Abstract
Purpose
To evaluate the safety and efficacy of initial treatment with imatinib mesylate 800 mg/d (400 mg twice daily) versus 400 mg/d in patients with newly diagnosed chronic myeloid leukemia in chronic phase.
Patients and Methods
A total of 476 patients were randomly assigned 2:1 to imatinib 800 mg (n = 319) or 400 mg (n = 157) daily. The primary end point was the major molecular response (MMR) rate at 12 months.
Results
At 12 months, differences in MMR and complete cytogenetic response (CCyR) rates were not statistically significant (MMR, 46% v 40%; P = .2035; CCyR, 70% v 66%; P = .3470). However, MMR occurred faster among patients randomly assigned to imatinib 800 mg/d, who had higher rates of MMR at 3 and 6 months compared with those in the imatinib 400-mg/d arm (P = .0035 by log-rank test). CCyR also occurred faster in the 800-mg/d arm (CCyR at 6 months, 57% v 45%; P = .0146). The most common adverse events were edema, gastrointestinal problems, and rash, and all were more common in patients in the 800-mg/d arm. Grades 3 to 4 hematologic toxicity also occurred more frequently in patients receiving imatinib 800 mg/d.
Conclusion
MMR rates at 1 year were similar with imatinib 800 mg/d and 400 mg/d, but MMR and CCyR occurred earlier in patients treated with 800 mg/d. Continued follow-up is needed to determine the clinical significance of earlier responses on high-dose imatinib.
INTRODUCTION
Imatinib mesylate (ie, imatinib) is the currently approved first-line treatment for chronic myeloid leukemia (CML).1,2 Seven-year data from the phase III International Randomized Study of Interferon and STI571 (IRIS), which compared imatinib with interferon-α plus cytarabine, demonstrated a cumulative best complete cytogenetic response (CCyR) rate of 82% in patients randomly assigned to imatinib.3 At 7 years, freedom from progression to accelerated phase/blast crisis (AP/BC) was 93%, and overall survival (OS) was 86%.3 Achievement of MMR on imatinib is predictive of long-term event-free and transformation-free survivals.4–8
The recommended starting doses of imatinib are 400 mg/d for patients with CML in chronic phase (CML-CP) and 600 mg/d for patients in AP or BC. Despite high response rates with imatinib at 400 mg/d, data from nonrandomized studies suggest that initial therapy with imatinib 800 mg/d might lead to higher CCyR and major molecular response (MMR) rates and that these responses might occur faster than with the standard 400 mg/d dose in patients with CML-CP.9–13
A phase III, randomized study was conducted to evaluate whether treatment with imatinib 800 mg/d improves outcomes compared with imatinib 400 mg/d in patients with newly diagnosed, previously untreated, Philadelphia chromosome–positive (Ph+) CML-CP, and the rate of MMR at 12 months was the primary end point.
PATIENTS AND METHODS
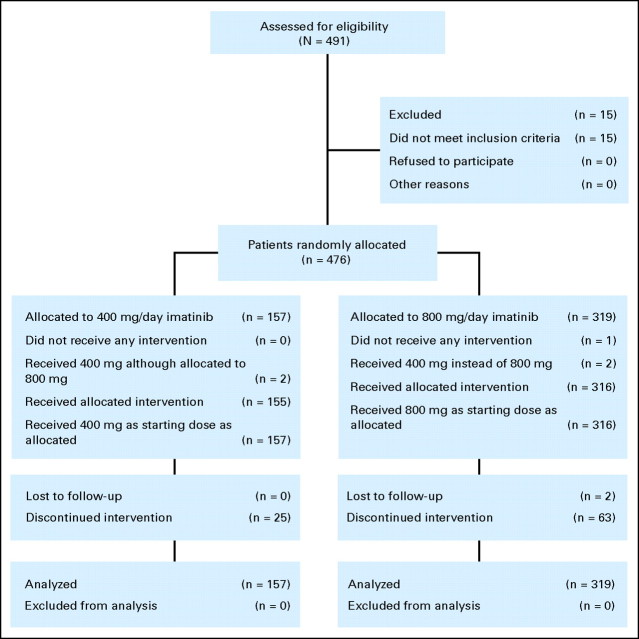
Inclusion criteria, response definitions, and a detailed description of molecular assessments are provided in the data supplement (online only). Patients with newly diagnosed, Ph+ CML-CP between the ages of 18 and 75 years were eligible. All patients were enrolled within 6 months of diagnosis. Patients had no prior treatment for CML (except hydroxyurea, anagrelide, or ≤ 2 weeks of prior imatinib; Fig 1).
Fig 1.
CONSORT diagram.
Trial Design
In this phase III, open-label, randomized, multicenter study, patients were randomly assigned 2:1 to imatinib 800 mg (400 mg twice daily) or 400 mg (once daily). Patients were stratified according to Sokal score at diagnosis.14
Complete blood counts were measured at baseline; at weeks 1, 2, and 4 monthly until month 6; and every 3 months thereafter until the end of the study. Conventional bone marrow cytogenetics were performed at baseline, at months 6 and 12, and every 6 months until CCyR was achieved. Molecular response was assessed by real-time, quantitative, reverse transcriptase polymerase chain reaction (RQ-PCR) at baseline, then monthly for 3 months, then every 3 months.
Patients were allowed to discontinue treatment in cases of progression or intolerance. In the absence of dose-limiting adverse events, patients in the imatinib 400-mg/d arm were allowed to escalate the dose to 800 mg/d if the following criteria for response failure were met: no complete hematologic response (CHR) by 3 months, no or minimal cytogenetic response (ie, > 65% Ph+ metaphases in bone marrow) by 6 months, or no CCyR (ie, ≥ 1% Ph+ metaphases in bone marrow) by 12 months. Patients in the 800-mg/d arm were not permitted to escalate the dose. Dose reductions were allowed for grades 3 to 4 toxicity or for persistent grade 2 toxicity.
