukemia; C/CMT, cytotoxic chemotherapy/combined-modality therapy; CMML, chronic myelomonocytic leukemia; fav, favorable; int, intermediate; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; NA, not available; UKMRC, United Kingdom Medical Research Council; XRT, external-beam radiation therapy.
Excluding cases with 11q23 (MLL) rearrangement.
Survival
There were no significant differences in the therapies used to treat AML/MDS, although there was a trend to treat the XRT patients less frequently with BMT (Table 4). There were no significant differences in OS between the XRT and de novo patients, but OS of the XRT patients was superior to that of the C/CMT patients (Fig 1A). Within the C/CMT group, there was a trend for longer OS in patients treated with chemotherapy alone versus CMT, but this did not reach statistical significance (data not shown). Among patients presenting with MDS/CMML, OS and PFS were similar in XRT and de novo groups, but markedly inferior in the C/CMT group (Fig 1B). When considering patients presenting with AML and patients in the MDS/CMML group who progressed to AML, OS rates from the time of AML diagnosis were similar between the XRT and de novo patients, but were significantly shorter in the C/CMT patients (Fig 1C); this survival difference was more marked when limiting the analysis to patients with AML patients who were 75 years of age or younger (P = .003; Table 4). There was no significant difference in OS for patients diagnosed at MGH versus MDACC in the XRT group (P = .7) and C/CMT group (P = .6). Within the de novo group, there was a trend for shorter OS in the 25 patients diagnosed with prior malignancies compared with those lacking prior malignancies (median, 9 v 26 months, respectively, P = .07).
Table 4.
Treatments and Outcome for Patients With MDS/CMML and AML
| Treatment Administered/Outcome | XRT (n = 47) |
C/CMT (n = 181) |
P (XRT v C/CMT) | De Novo (n = 222) |
P (XRT v De Novo) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Highest intensity treatment | .4 | .07 | ||||||
| Supportive care | 15 | 32 | 38 | 21 | 70 | 32 | ||
| Low-intensity therapies | 14 | 30 | 57 | 31 | 34 | 15 | ||
| Induction chemotherapy | 9 | 19 | 31 | 17 | 52 | 23 | ||
| Bone marrow transplant | 8 | 17 | 50 | 28 | 65 | 29 | ||
| Unknown | 1 | 2 | 5 | 3 | 1 | 0.5 | ||
| Median survival (all patients) | n = 47 | n = 181 | n = 222 | |||||
| OS, months | 22 | 11 | .006 | 22 | .8 | |||
| 95% CI | 15 to 52 | 9 to 13 | 17 to 29 | |||||
| Median survival of patients with MDS/CMML | n = 26 | n = 118 | n = 103 | |||||
| OS, months | 38 | 12 | < .001 | 30 | .9 | |||
| 95% CI | 19 to 63 | 8 to 13 | 21 to 39 | |||||
| PFS to AML or death, months | 33.0 | 9.4 | .002 | 22.8 | .8 | |||
| 95% CI | 9 to 57 | 6 to 12 | 12 to 30 | |||||
| Median survival of patients with AML* | n = 29 | n = 89 | n = 148 | |||||
| OS, months | 16 | 11 | .03 | 18 | .8 | |||
| 95% CI | 10 to 38 | 8 to 14 | 12 to 24 | |||||
| Median survival of patients age ≤ 75 with AML* | n = 20 | n = 82 | n = 124 | |||||
| OS, months | 38 | 12 | .003 | 20 | .2 | |||
| 95% CI | 15 to 98 | 8 to 15 | 16 to 28 | |||||
Abbreviations: AML, acute myeloid leukemia; C/CMT, cytotoxic chemotherapy/combined-modality therapy; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndrome; OS, overall survival; PFS, progression-free survival; XRT, external-beam radiation therapy.
From time of AML diagnosis, including patients with MDS/CMML who transformed to AML (eight patients in XRT group, 28 patients in C/CMT group, and 28 patients in de novo group).
Fig 1.
