Abstract
Purpose
Established guidelines recommend evaluation for hereditary cancer syndromes in patients younger than 50 years diagnosed with colorectal cancer (CRC). This group has been well described in the literature; however, patients diagnosed as adolescents and young adults are not well represented in CRC studies. Here, we define the clinical profile, including the extent of hereditary cancer syndromes and family history of cancer, in patients diagnosed with CRC at age 35 or younger.
Patients and Methods
We reviewed patients who underwent genetic counseling at our institution during 5 years (2009 to 2013). Data were collected regarding demographics, clinicopathologic information, tumor and genetic testing, and family history. Patients with an identified hereditary cancer syndrome were compared with those without a syndrome.
Results
Of the 193 patients with evaluable data, 35% had an identifiable hereditary cancer syndrome, including 23 with Lynch syndrome, 22 with mutation-negative Lynch syndrome, 16 with familial adenomatous polyposis, two with constitutional mismatch repair deficiency, two with biallelic MUTYH mutations, and one with Li-Fraumeni syndrome. Patients without a hereditary syndrome more frequently presented with metastatic disease, whereas patients with a syndrome were more likely to present at earlier stages and to have a family history of cancer. Nevertheless, a substantial proportion of the hereditary syndromes (19%) were diagnosed in individuals with no family history of the disease.
Conclusion
We conclude that patients diagnosed with CRC at age 35 years or younger should receive genetic counseling regardless of their family history and phenotype.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in incidence and the third leading cause of cancer-related deaths worldwide.1 Although most individuals with CRC do not have a family history of the disease, approximately 15% to 30% of patients with CRC have an aggregation of CRC among their family members, which merits the designation of familial CRC. Only 2% to 5% of CRCs are hereditary, caused by germline mutations in highly penetrant genes.2
CRC is widely considered to be a disease of individuals older than 50 years, and most diagnoses are made between ages 65 and 74.3 However, CRC diagnoses in adolescents and young adults have attracted attention4,5 because of the recent increase in the number of these occurrences.6 In addition, this young group is unique because of issues related to disease aggressiveness, the impact of treatment on fertility, and the potential genetic risk in family members.4
Although intestinal carcinogenesis is thought to be associated with environmental factors, such as increased red-meat intake and exposure to carcinogens,7 the impact of genetic risk factors is evident, especially in Lynch syndrome (LS), familial adenomatous polyposis (FAP), and, to a lesser extent, familial CRC. The prevalence of these genetic risk factors has been studied in the general population of individuals with CRC but has not been well defined in adolescents and young adults with CRC.
Most publications regarding young-onset CRC have focused on patients diagnosed at younger than 40 or 50 years and on tumor characteristics and survival rather than on family history and hereditary cancer syndromes.5,8 However, there is no consensus in the literature about the age that defines young-onset CRC. In addition, extreme phenotypic presentations in adolescents and young adults are not well understood, especially in terms of prevalence of family history of CRC and hereditary cancer syndromes. Here, we sought to better understand these variables in patients diagnosed with CRC at 35 years or younger, a population that accounted for less than 1.5% of CRC diagnoses,3 during a 5-year period in our institution. For this purpose, we hypothesized that the proportion of patients diagnosed with a hereditary cancer syndrome is higher among patients diagnosed with CRC at 35 years or younger than what has been previously reported in the general population of patients with CRC, because the average age at CRC diagnosis is younger in the context of hereditary cancer syndromes than in the general population.2
PATIENTS AND METHODS
Patient Selection
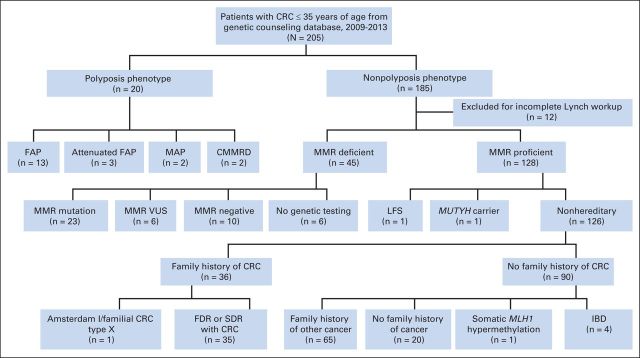