End Points
The primary end point of this study was the rate of MMR at 12 months, defined as a BCR-ABL:control gene ratio ≤ 0.1% by RQ-PCR in peripheral blood expressed on the international scale.15–17 Four regional laboratories were used to perform molecular analyses. Month-12 samples also were forwarded to the Institute of Medical and Veterinary Science (Adelaide, Australia) for central evaluation. If no 12-month PCR data were available and if results from both months 9 and 15 indicated MMR, patients were classified as having achieved MMR. Imputation of outcome was possible for 11 patients, which resulted in five additional imputed responses for the primary end point. The patient was classified as a nonresponder if no data were available at 12 months.
Secondary objectives included time to MMR, rate of CCyR, time to CCyR, progression-free survival (PFS, defined as progression to AP/BC or death as a result of any cause while on study treatment), OS, safety, and actual dose intensity delivered in each arm. Events were defined as the first occurrence of any of the following on study treatment: death as a result of any cause, progression to AP or BC, loss of major CyR, or loss of CHR.
Safety Analysis
Safety analyses included all patients who received at least one dose of study medication. Patients were analyzed according to the dose received at the start of the treatment. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Analysis
Efficacy analyses were performed on the intent-to-treat (ITT) regardless of dose escalation. When response rates at any particular time point were evaluated, patients with missing data at that assessment were treated like nonresponders in the ITT analysis (exceptions described in the End Points section).
A two-sided Fisher's exact test at the 5% alpha level was used for comparison of MMR rates. An exploratory Cochran-Mantel-Haenszel test stratified by baseline Sokal score was performed to account for the effect of Sokal score on MMR.
Time to response, duration of response, and long-term end points were analyzed by using the Kaplan-Meier method, and treatment differences were evaluated by log-rank test. Patients who discontinued study drug were observed for survival every 3 months.
Ethics and Study Management
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was reviewed by the ethics committee or review board at each participating institution. Patients were required to give written informed consent. This trial was registered at http://www.clinicaltrials.gov as NCT00124748.
RESULTS
Patients and Treatment
Between June 2005 and December 2006, 476 patients were randomly assigned to imatinib 800 mg/d (n = 319) or 400 mg/d (n = 157) at 103 sites in 19 countries. This analysis is based on data collected until December 31, 2007, after all patients had reached at least 1 year of study treatment or had discontinued treatment. The median follow-up was 17 months for both arms. There were no clinically significant differences in baseline characteristics between arms (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Patients by Imatinib Dose |
|||||
|---|---|---|---|---|---|---|
| 400 mg (n = 157) |
800 mg (n = 319) |
All (N = 476) |
||||
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 45 | 48 | 47 | |||
| Range | 18-75 | 18-75 | 18-75 | |||
| Male sex | 84 | 53.5 | 183 | 57.4 | 267 | 56.1 |
| ECOG performance status | ||||||
| 0 | 124 | 79.0 | 244 | 76.5 | 368 | 77.3 |
| 1 | 28 | 17.8 | 64 | 20.1 | 92 | 19.3 |
| 2 | 2 | 1.3 | 5 | 1.6 | 7 | 1.5 |
| Ethnicity | ||||||
| Asian | 22 | 14.0 | 42 | 13.2 | 64 | 13.4 |
| Black | 12 | 7.6 | 17 | 5.3 | 29 | 6.1 |
| White | 109 | 69.4 | 241 | 75.5 | 350 | 73.5 |
| Other | 14 | 8.9 | 19 | 6.0 | 33 | 6.9 |
| Time since diagnosis, days | ||||||
| Median | 28 | 28 | 28 | |||
| Range | −6 to 193* | 1 to 217 | −6 to 217* | |||
| Sokal risk group | ||||||
| Low | 62 | 39.5 | 135 | 42.3 | 197 | 41.4 |
| Intermediate | 53 | 33.8 | 111 | 34.8 | 164 | 34.5 |
| High | 42 | 26.8 | 73 | 22.9 | 115 | 24.2 |
| Prior treatment | ||||||
| Hydroxyurea | 109 | 69.4 | 227 | 71.2 | 336 | 70.6 |
| Anagrelide | 1 | 0.6 | 5 | 1.6 | 6 | 1.3 |
| Imatinib | 3 | 1.9 | 13 | 4.1 | 16 | 3.4 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
One patient was enrolled on the basis of first results received, and final cytogenetic confirmation was 6 days after random assignment.
Median duration of imatinib exposure was comparable between arms: 16.8 months in the 400-mg/d arm (range, 1.6 to 28.1 months) and 16.3 months in the 800-mg/d arm (range, 0.3 to 29.9 months). At data cutoff, 388 (81.5%) of 476 randomly assigned patients were still on study treatment (Table 2). Treatment discontinuation occurred in 15.9% of patients randomly assigned to imatinib 400 mg/d and in 19.7% of patients randomly assigned to imatinib 800 mg/d. The most frequent reasons for discontinuation were adverse events in the high-dose arm and unsatisfactory therapeutic effect in the 400-mg/d arm.
Table 2.