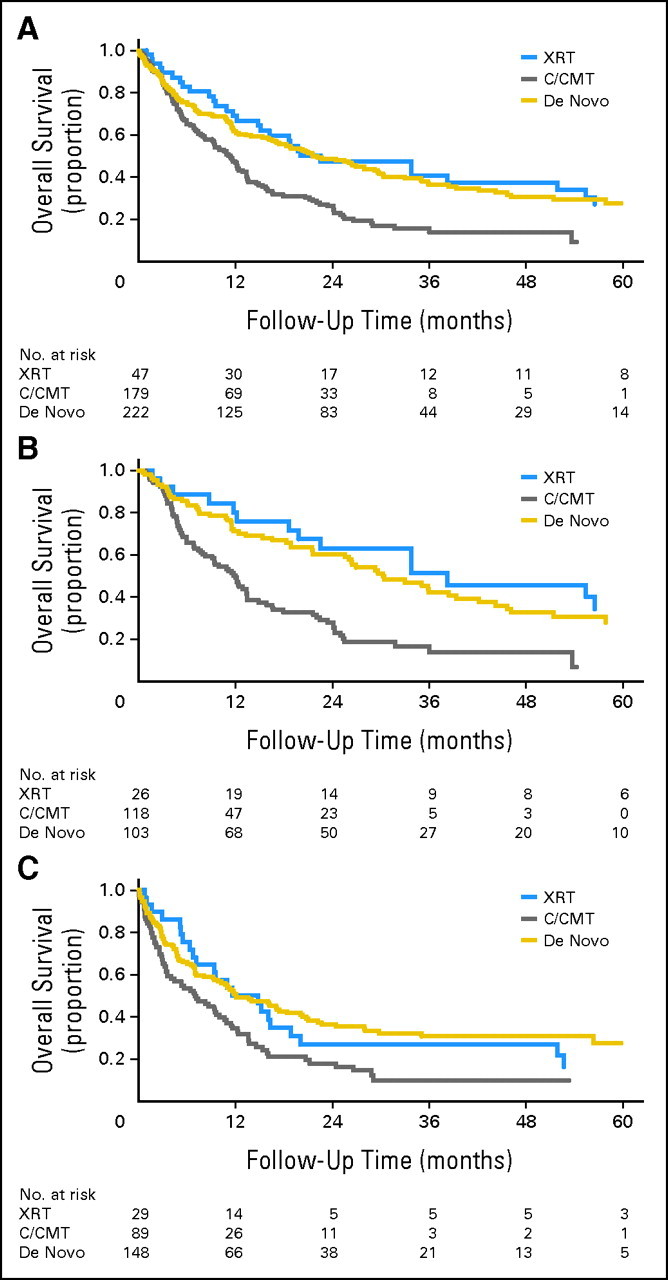
(A) Overall survival (OS) of the post–external-beam radiation therapy (XRT) patients was superior to that of the post–cytotoxic chemotherapy/combined-modality therapy (C/CMT) patients (P = .006), but was not significantly different from that of the de novo patients (P = .8). (B) OS of patients with myelodysplastic syndrome (MDS)/chronic myelomonocytic leukemia (CMML) after XRT was superior to that of patients after C/CMT (P < .001), but was not significantly different from that of patients with de novo MDS/CMML (P = .9). (C) For patients diagnosed with acute myeloid leukemia (either on presentation or as progression from prior MDS), OS of post-XRT patients was superior to that of post-C/CMT patients (P = .03) but was not different from that of de novo patients (P = .8).
In univariate analysis, older age, the presence of chromosome 5 or 7 abnormalities, and high-risk karyotype (both IPSS and United Kingdom Medical Research Council) were adverse prognostic factors, and treatment with BMT had a favorable impact on OS in all three groups (Appendix Table A1, online only). Bone marrow blast count was correlated with OS in the XRT (P = .04, Fig 2A) and de novo (P = .001, not shown) patients only. IPSS score for the patients with MDS/CMML was correlated with OS in the XRT (P < .001) and de novo (P < .001) patients only. Conversely, in the C/CMT group but not in the other groups, a lower bone marrow cellularity was associated with shorter OS (P = .02), whereas bone marrow blast count (Fig 2B) and total IPSS score did not influence survival. There was no association between the estimated percentage of hematopoietic marrow exposed to XRT and OS for patients in the XRT group (P = .7) or between latency and OS for patients in the XRT and C/CMT groups (P = .2).