We included 205 patients who were diagnosed with CRC at 35 years or younger and were evaluated by genetic counseling at the MD Anderson Cancer Center from 2009 to 2013. Approximately 225 new patients meeting this criterion were seen at our institution during this time period (average, 45 patients per year). This patient population is derived from our institutional catchment area of MD Anderson, which is focused in Texas. Patients were referred to genetic counseling by MD Anderson providers per established referral criteria or at the provider's discretion. All patients diagnosed with CRC at 35 years or younger met our referral criteria regardless of family history. All patients who underwent genetic counseling and were diagnosed with CRC within that age range were included regardless of whether the diagnosis was new in 2009 to 2013 or the patient was referred in 2009 to 2013 because of a previous diagnosis at age 35 or younger, even if the patient was over the cutoff age at the time of genetic counseling. Patients without an identifiable hereditary cancer phenotype (ie, polyposis) were excluded if they had an incomplete work-up for LS (absence of results for microsatellite instability [MSI] analysis by polymerase chain reaction or immunohistochemistry [IHC] of the mismatch repair genes [MMR] in the tumor, or germline genetic testing; Fig 1). Clinical data, including medical history and pathology data, were obtained from the electronic medical record. Family history data were obtained by a genetic counselor and recorded in a pedigree. This study was approved by the MD Anderson Cancer Center institutional review board.
Fig 1.
CONSORT diagram. CMMRD, constitutional mismatch repair deficiency; CRC, colorectal cancer; FAP, familial adenomatous polyposis; FDR, first-degree relative; IBD, inflammatory bowel disease; LFS, Li-Fraumeni syndrome; MAP, MUTYH-associated polyposis; MMR, mismatch repair; SDR, second-degree relative; VUS, variant of uncertain significance.
Genetic Evaluation and Patient Grouping
All patients underwent the following standard evaluation at the time of genetic counseling: Patients with a clear clinical phenotype for a hereditary cancer syndrome (ie, polyposis) underwent syndrome-specific genetic testing on the basis of phenotype. Genetic testing for other patients was recommended after genetic risk assessment on the basis of personal and family histories of cancer, as well as results of MSI and IHC tumor analyses. All patients without a clear syndromic phenotype underwent MSI and IHC analyses of the MMR proteins or germline genetic testing of the MMR genes for evaluation of LS at minimum; additional genetic testing was recommended on the basis of the results of these tests and the personal and family histories of cancer. All genetic testing was performed at Clinical Laboratory Improvement Amendments–certified laboratories.
According to the outcomes of the genetic work-up, patients with a confirmed genetic mutation, polyposis phenotype, or MMR-deficient tumor were placed in the hereditary group. Those without a detectable hereditary syndrome were place in the nonhereditary group (Fig 1).
Statistical Analysis
Demographics, clinicopathologic characteristics, and personal and family history variables were analyzed. Descriptive statistics were summarized as frequency distributions for categorical variables and as means for continuous variables. Wilcoxon rank sum tests were performed to compare continuous variables, and the χ2 or Fisher's exact test was performed to compare categorical variables; P values ≤ .05 were considered statistically significant. Data were processed and analyzed by using SAS software, version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patients
Of the 205 patients with CRC diagnosed at 35 years or younger who were assessed for genetic counseling in 2009 to 2013, 193 had sufficient genetic data and were included. The clinicopathologic characteristics of this population are described in Table 1. The mean age at diagnosis was 29 years, and most patients were women and white. The rectum was the most common tumor site, which was followed by the left and the right colon. Patients most frequently presented with stage IV disease at diagnosis. Forty-seven patients had poorly differentiated tumors, and 21 patients presented with signet ring cells. All patients met the Revised Bethesda guidelines solely on the basis of age at diagnosis,9 and 12 met Amsterdam I or II criteria.10 Twenty-three patients had a first-degree relative with CRC, 62 had a second-degree relative with CRC, and 33 patients (17%) had no relatives with any cancer.
