Abstract
Background:
Menthol is the most important active material in mint and different mechanisms have been suggested for the way mint functions, most of which emphasize its analgesic effect owing to the presence of a group of temporary protein receptors. This study investigates the efficacy of peppermint capsule in the treatment of primary dysmenorrhea, in comparison with Mefenamic Acid and placebo.
Materials and Methods:
This was a prospective, double-blinded, crossover study and was conducted on 127 girl students studying in Hamadan University of Medical Sciences who had experienced primary dysmenorrhea. Each participant was asked to take one of the drugs including Mefenamic Acid and Mint, starting from the first menstruation for 3 days. At the end of each period, a questionnaire was used to gather information; through the volunteer herself, pain intensity was recorded according to visual analog scale (VAS), duration of pain according to COX questionnaire, and bleeding amount according to pictorial blood loss assessment chart (PBAC) chart (Hygham).
Results:
Average pain intensity and duration of pain were significantly lower after intake of Mefenamic Acid and Mint (P < 0.05). Average bleeding was significantly lower in those taking Mefenamic Acid capsule than in those taking peppermint extract (P < 0.05). Nausea and diarrhea were lower in the mint group than in Mefenamic Acid group. But analgesic usage was lower in Mefenamic Acid group than in peppermint group (P < 0.05).
Conclusions:
While the bleeding amount did not significantly change, pain and its severity and all the clinical signs and symptoms decreased after taking peppermint extract. Because the side effect of herbal drugs is lower than other medicinal drugs, using mint is advised for treating dysmenorrhea symptoms.
Key words: Crossover study, dysmenorrhea, Iran, Mefenamic Acid, mint
INTRODUCTION
Dysmenorrhea is related to painful uterine contractions during menstruation.[1] Primary dysmenorrhea is a physiological phenomenon occurring in the absence of pelvic disease; this pain is associated with nausea, vomiting, and diarrhea.[2] Dysmenorrhea is a major cause of loss of efficacy in girls and their absence from school. This disease can disrupt girls’ quality of life.[3,4] The prevalence of primary dysmenorrhea among female adolescents is reported to be 74-86% in Iran.[5] The dysmenorrheal pain is believed to be caused by the release of prostaglandin F2α in the menstrual fluid. The most common treatment is the use of chemical agents, prostaglandin inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), and contraceptive pills. NSAIDs are among the main treatments for dysmenorrhea.[1,2] These drugs decrease the production of prostaglandins by cyclooxygenase (COX) enzymes and inhibit prostaglandin receptors in the uterus. The side effects of these drugs are numerous.[6] Much research has been done on medical treatments involving magnesium, zinc, vitamin B, vitamin E, and omega-3 fatty acids. Peppermint herbal products are usually used in treating diseases.[7,8] Peppermint is one among several plants used in traditional medicine. This plant exerts its effect on the myometrium contractile activity by inhibiting prostaglandin F2α and oxytocin.[9] Menthol is the most important active material in mint.[10] The most important effect of this treatment is on vomiting and diarrhea.[8] Furthermore, peppermint extract is identified to have analgesic effect and anti-inflammatory activity.[11] Due to probability of positive effects of peppermint in the treatment of dysmenorrhea against the effects of NSAIDs such as Mefenamic Acid, and also as only a few studies have been conducted on this in Iran, we performed this study in Hamadan. To compare the effects of herbal medicines to treat primary dysmenorrhea rather than medical drugs we have done this research.