Fig 2.
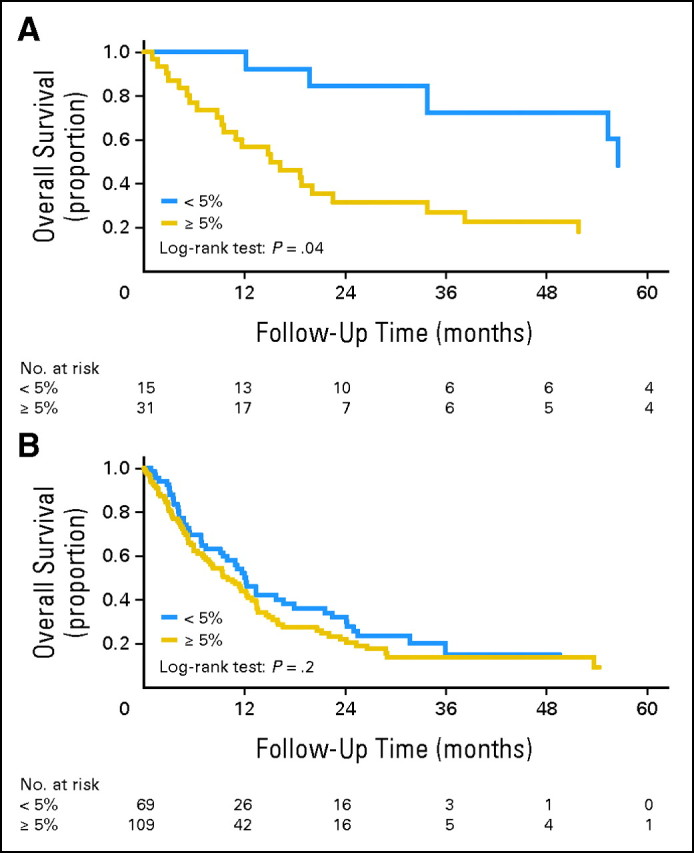
(A). Post–external-beam radiation therapy patients with < 5% bone marrow blasts had overall survival (OS) superior to that of patients with ≥ 5% bone marrow blasts (P = .04). (B) In contrast, post–cytotoxic chemotherapy/combined-modality therapy patients with < 5% or ≥ 5% blasts had similar OS (P = .7).
In a multivariate analysis, neither C/CMT nor XRT were associated with inferior OS in patients with MDS/CMML or AML independent of blast count (for patients with MDS/CMML), high-risk cytogenetics, and age (Appendix Tables 2 and 3, online only).
DISCUSSION
Our analysis of 450 patients confirms earlier data showing that t-MNs secondary to prior C/CMT are distinct from de novo AML and MDS, in terms of their cytogenetic characteristics and clinical behavior. However, we found that myeloid neoplasms arising after XRT differ from those arising after C/CMT. Smith et al25 failed to demonstrate any relationship between modality of primary therapy and cytogenetic features in secondary myeloid neoplasms. In contrast, Kantarjian et al24 described a lower frequency of abnormalities of chromosomes 5 and 7 after XRT relative to C/CMT. Although these prior studies failed to show any survival advantage for patients who received XRT alone versus C/CMT, in our series OS was significantly better for patients with myeloid neoplasms after XRT versus C/CMT and similar to those with de novo disease. This superior OS could be explained by the more favorable cytogenetic profile of the post-XRT patients compared with the C/CMT patients, as shown by multivariate analysis. Although we did find that treatment with BMT favorably affected survival in all three groups, BMT was not used more frequently in the XRT group and thus would not explain this difference.