Table 1.
Patient Demographics and Clinicopathologic Characteristics
| Characteristic | No. (%) of Patients |
P (hereditary v nonhereditary)* | ||
|---|---|---|---|---|
| With Hereditary Syndrome (n = 67) | Without Hereditary Syndrome (n = 126) | Total (N = 193) | ||
| Mean (range) age at diagnosis, years | 28.8 (17-35) | 29.1 (12-35) | 29 (12-35) | NS |
| Female sex | 33 (49.2) | 68 (54.0) | 101 (52.3) | NS |
| Race/ethnicity | NS | |||
| White | 51 (76.1) | 94 (74.6) | 145 (75.1) | |
| Black | 5 (7.5) | 9 (7.1) | 14 (7.3) | |
| Hispanic | 8 (11.9) | 18 (14.3) | 26 (13.5) | |
| Asian | 3 (4.5) | 5 (4.0) | 8 (4.1) | |
| Colorectal cancer site | < .001 | |||
| Right colon | 15 (22.4) | 25 (19.8) | 40 (20.7) | |
| Left colon | 12 (17.9) | 46 (36.5) | 58 (30.0) | |
| Rectum | 29 (43.3) | 54 (42.9) | 83 (43.0) | |
| Not specified | 11 (16.4) | 1(0.8) | 12 (6.2) | |
| Tumor stage | < .001 | |||
| 0/Tis | 1 (1.5) | 1 (0.8) | 2 (1.0) | |
| I | 8 (11.9) | 7 (5.6) | 15 (7.8) | |
| II | 11 (16.4) | 8 (6.3) | 19 (9.8) | |
| III | 20 (29.9) | 38 (30.2) | 58 (30.1) | |
| IV | 19 (28.4) | 71 (56.3) | 90 (46.6) | |
| Unknown | 8 (11.9) | 1 (0.8) | 9 (4.7) | |
| Grade of differentiation | < .001 | |||
| Moderately differentiated | 45 (67.2) | 84 (66.7) | 129 (66.8) | |
| Poorly differentiated | 8 (11.9) | 39 (31.0) | 47 (24.4) | |
| Unknown | 14 (20.9) | 3 (2.4) | 17 (10.4) | |
| Signet ring cells | < .001 | |||
| Yes | 3 (4.5) | 18 (14.3) | 21 (10.9) | |
| No | 50 (74.6) | 108 (85.7) | 158 (81.9) | |
| Unknown | 14 (20.9) | 0 (0) | 14 (7.2) | |
| Met Amsterdam I/II criteria | 11 (16.4) | 1 (0.8) | 12 (6.2) | |
| Family history | < .001 | |||
| FDR with CRC | 19 (28.4) | 4 (3.2) | 23 (11.9) | < .001 |
| FDR with other cancer | 20 (29.9) | 30 (23.8) | 50 (25.9) | NS |
| SDR with CRC | 28 (41.8) | 33 (26.2) | 62 (32.1) | < .047 |
| SDR with other cancer | 35 (52.2) | 95 (75.4) | 130 (67.4) | .001 |
| Personal history of other cancer | 22 (32.8) | 13 (10.3) | 35 (18.1) | < .001 |
Abbreviations: CRC, colorectal cancer; FDR, first-degree relative; NS, not significant; SDR, second-degree relative.
All tests were two sided.