Patient Disposition and Discontinuation Reasons
| Variable | Patients by Imatinib Dose |
|||
|---|---|---|---|---|
| 400 mg (n = 157) |
800 mg (n = 319) |
|||
| No. | % | No. | % | |
| Treatment disposition | ||||
| Continued initial treatment | 132 | 84.1 | 256 | 80.3 |
| Discontinued initial treatment | 25 | 15.9 | 63 | 19.7 |
| Reason for discontinuation | ||||
| Adverse events | 6 | 3.8 | 30 | 9.4 |
| Abnormal laboratory value(s) | 1 | 0.6 | 2 | 0.6 |
| Unsatisfactory therapeutic effect | 10 | 6.4 | 20 | 6.3 |
| Patient withdrew consent | 2 | 1.3 | 4 | 1.3 |
| Protocol violation | 3 | 1.9 | 1 | 0.3 |
| Administrative problems | 2 | 1.3 | 2 | 0.6 |
| No longer required study drug | 1 | 0.6 | 1 | 0.3 |
| Lost to follow-up | 0 | 2 | 0.6 | |
| Death | 0 | 1 | 0.3 | |
| Most frequent adverse events leading to discontinuation* | ||||
| Rash | 1 | 0.6 | 5 | 1.6 |
| Neutropenia | 1 | 0.6 | 4 | 1.3 |
| Nausea | 1 | 0.6 | 3 | 0.9 |
| Dyspnea | 0 | 3 | 0.9 | |
| Thrombocytopenia | 2 | 1.3 | 1 | 0.3 |
NOTE. Disposition in the intent-to-treat population as of December 31, 2007 (ie, data cutoff).
Safety population.
Efficacy
Molecular response.
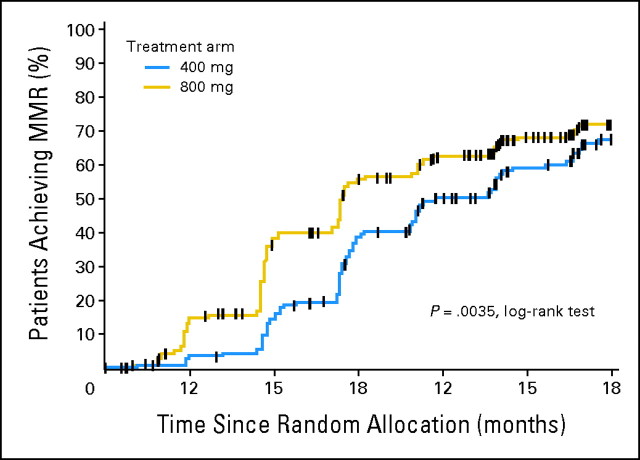
There was no statistically significant difference in the rate of MMR at 12 months between the two arms in the ITT analysis (40.1% v 46.4% for the 400-mg/d and 800-mg/d arms, respectively; P = .2035) or among evaluable patients (ie, those with PCR data available at each time point; P = .1386; Table 3). However, MMR was achieved more rapidly among patients randomly assigned to imatinib 800 mg/d compared with those randomly assigned to imatinib 400 mg/d (P = .0035 by log-rank test; Fig 2). This corresponded to significantly higher MMR rates for patients treated in the high-dose arm in earlier time periods (Table 3).
Table 3.
Response Rates Over Time and by Sokal Risk
| Variable | Patients by Imatinib Dose |
P | |||||
|---|---|---|---|---|---|---|---|
| 400 mg/d |
800 mg/d |
||||||
| No. With Response | No. Evaluated | % of Evaluated With Response | No. With Response | No. Evaluated | % of Evaluated With Response | ||
| MMR at time point, months | |||||||
| 3 | 5 | 157 | 3.2 | 39 | 319 | 12.2 | .001 |
| 6 | 27 | 157 | 17.2 | 107 | 319 | 33.5 | .0002 |
| 9 | 56 | 157 | 35.7 | 144 | 319 | 45.1 | .06 |
| 12* | 63 | 157 | 40.1 | 148 | 319 | 46.4 | .2035 |
| MMR in evaluable patients at time point, months | |||||||
| 3 | 5 | 137 | 3.6 | 39 | 283 | 13.8 | .001 |
| 6 | 27 | 135 | 20.0 | 107 | 276 | 38.8 | .001 |
| 9 | 56 | 136 | 41.2 | 144 | 267 | 53.9 | .0203 |
| 12 | 61 | 133 | 45.9 | 145 | 269 | 53.9 | .1386 |
| MMR at 12 months by Sokal risk group | |||||||
| Low | 26 | 62 | 41.9 | 68 | 135 | 50.4 | .286 |
| Intermediate | 24 | 53 | 45.3 | 48 | 111 | 43.2 | .867 |
| High | 11 | 42 | 26.2 | 29 | 73 | 39.7 | .160 |
| Cumulative incidence of CCyR by time point, months | |||||||
| 6 | 70 | 157 | 44.6 | 181 | 319 | 56.7 | .015 |
| 12 | 103 | 157 | 65.6 | 223 | 319 | 69.9 | .347 |
| CCyR by 12 months by Sokal risk group | |||||||
| Low | 43 | 62 | 69.4 | 105 | 135 | 77.8 | .2177 |
| Intermediate | 34 | 53 | 64.2 | 72 | 111 | 64.9 | 1.0 |
| High | 26 | 42 | 61.9 | 46 | 73 | 63.0 | 1.0 |
NOTE. Data are based on intent-to-treat populations unless otherwise specified.
Abbreviations: MMR, major molecular response; CCyR, complete cytogenetic response.
Primary end point, allowing for imputation.
Fig 2.
Time to first major molecular response (MMR) by treatment arm (intent-to-treat analysis).
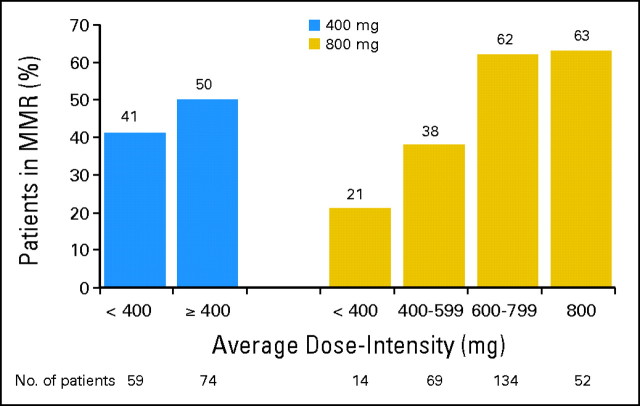
There was a correlation between MMR rate and average dose-intensity (ie, actual dose divided by time on treatment in mg/d) in the 800-mg/d arm. At 12 months, MMR was observed in 83 (62%) of 134 patients with an average dose intensity of 600 to 799 mg/d, and it was observed in 26 (38%) of 69 patients with an average dose intensity of 400 to 599 mg/d (Fig 3).