We also found that specific prognostic factors associated with outcome differed between the XRT and C/CMT patients. In particular, like de novo MDS and AML, bone marrow blast count risk stratified post-XRT patients, whereas there was no correlation between blast count and outcome in the C/CMT group, in keeping with prior studies.12,25 Similarly, IPSS score stratified the XRT and de novo patients, but not the C/CMT patients, despite the association of the IPSS cytogenetic risk group with outcome in the C/CMT patients. Taken together, these findings suggest that myeloid neoplasms developing after modern XRT regimens display biologic behavior akin to de novo AML and MDS and different from other t-MN, in which blast count and disease grade have little or no influence on patient outcome. Of note, a high-risk karyotype was associated with adverse outcome in all three groups and, after taking into account age and cytogenetic risk group, prior treatment with C/CMT did not adversely affect patient outcome in multivariate analysis. Although we had too few patients with favorable cytogenetics in our post-C/CMT AML group to analyze, others have found that t-AML patients with t(15;17) and inv(16) have an outcome similar to comparable patients with de novo disease.32 These findings suggest that the high incidence of adverse karyotype associated with cytotoxic chemotherapy underlies the generally poor outcome of t-MN.
One possible explanation for the difference in behavior of post-XRT myeloid neoplasms in our series is that prior studies reported patients diagnosed between 1972 and 2001,25 1973 to 1984,24 and before 2001,32 whereas our study reports patients diagnosed after 2001. A relatively small overall volume of hematopoietic marrow was exposed to radiation in most patients in our series, likely reflecting the more targeted radiation fields used in the past two decades. Although these data cannot be directly compared with those of prior studies, which did not provide these measurements, those earlier studies reported patients who had been treated with XRT between the 1960s and early 1990s.24,25,32 In more recent years, XRT field size and dose have decreased. This is well demonstrated by the evolution in lymphoma treatment, which has transformed from extended-field XRT through the 1990s to use of involved-field radiation,33,34 which has greatly reduced the amount of marrow exposed. In fact, only two patients in the XRT group in our series had been treated for lymphoma, and these patients had received only limited XRT that exposed less than 5% of the hematopoietic marrow. Advancements in imaging modalities and radiation planning capabilities, such as magnetic resonance imaging/computed tomography fusions, allow for more precise delineation of target volumes, and technological advancements in radiation therapy techniques (intensity-modulated radiation therapy, stereotactic radiotherapy, and proton therapy) have markedly enhanced the conformal delivery of radiation, thereby improving the dose-volume effect on normal tissues. The pelvis is rich in hematopoietic marrow; use of conformal XRT techniques in the treatment of pelvic tumors is known to significantly reduce the exposure of marrow35 and may account for some of the difference seen with our cohort as compared with older studies, which included a large proportion of patients radiated for pelvic tumors.25
The leukemogenic effect of ionizing radiation results from production of reactive oxygen species, which increase the frequency of double-strand DNA breaks.36 Through a different mechanism, alkylating agents cause double-strand DNA breaks, which, when not appropriately repaired, lead to chromosomal rearrangements37–39 and consequently to transformation of a bone marrow stem cell. Combinations of ionizing radiation and alkylating agents result in enhanced tumor radiosensitization.40 Although it is well-known that radiation-induced DNA damage can transform bone marrow stem cells and thereby cause myeloid neoplasms, we hypothesize that modern radiation therapy uses techniques that restrict dose to hematopoietic tissue; such a restricted dose thereby reduces the risk of reaching the cytotoxic threshold of radiation-induced DNA damage that leads to t-MN. Although a subset of our post-XRT cases displayed deletions and losses of chromosomes 5 and 7, these abnormalities can also occur in myeloid neoplasms that develop in patients with no prior history of cytotoxic therapy16,17 and indeed occurred with similar frequency in our post-XRT and de novo groups. It is important to note that our study was not prospective and did not evaluate whether the incidence of MDS or AML may be increased after exposure to XRT. Our study also did not directly evaluate whether XRT may potentiate the leukemogenic effects of cytotoxic chemotherapy in the setting of combined-modality therapy.