Outcomes of Genetic Counseling and Testing
After completion of the genetic evaluation, 67 patients in the study cohort had a hereditary cancer syndrome on the basis of positive genetic test results, polyposis phenotype, or a tumor displaying MMR deficiency (34.7% of the study population), and the other 126 patients (65.3%) had no identified hereditary syndromes. Four of the patients without a syndrome had a previous history of inflammatory bowel disease. Twenty patients in the study cohort presented with a polyposis phenotype; 13 patients (19.4% of the 67 with hereditary syndromes) were diagnosed with classic FAP with a pathogenic APC mutation. Two patients (3%) in the polyposis group tested negative for APC mutations but tested positive for biallelic MUTYH mutations. Three patients (4.5%) with polyposis had a clinical diagnosis of attenuated FAP and no identified germline mutations. Two patients with polyposis were evaluated for APC and MUTYH mutations and had negative results but, because of personal and family histories and/or tumor IHC results, underwent MMR gene testing and had biallelic mutations (one in MSH6 and the other in PMS2). Therefore, both patients were diagnosed with constitutional mismatch repair deficiency. One patient without polyposis had a phenotype consistent with Li-Fraumeni syndrome because of a personal history of cancers, including osteosarcoma and astrocytoma, and a germline TP53 mutation was identified. Finally, one patient had a monoallelic MUTYH mutation.
Patients without an identifiable polyposis or other phenotype underwent evaluation for LS at minimum through a combination of MSI and IHC tumor studies and/or germline genetic testing. Twenty-three of these patients had pathogenic germline mutations in MMR genes and therefore were diagnosed with LS (34.3% of 67 patients with hereditary syndromes): six had mutations in MLH1 (MutL homolog 1); nine, in MSH2; five, in MSH6; two, in PMS2; and one, in EPCAM. Another 22 patients (32.8%) without an identifiable phenotype had MMR-deficient tumors (demonstrated through the absence of IHC staining and/or high MSI) but did not harbor pathogenic mutations; therefore, they were diagnosed with mutation-negative LS.11 Six of the patients with MMR-deficient tumors had a variant of uncertain significance identified: three had a variant in MLH1; two, in MSH2; and one, in PMS2. These genetic alterations were still classified as variants of uncertain significance during preparation of this article. Of the 16 remaining patients with mutation-negative LS, 10 underwent genetic testing per the pattern of protein loss on IHC with uninformative negative germline genetic test results. The other six patients with mutation-negative LS had incomplete work-ups as a result of patient death, loss to follow-up, or patient refusal of germline testing.
The remaining patients without a hereditary cancer syndrome phenotype (n = 126) all were evaluated for LS through MSI and IHC tumor studies and/or germline MMR testing. Of the original cohort, 125 patients had normal tumor testing (ie, microsatellite stability or intact MMR proteins by IHC) or normal germline MMR genetic test results. One patient in this group with abnormal tumor testing had an absence of MLH1 and PMS2 protein expression in the rectal tumor; the tumor was positive for somatic MLH1 promoter hypermethylation and tested negative for MLH1 germline mutations. Four patients with inflammatory bowel disease who were in this nonhereditary group underwent no additional testing after normal MSI and/or IHC results. The other 122 patients in this group underwent a variety of different germline tests because of age, family history, or clinical phenotype, including testing of MLH1 (n = 19), MSH2/EPCAM (n = 18), MSH6 (n = 22), PMS2 (n = 11), APC (n = 2), MUTYH (n = 14), TP53 (n = 10), CDH1 (n = 1), PTEN (n = 1), SMAD4 (n = 2), BMPR1A (n = 2), STK11 (n = 1), CHEK2 (n = 1), and RET (n = 1). No pathogenic mutations or variants of uncertain significance were identified in this group. Eighty-five (67.5%) of the 126 patients did not undergo germline testing after MSI and IHC. Thus, on the basis of the normal tumor testing and negative genetic testing, these 126 patients were considered the nonhereditary group. Of these 126 patients, 36 (28.6%) had a history of CRC in first-degree or second-degree relatives. The other 90 patients had no family history of CRC, and 20 of these patients had no family history of any cancers. One of the 126 patients met Amsterdam I criteria10 but had normal MSI and IHC and negative comprehensive MMR germline testing, thus meriting the designation of familial CRC type X.12 Ten percent (13 of 126) of patients in the nonhereditary group had a history of adenomatous polyps (mean adenomas per patient, 1.8; range, 1 to 6 adenomas), and one patient had several synchronous hamartomas adjacent to the tumor.