Fig 3.
Major molecular response (MMR) at 12 months by average dose intensity (evaluable patients).
The rate of MMR at 12 months did not differ significantly between arms according to Sokal risk group (Table 3). However, when best molecular response by 12 months (ie, at any time during the first 12 months since random assignment) was evaluated in high-risk patients, 31.0% on imatinib 400 mg/d achieved an MMR compared with 50.7% on imatinib 800 mg/d (P = .05).
Cytogenetic Response
By 6 months, 70 (44.6%) of 157 patients randomly assigned to the standard dose of imatinib achieved CCyR compared with 181 (56.7%) of 319 patients randomly assigned to high-dose imatinib (P = .01; Table 3). By 12 months, CCyR rates were comparable between arms (65.6% and 69.9% in the 400-mg/d and 800-mg/d arms, respectively). There was no significant difference in CCyR rates within Sokal risk category (Table 3).
Long-Term Outcomes
The estimated PFS rates at 18 months were 95.0% (95% CI, 90.2% to 99.8%) for the 400-mg/d arm and 97.4% (95% CI, 95.3% to 99.6%) for the 800-mg/d arm (P = .63). Among patients with high Sokal risk, PFS rates were 88.1% versus 95.9% for the 400-mg/d arm and the 800-mg/d arm, respectively. Progression to AP/BC on treatment occurred in five (3.2%) of 157 patients in the 400-mg/d arm and in six (1.9%) of 319 patients in the 800-mg/d arm. The estimated OS rate at 18 months was 98.7% (98.7% v 98.2% for 400 mg v 800 mg, respectively; P = .56).
Bone Marrow Transplantation
Seven patients (1.5%) underwent bone marrow transplantation (BMT) after study discontinuation; one was in the 400-mg/d arm, and six were in the 800-mg/d arm. At the time of BMT, one patient was in MMR. The other six patients who received BMT had no or minimal CyR and no molecular response at the time of transplantation. Two patients underwent BMT after progression to BC (n = 1 in the 400-mg/d arm; n = 1 in the 800-mg/d arm).
Safety and Tolerability
Dose-intensity.
The average daily doses of imatinib were 388.4 mg in the 400-mg/d arm (range, 177 to 663 mg) and 662.0 mg in the 800-mg/d arm (range, 223 to 800 mg). This represents an average relative dose intensity of 97% and 83%, respectively. More than 50% of patients in the 800-mg/d arm received an average daily imatinib dose of at least 750 mg/d, 25% tolerated an average dose of at least 793 mg/d, and 75% tolerated 546 mg/d or more. Among the 433 patients still on treatment at 12 months, 61% of patients in the 800-mg/d arm were being treated with their assigned dose, and 78% of patients in this arm were receiving imatinib at least 600 mg/d.
Although dose increases were not permitted in the 800-mg/d arm, 11 patients (7.0%) in the 400-mg/d arm increased their doses to between 600 and 800 mg/d. Dose reductions were required in 28 patients (17.8%) in the 400-mg/d arm and in 194 patients (61.4%) in the 800-mg/d arm. In the 400-mg/d arm, 81 patients (52%) had at least 1 day of zero dose until data cutoff (with 7, 19, and 43 days representing the 25th, 50th, and 75th percentiles). Among patients in the 800-mg/d arm, 233 (73%) reported at least 1 day of zero dose (and 11, 21, and 38 days represented the 25th, 50th, and 75th percentiles). Half of patients (50.0%) in the 800-mg/d arm had at least one dose reduction to less than 600 mg/d. Dose interruptions lasting more than 5 days were experienced by 59 patients (37.6%) in the 400-mg/d arm and by 211 patients (66.8%) in the 800-mg/d arm. The most common reason for dose reduction was adverse event/laboratory abnormality, cited in 52 patients (33.1%) in the 400-mg/d arm and in 230 patients (72.8%) in the 800-mg/d arm. The median durations of imatinib dose reduction through month 12 were 95 days (range, 3 to 342 days) for the standard dose and 115 days (range, 1 to 335 days) for the high dose. The median durations of days without drug in the same time period were 15 days (range, 1 to 95 days) for the standard dose and 20 days (range, 1 to 160 days) for the high dose of imatinib.
Adverse Events
The majority of adverse events judged by the investigator to be related to study drug in both arms were mild or moderate in intensity and resolved spontaneously or with dose interruptions or reductions if needed. Overall, 147 patients (93.6%) in the 400-mg/d arm and 310 (98.1%) in the 800-mg/d arm reported adverse events (all grades), and 52 (33.1%) and 201 (63.6%) reported grades 3 to 4 adverse events, respectively. Rates of all-grade and grades 3 to 4 (Table 4) hematologic adverse events were higher in the 800-mg/d arm. The incidence of biochemical abnormalities did not differ according to dose. More patients in the 800-mg/d arm discontinued imatinib because of adverse events (3.8% v 9.4%; Table 2).
Table 4.
