Abstract
Background:
This study evaluated the efficacy of a sonic device (Vibringe), passive ultrasonic irrigation (PUI), and conventional syringe irrigation (CSI) in the removal of triple antibiotic paste (TAP) from artificial standardized grooves in the apical and coronal thirds of a root canal.
Materials and Methods:
One-hundred eighteen root canals were prepared using the ProTaper system. The roots were split longitudinally, and a standardized groove was prepared in the apical and coronal parts of one segment. The grooves were filled with TAP, and the roots were reassembled. The roots were randomly divided into nine experimental groups and two control groups, according to the following irrigation methods: (1) CSI with sodium hypochlorite (NaOCl) + ethylenediaminetetraacetic acid (EDTA), (2) CSI/EDTA, (3) CSI/NaOCl, (4) PUI/NaOCl + EDTA, (5) PUI/EDTA, (6) PUI/NaOCl, (7) Vibringe/NaOCl + EDTA, (8) Vibringe/EDTA, and (9) Vibringe/NaOCl. The amount of remaining medicament was evaluated under a stereomicroscope.
Results:
In the apical third, Vibringe/NaOCl + EDTA and PUI/NaOCl + EDTA were superior to the other groups (P < 0.05); there was no significant difference between the other experimental groups (P > 0.05). In the coronal third, there was no significant difference between the experimental groups (P > 0.05).
Conclusions:
The use of the NaOCl/EDTA combination together with sonic or ultrasonic agitation improved the removal of TAP from the apical third.
Keywords: Irrigation, root canal, triple antibiotic paste, vibringe
INTRODUCTION
The elimination of bacteria and their byproducts from the root canal system is one of the aims of root canal therapy. Thus, the combination of the instrumentation associated with various root canal irrigation solutions and medicaments has been suggested.[1,2] Calcium hydroxide is commonly used because of its antimicrobial efficacy against most bacterial species identified in endodontic infections. Recently, antibiotic pastes have been used for root canal therapy and especially for regenerative endodontic treatment because infections of the root canal system are regarded as polymicrobial (i.e., consisting of different species of bacteria and other microorganisms).[3,4] Triple antibiotic paste (TAP) containing metronidazole, ciprofloxacin, and minocycline is the most commonly used antibiotic paste.[5,6,7] Ruparel et al.[8] stated that TAP had a detrimental effect on human stem cells in the apical papilla when it was used in regenerative treatment. Thus, TAP should be removed completely from the root canal to inhibit its detrimental effects on stem cells in apical papilla. In addition, TAP may cause tooth discoloration if not removed completely from the root canal. Kim et al.[9] have demonstrated that the main reason for tooth discoloration after treatment was due to contact of the minocycline in TAP with coronal dentinal walls during treatment procedures. Various irrigating solutions, such as sodium hypochlorite (NaOCl), ethylenediaminetetraacetic acid (EDTA), and sterile saline in combination with ultrasonic, sonic, and laser devices were used in the removal of TAP from the root canal.[10,11,12] However, none of the irrigation techniques completely removed TAP from the root canal.
The Vibringe (Vibringe B.V. Corp, Amsterdam, The Netherlands) is a relatively new device on the market that combines manual delivery and sonic activation of the solution. It is a cordless handpiece that fits into a specially designed disposable syringe, that is, compatible with every irrigation needle. It continuously delivers irrigant in a pulsating manner directly into the root canal via a standard needle. It uses sonic flow technology combined with acoustic streaming.[13]
There is no study evaluating the efficiency of Vibringe and an irrigation solution combination of NaOCl/EDTA in the removal of TAP from root canals. Therefore, the purpose of this study was to evaluate the efficiency of different irrigation methods in the removal of TAP from artificial standardized grooves in the apical and coronal thirds of the root. The null hypothesis was that the removal of TAP is not affected by the irrigation technique used.