Mutations Detected in the Absence of Family History of CRC
Although some patients with a hereditary syndrome had a family history consistent with their diagnosis, several patients presented with germline mutations without a family history of CRC. Of the 16 patients with FAP or attenuated FAP, 10 apparently had de novo mutations, given the absence of polyposis and/or CRC in the family. Given the reported 25% de novo rate of APC mutations,13 new mutations are likely in this group. The other six patients each had a parent who had a known diagnosis of FAP, but the patients were not undergoing surveillance before CRC diagnosis. Of the 23 patients with mutation-positive LS, two had no family history of any LS cancer, which might have stemmed from incomplete penetrance. The other 21 patients with LS had a family history of LS cancers. Twenty patients were the first individuals in their families to be diagnosed with LS. One patient reported a sibling with a known LS mutation but did not undergo mutation testing before CRC diagnosis. The one patient with a TP53 mutation had an apparent de novo mutation.14 Overall, the hereditary group contained a high number of patients with no family history of disease, which represented a combination of de novo mutations and incomplete penetrance as well as index cases in families.
Comparison of Hereditary and Nonhereditary Groups
The hereditary and nonhereditary groups were compared with regard to their clinicopathologic characteristics, personal history, and family history (Table 1). No significant differences in age at diagnosis, sex, or race/ethnicity were noted between the groups. Nonhereditary cases were more likely to have a left-sided tumor (P < .001), to present with metastatic disease (P < .001), and to have poorly differentiated tumors (P < .001) or signet ring cells (P < .001), all features indicative of aggressive tumor behavior.
Regarding family history, as expected, patients with a hereditary syndrome were more likely than the nonhereditary group to meet Amsterdam I or II criteria (P < .001), to have a first-degree relative with CRC (P < .001), a second-degree relative with CRC (P = .047), or a second-degree relative with any non-CRC cancer (P = .001). There was no significant difference between the groups in first-degree relatives with any non-CRC cancer. Finally, patients with a hereditary syndrome were more likely to have a personal history of another cancer (P < .001). These findings are consistent with a stronger genetic predisposition toward developing CRC in the hereditary group.
DISCUSSION
This study, to our knowledge, consists of the largest reported cohort of patients diagnosed with CRC at age 35 years or younger in the United States. After undergoing a range of genetic testing, the majority of our cohort was not found to have a hereditary cancer syndrome; however, one third of our population had a hereditary cancer syndrome, which is much higher than the proportion with a hereditary cancer syndrome in the general CRC population (2% to 5%).2 It is also higher than the proportion reported in a recent study of patients diagnosed with CRC at 40 years or younger, in which 22.7% of patients had a hereditary syndrome.15 Of the patients with a hereditary cancer syndrome, one third were diagnosed with LS and one third had mutation-negative LS. Six patients with mutation-negative LS had incomplete work-ups for several reasons; therefore, we suspect that the number of patients with CRC who had mutation-positive LS could be higher than what is reported here. One fourth of the hereditary patients were diagnosed with a polyposis syndrome.
Patients without a hereditary syndrome were more likely to be diagnosed with a left-sided colon tumor, metastatic disease, and tumors with poor differentiation or signet ring cells. Patients with a hereditary syndrome were more likely to have a family history of cancer and personal history of another cancer. The difference between the hereditary and nonhereditary cases in stage at diagnosis may be related to a family history of cancer: We speculate that patients with a family history of cancer were diagnosed earlier because of surveillance measures undertaken as a result of family history or because of an earlier diagnosis when they presented with symptoms. Despite this pattern, our cohort included several patients with a known family history of FAP or LS who were not undergoing surveillance but were candidates for these measures. These findings underline the importance of surveillance observation of family members at risk for hereditary CRC.