MATERIALS AND METHODS
This was a prospective, randomized, cross-over study which was conducted from February 2012 to April 2013 on female students of Hamadan universities who had previously experienced primary dysmenorrhea. Sample size was calculated as 120 girls based on previous studies, with a 95% confidence coefficient and 5% probability of error. Taking into account a potential drop-out rate of 10%, the total number of girls required in the study was calculated as 144 (72 in each group). All outcomes were analyzed according to the intention-to-treat protocol. Students were asked two questions. The first question was related to whether or not they had experienced primary dysmenorrhea (primary dysmenorrhea was explained to the students) and the second question was concerned with their satisfaction regarding their participation in this study. Those who gave positive responses to both the above-mentioned questions were investigated in terms of inclusion and exclusion criteria. Having primary dysmenorrhea, regular menstruation, being single, and being between 18 and 25 years of age were among the inclusion criteria, while the exclusion criteria included absence of ban on mint use, being allergy to mint, patients with cholecystitis, gastroesophageal reflux or severe liver disease, sensitivity to Mefenamic Acid, ulcers, kidney and liver disease, blood dyscrasia, asthma, diabetes mellitus, and extreme sensitivity to aspirin.[12,13,14]
Randomization took place during the first visit and those who consented to participate in the study were randomized into two groups, Group 1 or Group 2, according to a computer-generated random number series. Group 1 subjects received three peppermint oil capsules (Colpermin; Tillotts Company is Switzerland and Razak company is Tehran) once a day for 3 days after menstruation started, followed by period of washout in the next cycle. Then in the third menstruation, they were given 250 mg of Mefenamic acid capsule (Ponstan; Razak Company) every 8 h for 3 days. Group 2 subjects received the same combination, but in the reverse order.
These two capsules were similar to each other and were prepared in the same package. These drugs were prescribed by project partners who were not aware of the code. Patients were unaware of the kind of drug taken and the results were evaluated by one of the research associates who were not aware of the study groups.
After taking drug, each volunteer responded to the questions posed in the questionnaire and filled in the demographic form. These questionnaires are standard and numerous studies have confirmed their reliability and validity. Pain intensity was assessed through visual analog scale (VAS) and dysmenorrhea timing through COX menstrual symptom scale.[15] The bleeding amount was measured using chart (PBAC).[16] VAS and COX questionnaires were filled in two times, at the beginning and at the end of each menstruation. The chart (PBAC) was completed on menstruation days. Cases were allowed to take sedatives 1 h after consuming the capsule, but they had to record the intensity and duration of their pain in the questionnaire before taking those sedative drugs. Nausea, vomiting, diarrhea, and analgesic uses were studied by enquiring the students.
The number of tranquilizers and their kinds had to be recorded in the questionnaire during the 2 months. Analysis of data was performed by STATA 11 software, while paired t-test and analysis of variance (ANOVA) were employed to analyze data quantitatively at the confidence level of 95%. Clinical signs appearing after using the treatment methods were compared with each other. The Vice Chancellor for Research and Technology of Hamadan University of Medical Sciences approved the study (137435-9-35-16). It was registered in the Iranian Registry of Clinical Trial (IRCT201011205206N1).
Ethical considerations
The study protocol was approved by the Institutional Review Board and the Human Research Ethics Committee in the Hamadan University of Medical Sciences.
RESULTS
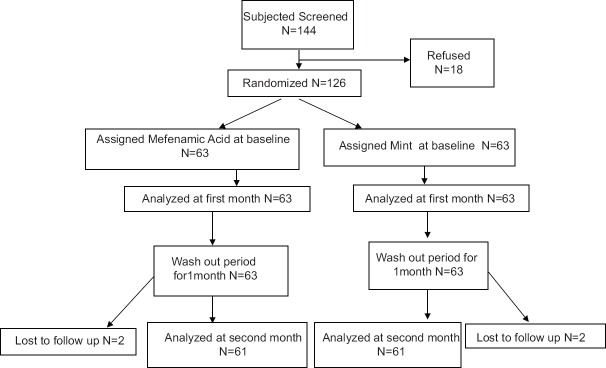
This study was conducted for 2 months on 144 female students aged 18-25 years who already had primary dysmenorrhea; Finally 122 girls were analyzed [Figure 1]. The mean age of the girls was 20.99 ± 0.15 years. Mean weight and height were 55.09 ± 5.81 kg and 141.63 ± 3.60 cm, respectively. The subjects of the study groups were similar in age, number of days of menstruation, age at first menstruation, and dysmenorrheal interval and the differences were statistically not significant. Clinical symptoms of the subjects after each of the two interventions (peppermint extract, Mefenamic Acid) are shown in Table 1.