MATERIALS AND METHODS
Ethical approval was obtained from the Review Committee of the Research Foundation of Erciyes University of Medical Sciences in Kayseri, Turkey (2015/412), before investigation. One hundred and eighteen extracted human single-rooted maxillary canine teeth with similar root lengths measured from the cementoenamel junction to the root apex were selected for this study. Criteria for tooth selection included a single root canal, no visible root caries, fractures, or cracks, no signs of internal or external resorption or calcification, a completely formed apex, and a curvature <5°, in accordance with the protocol prescribed by Schneider.[14] Preoperative radiographs were taken in the buccolingual and mesiodistal directions to confirm the presence of a single canal without previous root canal treatment, resorptions, or calcifications. The teeth were stored in purified filtered water until use. To ensure standardization, the crowns of the selected teeth were partially removed to achieve a final 22 mm length for each tooth. Endodontic access cavities were prepared using diamond burs (Diatech, Coltene Whaledent, Altstätten, Switzerland) with a high-speed handpiece under water-cooling. A size 15 K-file (Dentsply Maillefer, Ballaigues, Switzerland) was then placed in the canal until it was visible at the apical foramen. The working length (WL) was determined by substracting 1 mm from this measurement. The 118 roots were instrumented with the ProTaper rotary system (Dentsply Maillefer) up to size F5 (#50, 0.05 taper), which was chosen as the master apical file. During preparation, the root canal was irrigated with 2 mL of 2.5% NaOCl solution using a syringe and a 29-gauge needle (NaviTip; Ultradent, South Jordan, UT, USA) between each instrument change. After completion of the preparation, the root canals were irrigated with 5 mL of 17% EDTA for 1 min and 5 mL of 3% NaOCl for 1 min. The canals were finally rinsed with 10 mL of distilled water and then dried with sterile paper points.
After root canal preparation, the teeth were fixed in modified Eppendorf vials with silicone material (Coltene\Whaledent AG, Switzerland) [Figure 1]. After removal from the impression material, all teeth were grooved longitudinally on the buccal and lingual surfaces with a diamond disk under copious water irrigation, avoiding penetration into the root canal. The teeth were then split into 2 halves with a small chisel. Each root was split longitudinally through the canal, forming two halves. A standard groove of 4 mm in length, 0.2 mm in width and 0.5 mm in depth was cut in one canal wall 2–6 mm from the apex to simulate uninstrumented canal extensions in the apical third. Another longitudinal groove was created in the other half at a distance of 9–12 mm from the apex in the coronal third.[12] Five teeth were left and served as the negative control group. These teeth were not filled with TAP. The grooves in the remaining 113 teeth were filled with TAP. Equal portions of metronidazole (Eczacıbası, Istanbul, Turkey), ciprofloxacin (Biofarma, Istanbul, Turkey), and minocycline (Ratiopharm, Ulm, Germany) were mixed with distilled water, and TAP was packed into the apical and coronal grooves, using a plugger. Before reassembling, the apices were sealed with wax to prevent the overflow of the irrigation solution and to make a closed-end channel to create a vapor lock effect.[15] Then, the root halves were reassembled and the specimens were remounted in the Eppendorf vials [Figure 1]. Access cavities were sealed temporarily with a cotton pellet and temporary filling material (Cavit; 3M ESPE, Seefeld, Germany). The specimens were stored in an incubator at 37°C in 100% humidity for 28 days. 5 of the 113 teeth served as the positive control group and no TAP removal procedure was carried out on those teeth.
Figure 1.

The experimental model system used to fix teeth before splitting
Experimental groups and removal of triple antibiotic paste
After removal of the temporary filling material, specimens were divided into nine experimental groups as follows:
Group 1: Conventional syringe irrigation/sodium hypochlorite + ethylenediaminetetraacetic acid (n = 12)
To remove TAP from the grooves, irrigation with 5 mL of 3% NaOCl followed by 5 mL of 17% EDTA was performed using a syringe and a 29-gauge side-vented needle (NaviTip; Ultradent, South Jordan, UT, USA) placed 1 mm short of the WL.
Group 2: Conventional syringe irrigation/ethylenediaminetetraacetic acid (n = 12)
In this group, irrigation with 10 mL of 17% EDTA was performed using a syringe and a 29-gauge side-vented needle (NaviTip) placed 1 mm short of the WL.
Group 3: Conventional syringe irrigation/sodium hypochlorite (n = 12)
Irrigation with 10 mL of 3% NaOCl was performed using a syringe and a 29-gauge side-vented needle (NaviTip) placed 1 mm short of the WL.
Group 4: Passive ultrasonic irrigation/sodium hypochlorite + ethylenediaminetetraacetic acid (n = 12)
In this group, 5 mL of 3% NaOCl and 5 mL of 17% EDTA were each passively agitated using an ultrasonic device (EMS, Le Sentier, Switzerland). A smooth ultrasonic file size 15 and 0.02 taper (ESI instrument) was placed into the root canal to 1 mm short of the WL without touching the canal walls, enabling it to vibrate freely. The ultrasonic file was activated at power setting 6 for 1 min for each irrigation solution.