Most Frequently Reported Grades 3 to 4 Study Drug–Related Adverse Events
| Adverse Event | Patients by Imatinib Dose |
|||
|---|---|---|---|---|
| 400 mg (n = 157) |
800 mg (n = 319) |
|||
| No. | % | No. | % | |
| Nonhematologic* | ||||
| Nausea | 0 | 4 | 1.3 | |
| Periorbital edema | 0 | 3 | 0.9 | |
| Diarrhea | 0 | 8 | 2.5 | |
| Peripheral edema | 0 | 2 | 0.6 | |
| Rash | 4 | 2.5 | 18 | 5.7 |
| Muscle spasms | 1 | 0.6 | 3 | 0.9 |
| Fatigue | 3 | 1.9 | 5 | 1.6 |
| Vomiting | 1 | 0.6 | 3 | 0.9 |
| Arthralgia | 1 | 0.6 | 6 | 1.9 |
| Myalgia | 0 | 11 | 3.5 | |
| Face edema | 0 | 2 | 0.6 | |
| Pain in extremity | 0 | 7 | 2.2 | |
| Headache | 0 | 5 | 1.6 | |
| Eyelid edema | 0 | 2 | 0.6 | |
| Dyspepsia | 0 | 0 | ||
| Dizziness | 0 | 3 | 0.9 | |
| Hematologic† | ||||
| Leukopenia | 12 | 7.6 | 45 | 14.2 |
| Neutropenia | 29 | 18.5 | 90 | 28.5 |
| Thrombocytopenia | 16 | 10.2 | 57 | 18.0 |
| Anemia | 6 | 3.8 | 22 | 7.0 |
| Laboratory abnormalities† | ||||
| Hypophosphatemia | 23 | 14.6 | 38 | 12.0 |
| Hypocalcemia | 6 | 3.8 | 4 | 1.3 |
| Transaminase elevation | 7 | 4.5 | 9 | 2.8 |
| Alkaline phosphatase | 0 | 1 | 0.3 | |
| ALT | 7 | 4.5 | 8 | 2.5 |
| AST | 5 | 3.2 | 5 | 1.6 |
| Albumin | 0 | 1 | 0.3 | |
| Creatinine | 0 | 1 | 0.3 | |
| Total bilirubin | 0 | 0 | ||
| Hypercalcemia | 1 | 0.6 | 0 | |
NOTE. Most frequent adverse events occurred in at least 10% of patients from either treatment group.
Most frequently reported nonhematologic adverse events determined by the investigator to be study drug related.
Newly occurring or worsening abnormalities on the basis of laboratory values.
Death
Eight patients (1.7%) died; one died on study about 2 months after last study drug was taken, and seven died after discontinuation of study treatment. Of those who died, two were assigned to the 400-mg/d arm, and six were assigned to the 800-mg/d arm. Both patients on standard-dose imatinib who died discontinued treatment because of unsatisfactory therapeutic effect (n = 1 in CHR with no CyR at time of discontinuation; n = 1 with no CHR or CyR) and died during follow-up (n = 1 because of fungal infection 3.5 months after stopping imatinib; n = 1 because of CML 2 months after stopping study treatment). Of the six patients who died in the 800-mg/d arm, one patient died of pulmonary tuberculosis while on study but did not receive study drug for 2 months before death, and five died after discontinuation of treatment (n = 3 as a result of CML; n = 2 as a result of complications subsequent to bone marrow transplantation).
DISCUSSION
Data from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study suggest that imatinib 400 mg/d should remain the initial therapy for the majority of patients with CML. High-dose (ie, 800 mg/d) imatinib also was well tolerated in most patients and was associated with a more rapid response than the standard dose (ie, 400 mg/d) of imatinib, but MMR rates at 12 months—the primary end point of the study—were comparable between arms. Grades 3 to 4 adverse events were uncommon in this study, and there were no new adverse events associated with imatinib that had not been identified previously. Adverse events, although more frequent with imatinib 800 mg/d, were manageable in most cases with dose interruptions or dose reductions.
The majority of patients achieved a CyR by 6 months. The CCyR and MMR rates at 12 months for patients treated with 400 mg/d on the TOPS study were comparable with those seen on the IRIS trial in the same time period.4,18 Rates of discontinuation overall were comparable, but they did appear to be higher on the TOPS study (even for the standard-dose cohort) than on the IRIS trial for adverse events and unsatisfactory therapeutic effect; this is likely because of the availability of the second-generation tyrosine kinase inhibitors nilotinib and dasatinib. The estimated rates of PFS and OS at 18 months were greater than 95% for patients in both treatment arms on the TOPS study.
Although half of the patients in the 800-mg/d arm required dose reduction to less than 600 mg/d at some point on study, the average daily dose of imatinib for patients in the 800-mg/d arm was 662 mg/d. More than half of the patients in the 800-mg/d arm received an average dose of at least 750 mg/d, and 75% tolerated approximately 600 mg/d or more. These data suggest that the increased adverse events associated with imatinib 800 mg/d were manageable by following protocol dosing guidelines. Although the number of imatinib tablets dispensed and returned was recorded, and although imatinib blood levels were higher among patients randomly assigned to the 800-mg/d arm,19 the possibility that some patients may have had dose reductions or interruptions that were not reported cannot be excluded.
In the 800-mg/d arm, patients who received a median imatinib dose of at least 600 mg/d had the greatest probability of achieving an MMR, and patients who received less than 400 mg/d had the worst outcome (Fig 3). Interestingly, patients in the 800-mg/d arm who received an average dose intensity of less than 400 mg/d fared far worse than those randomly assigned to 400 mg/d who had the same dose intensity, as MMR rates were 21% and 41% at 12 months, respectively. This may be because patients randomly assigned to imatinib 800 mg/d who experienced dose reductions to less than 400 mg/d had more days off drug than patients randomly assigned to 400 mg/d with similar dose reductions. These data suggest that patients who can tolerate high-dose imatinib achieve superior cytogenetic and molecular responses compared with those who need dose modification and suggest that the number of days off medication may adversely impact outcomes. Other confounding factors that may contribute to a patient's ability to maintain dose intensity (eg, high plasma α-1-acid glycoprotein levels or other factors associated with increased adverse events20–23) were not investigated in this study.
In this study, imatinib 800 mg/day induced earlier CCyR and MMR in patients with newly diagnosed CML-CP. Differences in MMR rates at 3 and 6 months, time to first MMR, and CCyR rates at 6 months between the treatment arms were statistically significant (P = .001, .0002, .0035, and .013, respectively). A significantly greater proportion of patients in the 800-mg/d arm achieved an early (within 6 months) CCyR. By 12 months, MMR rates were comparable between arms. The reasons for this are unknown. It is possible that, over time, the inhibitory effect of imatinib reaches a plateau that is attained earlier with high-dose imatinib and later with the standard dose.