Figure 1.
Flow chart of the study
Table 1.
Mean and SD of severity and duration of pain and bleeding in the study groups that used mefenamic acid and peppermint as treatment
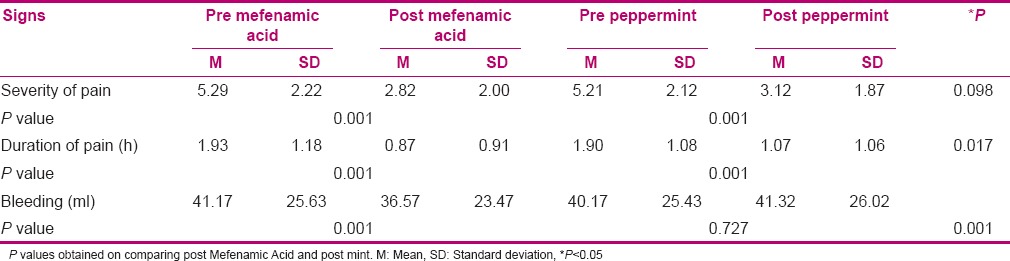
Table 1 shows that the intensity of pain after consumption of Mefenamic Acid and also mint capsules was less than before using the two drugs and this difference was significant (P < 0.05). But there was no significant difference between Mefenamic Acid and mint. Duration of pain after taking Mefenamic Acid and Mint was less than before (P < 0.05). Also, duration of pain was less in Mefenamic Acid group than in Mint group (P < 0.05). The amount of bleeding after using Mefenamic Acid and Mint was less than before, but Mefenamic Acid reduced the magnitude of bleeding compared to Mint.
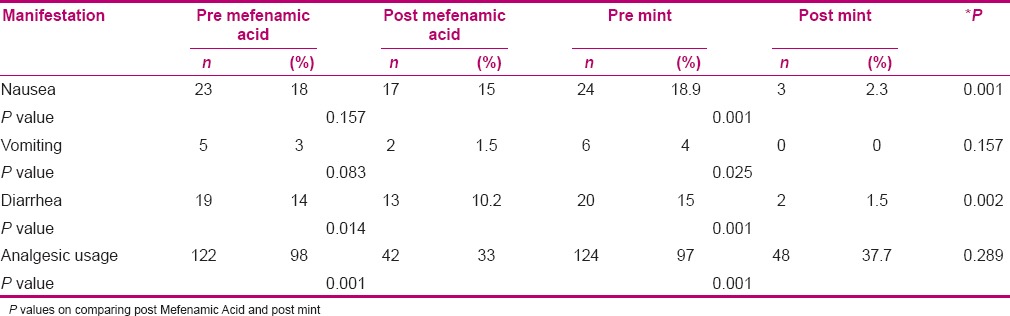
Results presented in Table 2 indicate that there was no significant difference in nausea and vomiting between before and after consumption of Mefenamic Acid (P > 0.05), but in the mint group, the difference was significant. Also, mint group had less nausea and vomiting (P < 0.05). Reduction in nausea and diarrhea due to mint was more than that due to Mefenamic Acid (P < 0.05). Also, consumption of analgesic during the treatment periods was decreased in both groups (P < 0.05).
Table 2.