In conclusion, we propose that AML and MDS diagnosed in the past decade after exposure to XRT alone have different clinicopathologic features from those occurring after exposure to cytotoxic chemotherapy (with or without XRT), but similar to de novo disease. t-MNs generally have a poor prognosis, and this consideration influences the therapeutic approach for MDS/AML and potentially affects management of the underlying primary malignancy. This adverse outcome seems to reflect the frequent high-risk karyotype in myeloid neoplasms after cytotoxic chemotherapy, whereas the incidence of high-risk karyotype in MDS and AML after XRT is similar to that of de novo disease. Furthermore, the clinical behavior of post-XRT MDS is influenced by bone marrow blast count and IPSS score, unlike post-C/CMT MDS. These data suggest that the clinical management of patients who develop MDS or AML after XRT alone should be based on de novo rather than on therapy-related disease.
Appendix
Table A1.
Univariate Analysis for Overall Survival in Each Group
| Variable |
P |
||
|---|---|---|---|
| XRT Group (n = 47) | C/CMT Group (n = 181) | De Novo Group (n = 222) | |
| For all patients | |||
| Patient age | .05 | .002 | < .001 |
| Patient sex | .2 | .5 | .9 |
| Percentage of marrow exposed to XRT | .7 | NA | NA |
| Abnormalities of chromosomes 5 or 7 | .02 | .007 | < .001 |
| UKMRC karyotype risk group (favorable v adverse/intermediate | .009 | .01 | ND |
| IPSS karyotype risk group (poor v good/intermediate) | .002 | .006 | ND |
| Treatment with BMT | .007 | < .001 | .02 |
| Bone marrow blast count | .03 | .7 | .001 |
| Bone marrow blasts < 5% v ≥ 5% | .04 | .7 | .001 |
| For patients with MDS/CMML only* | |||
| IPSS score | < .001 | .2 | < .001 |
| IPSS karyotype risk group (poor v good/intermediate) | .003 | .008 | < .001 |
| Bone marrow cellularity | .5 | .02 | .7 |
Abbreviations: BMT, bone marrow transplant; C/CMT, cytotoxic chemotherapy/combined-modality therapy; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; ND, not done; UKMRC, United Kingdom Medical Research Council; XRT, external-beam radiation therapy.
XRT group, n = 26; C/CMT group, n = 120; de novo group, n = 103.
Table A2.
Multivariate Cox Proportional Hazards Analysis for Overall Survival of Patients With MDS/CMML, All Groups (n = 249)
| Variable | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| Patient age | .001 | 1.029* | 1.012 to 1.046 |
| High-risk karyotype (IPSS) | < .001 | 4.310 | 2.752 to 6.750 |
| Bone marrow blast count | .004 | 1.049† | 1.016 to 1.083 |
| Prior chemotherapy or CMT‡ | .41 | 1.203 | 0.778 to 1.859 |
| Prior XRT‡ | .38 | 0.781 | 0.451 to 1.351 |
Abbreviations: CMT, combined-modality therapy; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; XRT, external-beam radiation therapy.
Hazard ratio per 1-year increase of age.
Hazard ratio per 1% increase in blasts.
Hazard ratio compared with de novo MDS/CMML group.
Table A3.
Multivariate Cox Proportional Hazard Analysis for Overall Survival of Patients With AML, All Groups (n = 201)
| Variable | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| Patient age | < .001 | 1.040* | 1.025 to 1.055 |
| High-risk karyotype (UKMRC) | < .001 | 2.710 | 1.870 to 3.928 |
| Prior chemotherapy or CMT† | .41 | 1.176 | 0.801 to 1.728 |
| Prior XRT† | .13 | 0.626 | 0.341 to 1.150 |
Abbreviations: AML, acute myeloid leukemia; CMT, combined modality therapy; UKMRC, United Kingdom Medical Research Council; XRT, external-beam radiation therapy.
Hazard ratio per 1-year increase of age.
Hazard ratio compared with de novo AML group.
Fig A1.