Group 5: Passive ultrasonic irrigation/ethylenediaminetetraacetic acid (n = 12)
In this group, 10 mL of 17% EDTA was used as the irrigant. Agitation of the irrigant was performed in the same manner as for the previous group. Agitation time was 2 min.
Group 6: Passive ultrasonic irrigation/sodium hypochlorite (n = 12)
TAP was removed through agitation of 10 mL of 3% NaOCl. Agitation of the irrigant was performed in the same manner as for Group 4. Agitation time of the irrigant was 2 min.
Group 7: Vibringe/sodium hypochlorite + ethylenediaminetetraacetic acid (n = 12)
The Vibringe device was attached to a 30-gauge Max-i-Probe side-vented needle. The needle was placed to 1 mm short of the WL, and the device was activated while delivering irrigant for 1 min for each irrigant.
Group 8: Vibringe/ethylenediaminetetraacetic acid (n = 12)
TAP was removed through the agitation of 10 mL of 17% EDTA. Agitation of the irrigant was performed in the same manner as for Group 7. Agitation time of the irrigant was 2 min.
Group 9: Vibringe/sodium hypochlorite (n = 12)
TAP was removed through the agitation of 10 mL of 3% NaOCl. Agitation of the irrigant was performed in the same manner as for Group 7. Agitation time of the irrigant was 2 min.
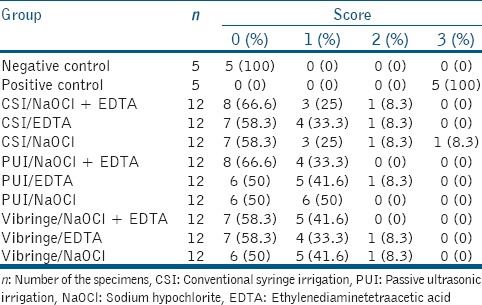
After the irrigation procedure, the canals were dried using paper points, and the roots were dissembled. Digital images were taken at ×25 magnification, using a digital camera (DP-70; Olympus, Tokyo, Japan) attached to a stereomicroscope (BX60; Olympus) and were transferred to the computer. Two calibrated examiners blinded to the TAP removal procedure then scored the amount of TAP remaining in the grooves using the scoring system described by van der Sluis et al.:[16]0 – the groove was empty, 1 – less than half of the groove was filled with TAP, 2 – more than half of the groove was filled with TAP, and 3 – the groove was completely filled with TAP.
RESULTS
A kappa test showed 95.5% inter-examiner agreement on the removal of TAP from artificial standardized grooves. Intraindividual reproducibility for the examiners was 98.3% (232/236) and 96.6% (228/236). Tables 1 and 2 show the distributions of the scores pooled for both examiners and the percentage values of residual TAP within the grooves. All of the experimental groups were significantly different from the positive and negative control groups (P < 0.05). None of the experimental groups showed complete removal of TAP from the artificial standardized grooves. There were statistically significant differences in the TAP removal results among the experimental groups (P < 0.05). In the apical third, Vibringe/EDTA + NaOCl and passive ultrasonic irrigation (PUI)/NaOCl + EDTA removed significantly more TAP than the other irrigation procedures (P < 0.05). However, there was no statistical difference between any of the other experimental groups (P > 0.05). In the coronal third, there was no significant difference among the experimental groups in the removal of TAP (P > 0.05).
Table 1.
Number and percentage of specimens at each score rank after different irrigation regimens in apical root third

Table 2.
Number and percentage of specimens at each score rank after different irrigation regimens in coronal root third
DISCUSSION
The design of the current study is comparable to that described by van der Sluis et al.[16] and seems well suited to evaluate the removal of TAP from artificial grooves. The advantage of the groove model is the standardized size and location of the grooves, allowing a standardized evaluation with high intra-examiner reproducibility and good inter-examiner agreement. The major disadvantage of this design is that the artificial standardized grooves do not represent the complexity of a natural root canal system. Therefore, it might be easier to remove TAP from artificial grooves than from isthmuses or oval extensions in vivo.[17]
Previous studies showed that TAP could be used clinically in the treatment of teeth with extensive periradicular lesions and as part of the endodontic regenerative technique.[18,19,20] TAP should be removed from the root canal before final treatment procedures because it causes discoloration and has a detrimental effect on stem cells in the apical papilla.[8,21] Researchers evaluated the effect of various irrigants and irrigation methods in removing TAP from the root canal. It has been determined that varying the irrigant and/or the irrigation technique will not ensure that TAP is completely removed from the root canal system.[10,11,22] Furthermore, the findings of the current study show that it is difficult to completely remove TAP from the apical and coronal thirds of root canals. In the apical third, there was a significant difference between the experimental groups as to how much TAP was removed. Therefore, the null hypothesis was rejected. Berkhoff et al.[10] determined that TAP was not completely removed from the root canal system, and they stated that this may be due to the apparent high diffusion and binding of TAP into root dentin. It has also been reported that minocycline in TAP binds to calcium ions via chelation to form an insoluble complex in the tooth matrix. Thakur et al.[23] compared the effectiveness of Canal Brushing technique, sonic technique (EndoActivator), and master apical file for the removal of TAP and determined that none of the techniques completely removed TAP from the root canal. Additionally, they determined that Canal Brush and EndoActivator showed better result than master apical file in the cervical and middle third of canal. Arslan et al.[22] compared the efficacy of various irrigation protocols (distilled water, 1% NaOCl, 2.5% NaOCl, 100% ethanol, 17% EDTA and PUI with 1% NaOCl) on the removal of TAP from artificial groove in the apical of root canal and determined that PUI with 1% NaOCl was more effective in removing TAP from artificial groove in root canal than other irrigating solutions.