Higher-risk patients may be potential candidates for initial therapy with high-dose imatinib, as more patients with high Sokal risk in the 800-mg/d arm achieved an MMR compared with the 400-mg/d arm when best molecular response by 12 months was evaluated. In a recent study conducted by the European LeukemiaNet, rates of MMR and CCyR were comparable between patients with newly diagnosed CML-CP who had high Sokal risk and who were treated with 800 mg/d versus 400 mg/d imatinib.24 However, as in the TOPS study, CCyR rates appeared to be related to the actual dose received (ie, 91% for 700 to 800 mg/d, 73% for 400 to 699 mg/d, and 20% for < 400 mg/d). Dose-intensity also correlated with response in other trials of higher-dose imatinib in patients with newly diagnosed CML-CP.11,12
The safety and efficacy of imatinib 400 mg/d for patients with newly diagnosed CML-CP was confirmed in the TOPS study. Response and safety findings for patients treated with standard-dose imatinib were similar to those seen in the IRIS trial. Longer follow-up is required to determine whether the earlier responses induced by high-dose imatinib will translate into improved long-term outcomes.
Supplementary Material
Acknowledgment
We thank Erinn Goldman, PhD (Articulate Science), for her medical editorial assistance with this manuscript.
Appendix
The following investigators participated in the TOPS study: Argentina—Academia Nacional de Medicina, Buenos Aires: R. Bengio; Hospital Jose Maria Ramos Mejia, Buenos Aires: B. Moiraghi; Fundaleu, Buenos Aires: S. Pavlovsk; Hospital Rodolfo Rossi, La Plata: M. Perez. Australia—Royal North Shore Hospital, St Leonards: C. Arthur; Westmead Hospital, Westmead: M. Hertzberg; Calvary Mater Newcastle, Waratah: P. Rowling; Peter MacCallum Cancer Institute, East Melbourne: J. Seymour; Royal Melbourne Hospital, Parkville: A. Grigg; St Vincent's Hospital, Fitzroy: R. Filshie; The Alfred Hospital, Prahran: A. Schwarer; Frankston Hospital, Frankston: J. Catalano; Royal Brisbane Hospital, Herston: S. Durrant; Haem & Oncology Clinics of Australasia, Mater Medical Centre, South Brisbane: K. Taylor; Princess Alexandra Hospital, Wooloongabba: A. Mills; Royal Adelaide Hospital, Adelaide: T. Hughes; Fremantle Hospital, Fremantle: M. Leahy. Brazil—Irmandade da Santa Casa de Misericordia de Sao Paulo, Sao Paulo: V. T. Hungria; Hospital Brigadeiro, Sao Paulo: M. A. Zanichelli; Hospital das Clinicas da Faculdade de Medicina da USP, Sao Paulo: P. D. Llacer; Hematology and Hemotherapy Center, University of Campinas, Campinas: C. De Souza; Hospital de Base do Distrito Federal, Brasilia: A. Nonino; Hospital de Clinicas da Universidade Federal do Parana, Curitiba: R. Pasquini. Canada—Hospital du Saint-Sacrement, Quebec: R. Delage; Hôpital Notre-Dame du CHUM, Montreal: H. Olney; Ottawa General Hospital, Ottawa: I. B. Bruckler; Tom Baker Cancer Center, Calgary: D. Stewart. Chile—Hospital Clinico Universidad de Chile, Santiago: L. G. Conte; Hospital del Salvador, Santiago: M. E. Cabrera; Colombia—Hospital San Jose, Bogota: V. Abello. Czech Republic—University Hospital, Olomouc: E. Faber. France—Institut Bergonie, Bordeaux: J. Reiffers; Hopital Saint Louis, Paris: D. Rea; CHU Hospital Jean Bernard, Poitiers: F. Guilhot. Israel—Rambam Medical Center, Haifa: J. M. Rowe; Rabin Medical Center, Petach-Tikva: P. Raanani; Hadassah Medical Organization, Ein Karem, Jerusalem: D. B. Yehuda. Italy—Ospedale S. Eugenio, Roma: E. Abruzzese; Az. Ospedaliero-Universitaria S. Luigi Gonzaga, Orbassano: G. Saglio; Az. Ospedaliera Universit. S. Martino-Universita degli Stud, Genova: M. Gobbi, Az. Ospedaliera University. S. Martino-Universita degli Stud, GE: M. Carella; Az. Osp. Niguarda Ca Granda, Milano: E. Morra; Fodazione IRCCS Policlinico S. Matteo, Pavia: M. Lazzariono; Istituto di Ematologia “L. e A. Seràgnoli,” Policlinico S. Orsola-Malpighi, Bologna: M. Baccarani; Azienda Ospedaliera di Rilievo Nazionale A. Cardarelli, Napoli: F. Ferrara. Korea—Seoul National University Hospital, Seoul: I-H. Kim; Samsung Medical Center, Korea: C-W. Jung; Seoul St Mary's Hospital, The