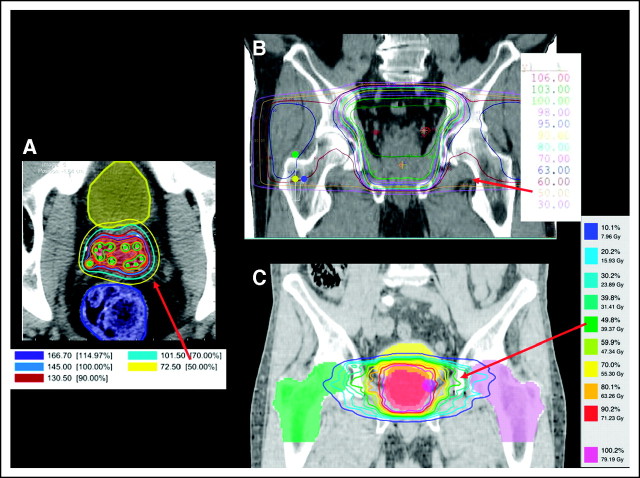
Estimating marrow exposure from radiation therapy: example from treatment of prostate cancer. Radiation therapy planning generates a set of isodose lines that indicate the dosage to the tissue contained therein. Each colored line represents a different percentage of the total prescribed dose. For example, the red arrow in each picture points to the 50% isodose line; the tissue contained within that line will receive 50% of the prescribed dose. More conformal treatment techniques expose less normal tissue to radiation, including marrow. (A) Axial image representing postimplant dosimetry after prostate seed brachytherapy. (B) Coronal image depicting treatment of a small pelvic field using four beams (similar to the “four-field box” plans that were more common before the year 2000). (C) Coronal images of an intensity-modulated radiation therapy plan to treat the prostate (typical treatment after the year 2000).
Footnotes
See accompanying editorial on page 2300
Presented in part as a platform presentation at the 2011 Annual Meeting of the United States and Canadian Academy of Pathology, San Antonio, TX, February 26-March 4, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Valentina Nardi, Robert P. Hasserjian
Collection and assembly of data: Valentina Nardi, Karen M. Winkfield, Chi Young Ok, Andrzej Niemierko, Michael J. Kluk, Sa A. Wang, Robert P. Hasserjian
Data analysis and interpretation: Valentina Nardi, Karen M. Winkfield, Andrzej Niemierko, Eyal C. Attar, Guillermo Garcia-Manero, Robert P. Hasserjian
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Allan WS. Acute myeloid leukaemia after treatment with cytostatic agents. Lancet. 1970;2:775. doi: 10.1016/s0140-6736(70)90250-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown WM, Doll R. Mortality from cancer and other causes after radiotherapy for ankylosing spondylitis. BMJ. 1965;2:1327–1332. doi: 10.1136/bmj.2.5474.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit CG, Meyler L. Acute myeloid leukaemia after treatment with cytostatic agents. Lancet. 1970;2:671–672. doi: 10.1016/s0140-6736(70)91448-0. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Golomb HM, Rowley JD, et al. Acute nonlymphocytic leukemia in malignant lymphoma: A morphologic study. Cancer. 1978;42:229–242. doi: 10.1002/1097-0142(197807)42:1<229::aid-cncr2820420137>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Damiani D, Michieli M, Ermacora A, et al. P-glycoprotein (PGP), and not lung resistance-related protein (LRP), is a negative prognostic factor in secondary leukemias. Haematologica. 1998;83:290–297. [PubMed] [Google Scholar]
- 6.Estey EH. Prognosis and therapy of secondary myelodysplastic syndromes. Haematologica. 1998;83:543–549. [PubMed] [Google Scholar]
- 7.Leone G, Mele L, Pulsoni A, et al. The incidence of secondary leukemias. Haematologica. 1999;84:937–945. [PubMed] [Google Scholar]
- 8.Leoni F, Ciolli S, Nozzoli C, et al. Idarubicin in induction treatment of acute myeloid leukemia in the elderly. Haematologica. 1997;82:13–18. [PubMed] [Google Scholar]
- 9.Phillips MJ, Cull GM, Ewings M. Establishing the incidence of myelodysplasia syndrome. Br J Haematol. 1994;88:896–897. doi: 10.1111/j.1365-2141.1994.tb05138.x. [DOI] [PubMed] [Google Scholar]
- 10.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35:418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litzow MR, Tarima S, Pérez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh ZN, Huo D, Anastasi J, et al. Therapy-related myelodysplastic syndrome: Morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197–205. doi: 10.1309/NQ3PMV4U8YV39JWJ. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman JW, Matutes E, Arber DA, et al. Therapy related myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. pp. 127–129. [Google Scholar]