Our results support a referral to genetic counseling for hereditary cancer syndromes for all patients diagnosed with CRC at 35 years or younger, regardless of family history of CRC, even when patients have normal MSI and IHC tumor study results, because 13 patients in our study cohort had germline mutations and no family history of disease. Our findings also support tumor testing in this young cohort to evaluate for LS, as recommended by the revised Bethesda guidelines9; however, evaluation for hereditary syndromes should not be limited to LS only. Although the incidence in CRC diagnosis is increasing in the adolescent and young adult population, perhaps as a result of diet and lifestyle factors,6 genetic evaluation is still warranted for this population.
One limitation of this study is that this group did not undergo uniform genetic testing, so there may be patients in this cohort whose underlying hereditary predispositions were not identified. Only two patients in this group underwent testing by using a panel of multiple hereditary cancer genes. Future studies in young populations may benefit from implementation of a comprehensive hereditary CRC panel to identify young patients who may present with an atypical phenotype or young patients with CRC without a family history of cancer who may harbor a de novo mutation or a syndrome with incomplete penetrance.
Although patients with a hereditary syndrome were diagnosed at earlier stages than those without a syndrome, nearly half of our cohort was diagnosed with metastatic disease. For these patients, genetic assessment may be seen as low priority, because oncologic care tends to be prioritized. However, referral for genetic counseling is critical, because it may help diagnose a genetic condition that will affect the care of family members and may trigger needed surveillance or chemopreventive treatments for those relatives.
Another limitation of our study is a possible referral bias: Patients with a family history of cancer might have been referred to genetic counseling more frequently than patients without a family history of cancer, potentially skewing the frequency of hereditary syndromes in our population. However, regardless of family history, patients diagnosed with CRC at 35 years or younger meet the revised Bethesda guidelines and met institutional referral criteria for genetic evaluation; therefore, all patients evaluated in our center who were diagnosed with CRC in this age group should have been referred for genetic counseling.
In conclusion, patients diagnosed with CRC at 35 years or younger have a higher frequency of hereditary predispositions to CRC than the general population of patients with CRC. Hereditary syndromes in these patients are not limited to LS or FAP, nor to those with a family history of cancer. Adolescent and young adult patients with CRC should be referred for a hereditary cancer work-up and may benefit from the use of comprehensive hereditary CRC genetic testing panels, especially in the absence of a clinical phenotype or with normal MSI and IHC tumor studies.
Acknowledgment
We thank Sarah Bronson for editorial assistance.
Glossary Terms
- APC:
a tumor suppressor gene. Mutations in the APC gene are responsible for familial adenomatous polyposis (germline mutations) or sporadic (somatic mutations) colorectal tumors. The gene product is known to interact with adherens junction proteins, a- and b-catenins, suggesting a role in cell adhesion.
- biallelic:
the condition in which both alleles of a gene are mutated.
- germline mutation:
an inherited variation in the lineage of germ cells. Germline mutations can be passed on to offspring.
- immunohistochemistry:
the application of antigen-antibody interactions to histochemical techniques. Typically, a tissue section is mounted on a slide and incubated with antibodies (polyclonal or monoclonal) specific to the antigen (primary reaction). The antigen-antibody signal is then amplified using a second antibody conjugated to a complex of peroxidase-antiperoxidase, avidin-biotin-peroxidase, or avidin-biotin alkaline phosphatase. In the presence of substrate and chromogen, the enzyme forms a colored deposit at the sites of antibody-antigen binding. Immunofluorescence is an alternate approach to visualize antigens. In this technique, the primary antigen-antibody signal is amplified using a second antibody conjugated to a fluorochrome. On ultraviolet light absorption, the fluorochrome emits its own light at a longer wavelength (fluorescence), thus allowing localization of antibody-antigen complexes.
- Lynch syndrome:
hereditary nonpolyposis colorectal cancer (HNPCC). A cancer syndrome characterized by Henry T. Lynch in 1966, this genetic condition has a high risk of colon cancer as well as other cancers including endometrial, ovary, stomach, small intestine, hepatobiliary tract, upper urinary tract, brain, and skin.