In the current study, Vibringe and PUI exhibited performance similar to conventional syringe irrigation (CSI) in the coronal part, but Vibringe and PUI removed significantly more TAP in the apical part. A possible explanation is that the oscillation amplitude of the sonically activated irrigation needle or ultrasonically activated file is higher at the tip than at the attached end,[24,25] resulting in increased fluid velocity at that point. In the coronal part, the greater distance of the needle or file tips from the artificial groove seems to reduce the efficacy of the agitation of the irrigant.
When the type of irrigant is considered, in the coronal third, no difference was found between the NaOCl/EDTA combination, EDTA, and NaOCl irrigants in the removal of TAP. In the apical third, the sonic or ultrasonic agitation of the NaOCl/EDTA combination compared with CSI showed better removal of TAP from the artificial groove. In previous in vitro studies,[11,22] only one irrigant (NaOCl or EDTA) with or without agitation was used to remove TAP from the root canal. The present study is the first study to evaluate the NaOCl/EDTA combination in the removal of TAP from simulated root canal irregularities. An important finding of this study is that the use of the NaOCl/EDTA combination together with sonic or ultrasonic agitation in the apical third, where removal of TAP is difficult, is more effective than either EDTA or NaOCl alone. In a study by Akman et al.,[11] it was determined that agitation of NaOCl with PUI removes significantly more modified TAP medicament than the CSI group at the apical third. This is in agreement with the current study.
PUI is based on the principle of cavitation and acoustic streaming. Because of the microstreaming generated, more dentinal debris can be removed from the root canal system compared with syringe delivery of an irrigant.[26] The same mechanisms are probably responsible for the more effective removal of TAP during PUI in comparison with CSI. The higher velocity of irrigant flow generated by PUI may explain its efficiency in TAP removal from artificial grooves in the apical part of a root canal.[27]
CONCLUSIONS
Under the conditions of this in vitro study, it can be concluded that none of the irrigation methods completely removed TAP from the artificial standardized grooves in the apical and coronal root thirds. In the apical third, however, the sonic or ultrasonic agitation of the irrigation solution combination NaOCl/EDTA can improve the removal of TAP compared with CSI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Estrela C, Holland R, Bernabé PF, de Souza V, Estrela CR. Antimicrobial potential of medicaments used in healing process in dogs' teeth with apical periodontitis. Braz Dent J. 2004;15:181–5. doi: 10.1590/s0103-64402004000300003. [DOI] [PubMed] [Google Scholar]
- 2.Nair PN. On the causes of persistent apical periodontitis: A review. Int Endod J. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 3.Kusgoz A, Yildirim T, Er K, Arslan I. Retreatment of a resected tooth associated with a large periradicular lesion by using a triple antibiotic paste and mineral trioxide aggregate: A case report with a thirty-month follow-up. J Endod. 2009;35:1603–6. doi: 10.1016/j.joen.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Bezgin T, Yilmaz AD, Celik BN, Sönmez H. Concentrated platelet-rich plasma used in root canal revascularization: 2 case reports. Int Endod J. 2014;47:41–9. doi: 10.1111/iej.12144. [DOI] [PubMed] [Google Scholar]
- 5.Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35:160–4. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Saoud TM, Zaazou A, Nabil A, Moussa S, Lin LM, Gibbs JL. Clinical and radiographic outcomes of traumatized immature permanent necrotic teeth after revascularization/revitalization therapy. J Endod. 2014;40:1946–52. doi: 10.1016/j.joen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: A case series. J Endod. 2010;36:536–41. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: A case report. J Endod. 2010;36:1086–91. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Berkhoff JA, Chen PB, Teixeira FB, Diogenes A. Evaluation of triple antibiotic paste removal by different irrigation procedures. J Endod. 2014;40:1172–7. doi: 10.1016/j.joen.