Comparison of nausea, vomiting, diarrhea, and analgesic usage in the study population before and after using Mefenamic Acid and mint extract
DISCUSSION
This study compared the effects of Peppermint and Mefenamic acid on the treatment of primary dysmenorrhea in young girls. Sometimes the severity of symptoms in dysmenorrhea impairs the daily activities, and therefore, proper treatment is necessary.[7] In our study, duration and severity of dysmenorrhea was reduced with the consumption of Mefenamic Acid and mint. Many studies have attempted to find better drugs and methods with the same effect.[15] Considering menthol's mechanism, it is predicted that it could be useful in the treatment of primary dysmenorrhea in appropriate concentrations. The findings of our study show that peppermint can reduce the duration and severity of menstrual cramps. The cooling effect of menthol has been explained by its direct effect on the “cold receptor” TRPM8, a distant relative of the vanilloid receptors that sense pain and noxious high temperatures. Binding of menthol to TRPM8 induces calcium release from the endoplasmic reticulum and Golgi apparatus in the cell. Menthol is an agonist of TRPM8. Peppermint oil exerts an antispasmodic action on smooth muscle, considered to be caused by calcium channel blockade. Wasner et al., in a double-blind clinical trial study done on 10 people, found that menthol significantly increases block fiber.[17] Kline et al. investigated the effect of peppermint oil capsules on 42 children suffering from irritable bowel syndrome and reported 75% pain decrease in these patients after using the drug.[18] Also, prostaglandin inhibitors are the most effective drugs for the treatment of primary dysmenorrhea.[19] Anti-prostaglandin drugs such as NSAIDs are effective in reducing the pain in dysmenorrhea. Mefenamic Acid belongs to the category of fenamate drugs and is an inhibitor of prostaglandin synthesis.
One study shows that Mefenamic Acid is a suitable drug for the treatment of primary dysmenorrhea, especially in those suffering from moderate pain.[20] In another study, Mefenamic Acid has been proposed as a dominant treatment for dysmenorrhea.[21] The results of our study show that nausea, vomiting, and diarrhea were lesser after mint consumption than after consumption of Mefenamic Acid. Peppermint oil relaxes the smooth muscle; its active principle is menthol which contains a cyclic monoterpene that has anti-spasmodic properties due to its ability to block the calcium channel of intestinal smooth muscles, and it is commonly used for Irritable bowel syndrome (IBS) in Europe.[22] Shavakh et al. also reported that peppermint capsules can greatly reduce nausea and vomiting during colonoscopy.[23] In another study, reduction of diarrhea was reported in patients with irritable bowel syndrome.[24] According to the findings of this study, mint and Mefenamic Acid have a positive effect on the symptoms of dysmenorrhea. But due to the complications of Mefenamic Acid including gastrointestinal bleeding, gastrointestinal ulcers, flatulence, indigestion, stomach pain, melaena, vomiting blood, inflammation of the mouth, worsening of Crohn's disease, and worsening of colitis, peppermint herbal drug is better to treat dysmenorrhea. Thus, it has been suggested that more studies should be done concerning the effects of peppermint capsule on decreasing dysmenorrhea symptoms. Also, we suggest to the interested researchers in this field to use higher dosages of mint capsules or use them in the luteal phase.
CONCLUSION
Although the average pain intensity and bleeding was significantly lower in Mefenamic Acid group than in peppermint extract group, due to the complications caused by the consumption of Mefenamic Acid capsules, peppermint extract is recommended for dysmenorrhea.
Financial support and sponsorship
This article was supported by Hamadan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the Vice-Chancellery of Research and Technology, Hamadan University of Medical Sciences, which approved and financially supported this project. They would also like to thank all the students who participated in this study for their cooperation.