- microsatellite instability:
the genomic instability associated with the presence of microsatellites (repeating units in DNA of 1-5 basepairs that are ubiquitous, abundant, and repeated several times in eukaryotic genomes), giving rise to mutations that involve the addition or subtraction of one or two repeat units.
- mismatch repair genes (MMR):
genes that recognize and correct errors in DNA replication leading to single base-pair mismatches or insertions/deletions in small repetitive tracts of DNA known as microsatellites.
- MLH1 (MutL homolog 1):
a DNA mismatch repair enzyme. MLH1 is responsible for overall fidelity of DNA replication.
- promoter hypermethylation:
methylation of the promoter region of a gene, which can lead to DNA silencing as a consequence of the inability of activating transcriptional factors to bind to the promoter region, a process important in gene transcription. In addition, repressor complexes may be attracted to sites of promoter methylation, leading to the formation of inactive chromatin structures.
Footnotes
See accompanying editorial on page 3525
Supported by a gift from the Feinberg Family Fund (to E.V).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The author(s) disclose no potential conflicts of interest.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Maureen E. Mork, Eduardo Vilar
Financial support: Eduardo Vilar
Administrative support: Eduardo Vilar
Provision of study materials or patients: Maureen E. Mork, Y. Nancy You, Sarah A. Bannon, Patrick M. Lynch, Miguel A. Rodriguez-Bigas, Eduardo Vilar
Collection and assembly of data: Maureen E. Mork, Y. Nancy You, Sarah A. Bannon, Patrick M. Lynch, Miguel A. Rodriguez-Bigas, Eduardo Vilar
Data analysis and interpretation: Maureen E. Mork, Jun Ying, Eduardo Vilar
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Maureen E. Mork
No relationship to disclose
Y. Nancy You
No relationship to disclose
Jun Ying
No relationship to disclose
Sarah A. Bannon
No relationship to disclose
Patrick M. Lynch
No relationship to disclose
Miguel A. Rodriguez-Bigas
No relationship to disclose
Eduardo Vilar
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance, Epidemiology, and End Results Program. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Factsheets: Colon and rectum cancer. http://www.seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 4.Hubbard JM, Grothey A. Adolescent and young adult colorectal cancer. J Natl Compr Canc Netw. 2013;11:1219–1225. doi: 10.6004/jnccn.2013.0144. [DOI] [PubMed] [Google Scholar]
- 5.Stigliano V, Sanchez-Mete L, Martayan A, et al. Early-onset colorectal cancer: A sporadic or inherited disease? World J Gastroenterol. 2014;20:12420–12430. doi: 10.3748/wjg.v20.i35.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slattery ML, Boucher KM, Caan BJ, et al. Eating patterns and risk of colon cancer. Am J Epidemiol. 1998;148:4–16. doi: 10.1093/aje/148.1.4-a. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Bigas MA, Mahoney MC, Weber TK, et al. Colorectal cancer in patients aged 30 years or younger. Surg Oncol. 1996;5:189–194. doi: 10.1016/s0960-7404(96)80043-0. [DOI] [PubMed] [Google Scholar]
- 9.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasen HF. Clinical diagnosis and management of hereditary colorectal cancer syndromes. J Clin Oncol. 2000;18:81S–92S. [PubMed] [Google Scholar]
- 11.You YN, Vilar E. Classifying MMR variants: Time for revised nomenclature in Lynch syndrome. Clin Cancer Res. 2013;19:2280–2282. doi: 10.1158/1078-0432.CCR-13-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: Familial colorectal cancer type X. JAMA. 2005;293:1979–1985. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisgaard ML, Fenger K, Bulow S, et al. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121–125. doi: 10.1002/humu.1380030206. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet. 2009;46:689–693. doi: 10.1136/jmg.2008.058958. [DOI] [PubMed] [Google Scholar]
- 15.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]