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Akman M, Akbulut MB, Aydinbelge HA, Belli S. Comparison of different irrigation activation regimens and conventional irrigation techniques for the removal of modified triple antibiotic paste from root canals. J Endod. 2015;41:720–4. doi: 10.1016/j.joen.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Arslan H, Akcay M, Capar ID, Ertas H, Ok E, Uysal B. Efficacy of needle irrigation, EndoActivator, and photon-initiated photoacoustic streaming technique on removal of double and triple antibiotic pastes. J Endod. 2014;40:1439–42. doi: 10.1016/j.joen.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Bolles JA, He J, Svoboda KK, Schneiderman E, Glickman GN. Comparison of Vibringe, EndoActivator, and needle irrigation on sealer penetration in extracted human teeth. J Endod. 2013;39:708–11. doi: 10.1016/j.joen.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32:271–5. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 15.Pedullà E, Genovese C, Campagna E, Tempera G, Rapisarda E. Decontamination efficacy of photon-initiated photoacoustic streaming (PIPS) of irrigants using low-energy laser settings: An ex vivo study. Int Endod J. 2012;45:865–70. doi: 10.1111/j.1365-2591.2012.02044.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Sluis LW, Wu MK, Wesselink PR. The evaluation of removal of calcium hydroxide paste from an artificial standardized groove in the apical root canal using different irrigation methodologies. Int Endod J. 2007;40:52–7. doi: 10.1111/j.1365-2591.2006.01182.x. [DOI] [PubMed] [Google Scholar]
- 17.Rödig T, Vogel S, Zapf A, Hülsmann M. Efficacy of different irrigants in the removal of calcium hydroxide from root canals. Int Endod J. 2010;43:519–27. doi: 10.1111/j.1365-2591.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 18.Er K, Kustarci A, Ozan U, Tasdemir T. Nonsurgical endodontic treatment of dens invaginatus in a mandibular premolar with large periradicular lesion: A case report. J Endod. 2007;33:322–4. doi: 10.1016/j.joen.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Taneja S, Kumari M. Use of triple antibiotic paste in the treatment of large periradicular lesions. J Investig Clin Dent. 2012;3:72–6. doi: 10.1111/j.2041-1626.2011.00082.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagata JY, Gomes BP, Rocha Lima TF, Murakami LS, de Faria DE, Campos GR, et al. Traumatized immature teeth treated with 2 protocols of pulp revascularization. J Endod. 2014;40:606–12. doi: 10.1016/j.joen.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Akcay M, Arslan H, Yasa B, Kavrik F, Yasa E. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod. 2014;40:845–8. doi: 10.1016/j.joen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Arslan H, Capar ID, Saygili G, Uysal B, Gok T, Ertas H, et al. Efficacy of various irrigation protocols on the removal of triple antibiotic paste. Int Endod J. 2014;47:594–9. doi: 10.1111/iej.12194. [DOI] [PubMed] [Google Scholar]
- 23.Thakur DA, Patil S, Gade V, Jogad N, Gangrade A, Sinkar R. Comparative scanning electron microscopy evaluation of Canal Brushing technique, sonic activation, and master apical file for the removal of triple antibiotic paste from root canal (in vitro study) Contemp Clin Dent. 2015;6:517–21. doi: 10.4103/0976-237X.169852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang LM, Verhaagen B, Versluis M, van der Sluis LW. Evaluation of a sonic device designed to activate irrigant in the root canal. J Endod. 2010;36:143–6. doi: 10.1016/j.joen.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad M, Pitt Ford TJ, Crum LA. Ultrasonic debridement of root canals: Acoustic streaming and its possible role. J Endod. 1987;13:490–9. doi: 10.1016/s0099-2399(87)80016-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Wu MK, Wesselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from different-sized simulated plastic root canals. Int Endod J. 2004;37:607–12. doi: 10.1111/j.1365-2591.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang LM, Verhaagen B, Versluis M, van der Sluis LW. Influence of the oscillation direction of an ultrasonic file on the cleaning efficacy of passive ultrasonic irrigation. J Endod. 2010;36:1372–6. doi: 10.1016/j.joen.2010.03.019. [DOI] [PubMed] [Google Scholar]