Catholic University of Korea, Seoul: D-W. Kim; Kyung Pook National University Hospital, Taegu: S-K. Sohn. Poland—Collegium Medicum Uniwersytetu Jagielonskiego, Krakow: A. Skotnicki; Slaska Akademia Medyczna w katowicach, Katowice: J. Holowiecki; Klinika Hematologii, Nowotworow Krwi I Transplantacji Szpiku, Wroclaw: K. Kuliczkowski; Akademia Medyczna im. Karola Marcinkowskiego w Poznaniu, Poznan: M. Komarnicki; Klinika Hematologii AM, Gdansk: A. Hellmann. Singapore—Singapore General Hospital, Singapore: C. Chuah. Slovakia—Fakultna Nemocnica L. Pasteura, Kosice: E. Tothova. South Africa—Chris Hani Baragwanath Hospital, Johannesburg: M. Patel; Johannesburg Hospital, Johannesburg: P. Ruff; University of Cape Town, Cape Town: N. Novitzky; Faculty of Health Sciences, Bloemfontein: V. Louw. Spain—Hospital de la Santa Creu I Sant Pau, Barcelona: A. Sureda; Hospital Durans I Reynals-ICO, Spain: C. Boque; Hospital Clinico Universitario Santiago De Compostela, Santiago de Compostela: M. P. Encinas; Hospital Gregorio Maranon, Madrid: G. P. Rus; Hospital Ramon Y Cajal, Madrid: P. Herrera; Hospital Universitario La Paz, Madrid: R. De Paz; Hospital Clinical Universitario, Salamanca: C. Canizo; Hospital Clinic I Provincial De Barcelona, Spain: F. Cervantes. Thailand—Siriraj Hospital, Bangkok: S. Issaragrisil. Taiwan—Chang-Gung Memorial Hospital-Linko, Lin-Ko: L-Y. Shih; Taichung Veteran's General Hospital, Taichung: T-H. Lin; Chi Mei Fundation Hospital, Yungkang: C-J. Tsao. United States—The Cancer Center, Hackensack University Medical Center, Hackensack: S. L. Goldberg; Roswell Park Cancer Institute, Buffalo: M. Wetzler; University of Pittsburgh Cancer Institute, Pittsburgh: M. Agha; St Agnes Hospital, Baltimore: C. Miller; University of Virginia Health Systems, Charlottesville: M. G. Douvas; Wake Forest University Health Sciences, Winston-Salem: B. L. Powell; Duke University Medical Center, Durham: D. A. Rizzieri; MUSC Hollings Cancer Center, Charleston: R. K. Stuart; Cancer Centers of the Carolinas, Greensville: J. J. Stephenson, Jr.; Integrated Community Oncology Network, Orange Park: M. Moezi; Advanced Medical Specialties, Miami: S. Garrido; Palm Beach Cancer Institute, West Palm Beach: D. L. Spitz; University of South Alabama/Mitchell Cancer Institute, Mobile: H. T. Khong; Sarah Cannon Cancer Center, Nashville: I. W. Flinn; The Jones Clinic, Germantown: C. M. Jones; Louisville Oncology Associates, Louisville: M. Choudry; University of Kentucky, Lexington: K. T. McDonagh; University Hospitals of Cleveland, Cleveland: H. M. Lazarus; Cleveland Clinic Foundation, Cleveland: M. Kalaycio; Indiana Blood and Marrow Institute, Beach Grove: L. P. Akard; University of Iowa Hospitals & Clinics, Iowa City: R. D. Gingrich; University of Minnesota, Masonic Cancer Center, Minneapolis: B. Peterson; Rush-Presbyterian St Luke's Medical Center, Chicago: J. Shammo; Hematology and Oncology Specialists, Metairie: T. F. Roberts; LSU Health Sciences Center, Shreveport: J. Glass; The University of Texas M. D. Anderson Cancer Center, Houston: J. E. Cortes; Rocky Mountain Cancer Centers-Midtown, Denver: M. W. Brunvand; University of California, Los Angeles Medical Center, Los Angeles: R. Paquette; Alta Bates Cancer Center, Berkeley: U. Sunkara; Cancer Research Center of Hawaii, Honolulu: J. Cho; Kaiser Permanente Northwest Region, Portland: M. U. Rarick. Study Management Committee—The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA: J. E. Cortes; Oregon Health Sciences University Knight Cancer Institute, Portland, OR, USA: B. J. Druker; Clinical Investigation Centre CIC P 802 INSERM, CHU de Poitiers, Poitiers, France: F. Guilhot; Royal Adelaide Hospital, Adelaide, Australia: T. P. Hughes; University of Bologna, Bologna, Italy: M. Baccarani. PCR Committee—SA Pathology, Adelaide, Australia: S. Branford; Royal Adelaide Hospital, Adelaide, Australia: T. P. Hughes; Fred Hutchinson Cancer Research Center, Seattle, WA, USA: J. P. Radich; Seoul St Mary's Hospital, The Catholic University of Korea, Seoul, Korea: D-W. Kim; University of Naples, Naples, Italy: F. Pane.