REFERENCES
- 1.Berek J, Novak E. Berek and Novak's Gynecology. 14th ed. Tehran: Gholban Arianteb; 2007. pp. 481–6. [Google Scholar]
- 2.Ryan K, Berkowitz R, Barbieri R. Kistner's Gynecology and Women's Health. 7th ed. USA: Mosby; 1999. pp. 45–6. [Google Scholar]
- 3.Gagua T, Tkeshelashvili B, Gagua D. Primary dysmenorrhea: Prevalence in adolescent population of Tbilisi, Georgia and risk factors. J Turk Ger Gynecol Assoc. 2012;13:162–8. doi: 10.5152/jtgga.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandi G, Ferrari S, Xholli A, Cannoletta M, Palma F, Romani C, et al. Prevalence of menstrual pain in young women: What is dysmenorrhea? J Pain Res. 2012;5:169–74. doi: 10.2147/JPR.S30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodakarami B, Masoumi SZ, Faradmal J, Nazari M, Saadati M, Sharifi F, et al. The severity of dysmenorrhea and its relationship with body mass index among female adolescents in Hamadan, Iran. J Midwifery Reproductive Health. 2015;3:444–50. [Google Scholar]
- 6.Murphy Goodwin T, Montoro MN, Muderspach L, Paulson R. Management of Common Problems in Obstetrics and Gynecology. 5th ed. North America: Wiley-Blackwell; 2010. pp. 97–100. [Google Scholar]
- 7.Fletcher HM, Dawkins J, Rattray C, Wharfe G, Reid M, Gordon-Strachan G. Morinda citrifolia (Noni) as an anti-inflammatory treatment in women with primary dysmenorrhoea: A randomised double-blind placebo-controlled trial. Obstet Gynecol Int. 2013;2013:195454. doi: 10.1155/2013/195454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves JG, de Brito Rde C, Cavalcanti TS. Effectiveness of Mentha piperita in the treatment of infantile colic: A crossover stud. Evid Based Complement Alternat Med. 2012;2012:981352. doi: 10.1155/2012/981352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalilzadeh-Amin G, Mahama M. Evaluation of pulegone on transit time and castor-oil induced diarrhea in rat. Pharmaceutical Sci. 2013;19:77–82. [Google Scholar]
- 10.Hiki N, Kaminishi M, Hasunuma T, Nakamura M, Nomura S, Yahagi N, et al. A phase I study evaluating tolerability, pharmacokinetics, and preliminary efficacy of L-menthol in upper gastrointestinal endoscopy. Clin Pharmacol Ther. 2011;90:221–8. doi: 10.1038/clpt.2011.110. [DOI] [PubMed] [Google Scholar]
- 11.Taher YA. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J Med. 2012;7 doi: 10.3402/ljm.v7i0.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kligler M, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1028–30. [PubMed] [Google Scholar]
- 13.Mills S, Bone K. Principles and Parctice of Phytotherapy: Modern Herbal Medicine. 2nd ed. London: Churchill Livingstone; 2013. pp. 1–110. [Google Scholar]
- 14.Somerville KW, Richmond CR, Bill GD. Delayed release peppermint oil capsules (Colpermin) for the spastic colon syndrome: A pharmacokinetic study. Br J Clin Pharmacol. 1984;18:638–40. doi: 10.1111/j.1365-2125.1984.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooshk A, Tofighiyan T, Rakhshani MH. Comparison of the effects of marine omega-3 fatty acids and ibuprofen on primary dysmenorrehea. Life Sci J. 2013;10:1–3. [Google Scholar]
- 16.Hoaglin DC, Filonenko A, Glickman ME, Wasiak R, Gidwani R. Use of mixed-treatment-comparison methods in estimating efficacy of treatments for heavy menstrual bleeding. Eur J Med Res. 2013;18:17. doi: 10.1186/2047-783X-18-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol-a human model for cold pain by activation and sensitization of C nociceptors. Brain. 2004;127:1159–71. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- 18.Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138:125–8. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 19.French L. Dysmenorrhea in adolescents: Diagnosis and treatment. Paediatr Drugs. 2008;10:1–7. doi: 10.2165/00148581-200810010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Khodakrami N, Moatar F, Ghahiri A. Comparison of the effect of an herbal drug (SCA) and mefenamic acid on primary dysmenorrhoea a clinical control trial. Horizon Med Sci. 2008;14:11–9. [Google Scholar]
- 21.Asif M. Study of anthranylic acid derivatives: Mefenamic acid and its various analogues. Am J Med Studies. 2014;2:24–30. [Google Scholar]
- 22.Taylor T. Treatment of nausea and vomiting in pregnancy. Aust Prescr. 2014;37:42–5. [Google Scholar]
- 23.Shavakh A, Ardestani SK, Taki M, Goli M, Kashteli AH. Premedication with Peppermint oil capsules in colonoscopy: A double blind placebo-controlled randomazed trial study. Acta Gastroenterol Belg. 2012;75:349–53. [PubMed] [Google Scholar]
- 24.Cappelo G, Spezzaferro M, Grossi L, Manzoli L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–6. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]