- 14.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 15.Seedhouse C, Russell N. Advances in the understanding of susceptibility to treatment-related acute myeloid leukaemia. Br J Haematol. 2007;137:513–529. doi: 10.1111/j.1365-2141.2007.06613.x. [DOI] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern W, Haferlach T, Schnittger S, et al. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 18.Schoch C, Kern W, Schnittger S, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): An analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen-Bjergaard J, Christiansen DH, Desta F, et al. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 20.Cronkite EP, Moloney W, Bond VP. Radiation leukemogenesis: An analysis of the problem. Am J Med. 1960;28:673–682. doi: 10.1016/0002-9343(60)90126-1. [DOI] [PubMed] [Google Scholar]
- 21.von Jagie N, Schwarz G, von Siebenbach L. Blutbefunde bei Roentgenologen. Berl klin Wchnschr. 1911;48:1220. [Google Scholar]
- 22.Mole RH. The development of leukaemia in irradiated animals. Br Med Bull. 1958;14:174–177. doi: 10.1093/oxfordjournals.bmb.a069664. [DOI] [PubMed] [Google Scholar]
- 23.Abdelhameed A, Pond GR, Mitsakakis N, et al. Outcome of patients who develop acute leukemia or myelodysplasia as a second malignancy after solid tumors treated surgically or with strategies that include chemotherapy and/or radiation. Cancer. 2008;112:1513–1521. doi: 10.1002/cncr.23325. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian HM, Keating MJ, Walters RS, et al. Therapy-related leukemia and myelodysplastic syndrome: Clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4:1748–1757. doi: 10.1200/JCO.1986.4.12.1748. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 26.Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CK. Evolving role of radiation therapy for hematologic malignancies. Hematol Oncol Clin North Am. 2006;20:471–503. doi: 10.1016/j.hoc.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 30.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 31.Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–258. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 32.Andersen MK, Larson RA, Mauritzson N, et al. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: Report from an international workshop. Genes Chromosomes Cancer. 2002;33:395–400. doi: 10.1002/gcc.10043. [DOI] [PubMed] [Google Scholar]
- 33.Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin's lymphoma: Results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2003;21:3601–3608. doi: 10.1200/JCO.2003.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin's lymphoma: Long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol. 2006;24:3128–3135. doi: 10.1200/JCO.2005.05.2746. [DOI] [PubMed] [Google Scholar]
- 35.Fiorino C, Valdagni R, Rancati T, et al. Dose-volume effects for normal tissues in external radiotherapy: Pelvis. Radiother Oncol. 2009;93:153–167. doi: 10.1016/j.radonc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Rassool FV, Gaymes TJ, Omidvar N, et al. Reactive oxygen species, DNA damage, and error-prone repair: A model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007;67:8762–8771. doi: 10.1158/0008-5472.CAN-06-4807. [DOI] [PubMed] [Google Scholar]
- 37.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 38.Rothkamm K, Kühne M, Jeggo PA, et al. Radiation-induced genomic rearrangements formed by nonhomologous end-joining of DNA double-strand breaks. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 39.Philip P, Pedersen-Bjergaard J. Cytogenetic, clinical, and cytologic characteristics of radiotherapy-related leukemias. Cancer Genet Cytogenet. 1988;31:227–236. doi: 10.1016/0165-4608(88)90221-x. [DOI] [PubMed] [Google Scholar]
- 40.Choy H, Kim DW. Chemotherapy and irradiation interaction. Semin Oncol. 2003;30:3–10. doi: 10.1016/s0093-7754(03)00268-9. [DOI] [PubMed] [Google Scholar]