Footnotes
Supported by Novartis Pharmaceuticals Corporation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00124748.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marc Rudoltz, Novartis (C); Richard Yu, Novarits (C); Tillmann Krahnke, Novartis (C) Consultant or Advisory Role: Michele Baccarani, Novartis (C), Bristol-Myers Squibb (C); François Guilhot, Novartis (C), Bristol-Myers Squibb (C); Brian J. Druker, Novartis (U); Susan Branford, Novartis (C), Bristol-Myers Squibb (C); Dong-Wook Kim, Novartis (C), Bristol-Myers Squibb (C); Fabrizio Pane, Novartis (C), Bristol-Myers Squibb (C); Beatriz Moiraghi, Bristol-Myers Squibb (C); Jerald P. Radich, Novartis (C); Timothy P. Hughes, Bristol-Myers Squibb (C), Novartis (C) Stock Ownership: Brian J. Druker, MolecularMD; Marc Rudoltz, Novartis; Richard Yu, Novartis; Tillmann Krahnke, Novartis Honoraria: Michele Baccarani, Novartis, Bristol-Myers Squibb; François Guilhot, Novartis, Bristol-Myers Squibb; Susan Branford, Novartis, Bristol-Myers Squibb; Dong-Wook Kim, Novartis, Wyeth, Bristol-Myers Squibb; Ricardo Pasquini, Novartis, Bristol-Myers Squibb; Stuart L. Goldberg, Novartis; Matt Kalaycio, Novartis; Jerald P. Radich, Novartis; Timothy P. Hughes, Novartis, Bristol-Myers Squibb Research Funding: Jorge E. Cortes, Novartis, Bristol-Myers Squibb, Wyeth; Michele Baccarani, Novartis; Brian J. Druker, Novartis, Bristol-Myers Squibb; Susan Branford, Novartis, Bristol-Myers Squibb; Dong-Wook Kim, Novartis, Wyeth, Bristol-Myers Squibb; Fabrizio Pane, Novartis; Stuart L. Goldberg, Novartis; Matt Kalaycio, Novartis; Hagop M. Kantarjian, Genzyme, Novartis, Bristol-Myers Squibb; Jerald P. Radich, Novartis; Timothy P. Hughes, Novartis, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: Brian J. Druker, Dana-Farber Cancer Institute, Oregon Health Sciences University (Patent No. 843)
AUTHOR CONTRIBUTIONS
Conception and design: Jorge E. Cortes, Michele Baccarani, François Guilhot, Brian J. Druker, Marc Rudoltz, Richard Yu, Hagop M. Kantarjian, Jerald P. Radich, Timothy P. Hughes
Administrative support: Richard Yu
Provision of study materials or patients: Jorge E. Cortes, Michele Baccarani, François Guilhot, Brian J. Druker, Dong-Wook Kim, Ricardo Pasquini, Stuart L. Goldberg, Matt Kalaycio, Beatriz Moiraghi, Jacob M. Rowe, Elena Tothova, Carmino De Souza, Hagop M. Kantarjian, Timothy P. Hughes
Collection and assembly of data: Jorge E. Cortes, Michele Baccarani, Susan Branford, Dong-Wook Kim, Fabrizio Pane, Stuart L. Goldberg, Elena Tothova, Carmino De Souza, Marc Rudoltz, Jerald P. Radich, Timothy P. Hughes
Data analysis and interpretation: Brian J. Druker, Susan Branford, Dong-Wook Kim, Fabrizio Pane, Marc Rudoltz, Tillmann Krahnke, Jerald P. Radich, Timothy P. Hughes
Manuscript writing: Brian J. Druker, Susan Branford, Stuart L. Goldberg, Jacob M. Rowe, Marc Rudoltz, Tillmann Krahnke, Jerald P. Radich, Timothy P. Hughes
Final approval of manuscript: Jorge E. Cortes, Michele Baccarani, François Guilhot, Brian J. Druker, Susan Branford, Dong-Wook Kim, Fabrizio Pane, Ricardo Pasquini, Stuart L. Goldberg, Matt Kalaycio, Jacob M. Rowe, Elena Tothova, Carmino De Souza, Marc Rudoltz, Jerald P. Radich, Timothy P. Hughes
REFERENCES
- 1.Baccarani M, Saglio G, Goldman J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN: Clinical practice guidelines in oncology—Chronic myelogenous leukemia, version 2. 2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 3.O'Brien SG, Guilhot F, Goldman JM, et al. International Randomized Study of Interferon Versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib. Blood. 2008;112(suppl):76. abstr 186. [Google Scholar]
- 4.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TP, Hochhaus A, Branford S, et al. Reduction of BCR-ABL transcript levels at 6, 12, and 18 months correlates with long-term outcomes on imatinib at 72 mo: An analysis from the International Randomized Study of Interferon versus STI571 (IRIS) in patients with chronic phase chronic myeloid leukemia. Blood. 2008;112(suppl):129. abstr 334. [Google Scholar]
- 6.Iacobucci I, Saglio G, Rosti G, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006;12:3037–3042. doi: 10.1158/1078-0432.CCR-05-2574. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 8.Press RD, Love Z, Tronnes AA, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–4256. doi: 10.1182/blood-2005-11-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki E, Kantarjian H, O'Brien S, et al. High-dose imatinib mesylate treatment in patients with untreated early chronic phase chronic myeloid leukemia. J Clin Oncol. 2006;24(suppl):345s. abstr 2143. [Google Scholar]
- 10.Cortes J, Giles F, O'Brien S, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003;102:83–86. doi: 10.1182/blood-2003-01-0025. [DOI] [PubMed] [Google Scholar]
- 11.Cortes J, Kantarjian H, Goldberg S, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: High rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes TP, Branford S, White DL, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic- phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–3973. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Talpaz M, O'Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 14.Sokal JE, Baccarani M, Russo D, et al. Staging and prognosis in chronic myelogenous leukemia. Semin Hematol. 1988;25:49–61. [PubMed] [Google Scholar]
- 15.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branford S, Cross NC, Hochhaus A, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006;20:1925–1930. doi: 10.1038/sj.leu.2404388. [DOI] [PubMed] [Google Scholar]
- 17.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112:3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 19.Guilhot F, Hughes TP, Cortes J, et al. Imatinib pharmacokinetic (PK) exposure and its correlation with clinical outcome in patients with chronic-phase chronic myeloid leukemia for 400 mg and 800 mg daily doses (Tyrosine Kinase Inhibitor Optimization and Selectivity [TOPS]) Blood. 2008;112:170. [Google Scholar]
- 20.Menon-Andersen D, Mondick JT, Jayaraman B, et al. Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol. 2009;63:229–238. doi: 10.1007/s00280-008-0730-x. [DOI] [PubMed] [Google Scholar]
- 21.Petain A, Kattygnarath D, Azard J, et al. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res. 2008;14:7102–7109. doi: 10.1158/1078-0432.CCR-08-0950. [DOI] [PubMed] [Google Scholar]
- 22.Delbaldo C, Chatelut E, Re M, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006;12:6073–6078. doi: 10.1158/1078-0432.CCR-05-2596. [DOI] [PubMed] [Google Scholar]
- 23.Widmer N, Decosterd LA, Csajka C, et al. Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol. 2006;62:97–112. doi: 10.1111/j.1365-2125.2006.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: A European LeukemiaNet Study. Blood. 2009;113:4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.