Abstract
Background:
Caries is the most common dental disease facing the world population. Caries can be prevented by remineralizing early enamel lesions.
Aim:
To evaluate remineralization efficacy of stannous fluoride (SnF2), casein phosphopeptide-amorphous calcium phosphate with fluoride (CPP-ACPF) and calcium sucrose phosphate (CaSP).
Materials and Methods:
Fifty enamel samples were taken; they were divided into five groups (n = 10). Demineralization was carried out with Groups A, B, C, and E. Remineralization was carried out with Groups A, B, and C for 7 days using SnF2, CPP-ACPF, and CaSP, respectively. In Group D, no surface treatment was carried out, to mark as positive control whereas Group E was kept as negative control with only surface demineralization of enamel. Enamel microhardness was tested using Vickers's microhardness tester after 7 day remineralization regime.
Statistical Analysis:
One-way analysis of variance and post hoc Tukey tests were performed.
Results:
The mean microhardness values in descending order: Positive control > SnF2> CaSP > CPP-ACPF > negative control.
Conclusion:
All remineralizing agents showed improved surface remineralization. However, complete remineralization did not occur within 7 days. SnF2 showed the highest potential for remineralization followed by CaSP and CPP-ACPF.
Keywords: Calcium sucrose phosphate, caries prevention, casein phosphopeptide-amorphous calcium phosphate with fluoride, microhardness, remineralization, stannous fluoride
INTRODUCTION
The most common oral disease affecting a large number of people is dental caries.[1] The occurrence of caries is pH dependent. When pH drops below 5.5, enamel dissolution starts, embarking demineralization. It marks the beginning of early enamel caries.[2] During demineralization, subsurface layer gets demineralized while the enamel surface layer stays consistently unmutilated.[1] Neutralizing the oral pH opposes the process of demineralization. This can be achieved by increasing calcium and phosphate ions. This process is called as remineralization, which involves rebuilding of partly dissolved apatite crystals.[2]
Numerous mechanisms are available for accelerated remineralization. It involves a delivery mechanism of ions to the affected area. Topical fluorides have been well known and proven as delivery system for prevention of caries.[1] Restriction of initiation of dental caries can be achieved by milk and its products. Casein, calcium, and phosphate provide this anticariogenic property to milk, imparting its protective action.[3] Recently, calcium sucrose phosphate (CaSP) was reintroduced as a promising remineralizing agent. It decomposes to calcium, phosphate, and sucrose ions, thus resulting in increased rate of remineralization.[4]
This study focuses on evaluating the enamel remineralizing potential of CaSP (EnaFix, Group Pharmaceuticals Limited, Bengaluru, India), Casein phosphopeptide-amorphous calcium phosphate with fluoride (CPP-ACPF, GC Tooth Mousse Plus, Leuven, Belgium) and stannous fluoride (SnF2, Gel Kam Colgate, Palmolive Limited, India) using surface microhardness (SMH) analysis (Vickers hardness test).
MATERIALS AND METHODS
Fifteen freshly extracted sound maxillary premolars, extracted for orthodontic reasons, were used. Teeth with any visible or detectable caries or any white spot lesions were excluded.[5]
The teeth were handled according to OSHA and CDC criterion.[5]
The teeth were decoronated and the crown portions were divided into four segments of two buccal and two palatal halves each using a double-faced diamond disc mounted on a contra-angle hand piece.[5]
Enamel samples were embedded in self-cure acrylic with the enamel surface exposed. These samples were stored in deionized water until further use. A total of fifty enamel samples embedded in acrylic slabs were produced, and were then randomly divided into five groups of 10 samples each [Table 1].
Table 1.
Division of groups and one-way analysis of variance

Demineralizing solution was prepared in the Department of Biochemistry. A digital pH meter (Slope Labtronics, Model LT-11, Panchkula, Haryana, India) was used to check pH during and after preparation of solution. The composition of demineralizing solution used was as follows:
2.2 mM calcium chloride, CaCl2·2H2O (Loba Chemie Pvt. Ltd., Mumbai, Maharashtra, India)
2.2 mM monosodium phosphate, NaH2PO4·7H2O (Loba Chemie Pvt. Ltd., Mumbai, Maharashtra, India)
0.05 M lactic acid, C3H6O3(Loba Chemie Pvt. Ltd., Mumbai, Maharashtra, India).
The final pH was adjusted to 4.5 with 50% sodium hydroxide, NaOH (Loba Chemie Pvt. Ltd., Mumbai, Maharashtra, India).[1]
All the samples of Groups A, B, C, and E were then immersed into a glass container containing 50 ml of prepared demineralizing solution for a period of 48 h at 37°C inside universal incubator (Coslab, ISO 9001:2000, Ambala Cant, Haryana, India). This demineralizing procedure was contemplated to produce a consistent subsurface lesion. After 48 h of incubation, the teeth were washed with deionized water, dried with the help of an air syringe, and placed in respective glass containers until further evaluation.[1]
The samples in Groups A, B, and C were treated with respective remineralizing agents at every 24 h for 7 days. Samples were rubbed with respective remineralizing agent with the help of polishing cup attached to a contra-angle hand piece for 4 min, washed with deionized water, and then placed in artificial saliva. All samples were placed in universal incubator at 37°C between each remineralizing cycle. In the control groups, samples were only washed with deionized water and placed in artificial saliva. Artificial saliva was renewed every 24 h just before immersion of freshly treated samples.[1]
After seven cycles of remineralization, the SMH of the specimens were determined using Vickers microhardness testing machine (Mitutoyo, Kawasaki, Japan). A load of 100 g was exercised steadily to the surface of specimens for 10 s using Vickers elongated diamond pyramid indenter under a ×40 objective lens. Accuracy of values of diagonal length of indentations was determined under high magnification of ×400. The depth of the indentations was measured via a built-in scaled microscope and the values were converted to Vickers microhardness values. Five indentations were placed on the surface and the average value was considered for each specimen. Statistical analysis was done using one-way analysis of variance (ANOVA), post hoc Tukey test, and SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
Statistical methods
The SMH of samples was compared across study groups. The mean and standard deviation (SD) of microhardness of samples were obtained for each group and comparison was performed using one-way ANOVA. The pair-wise comparison of mean microhardness between groups was carried out using Tukey's post hoc test. The statistical significance was tested at 5%, and the analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA) software.
RESULTS
The statistical parameters such as mean and SD of microhardness of samples were obtained for each group as shown in Table 1. The mean for positive control was the highest 282.35 ± 28.97 MPa, while that of negative control was the lowest 198.74 ± 25.46 MPa. In case of materials used, SnF2 indicated highest mean 239.78 ± 23.4 MPa, followed by CaSP with a mean value of 234.24 ± 19.05 MPa, and CPP-ACPF with the least mean value of 213.91 ± 31.81 MPa [Table 1]. One-way ANOVA resulted into the F-statistic of 14.73 with a corresponding P < 0.0001. The test indicated statistically significant difference of mean microhardness across the groups. Accordingly, pair-wise comparison of microhardness was performed between groups to determine which groups differed significantly from other, with results shown in Table 2. The test revealed that the mean microhardness of positive control group was significantly higher than all other groups as indicated by P < 0.05 for all comparisons. The mean microhardness of SnF2 was higher than other two experimental groups; however, the difference was statistically insignificant as indicated by P > 0.05. But, its mean differed significantly from that of negative control (P < 0.05). The mean microhardness of CaSP and CPP-ACPF was insignificantly different (P > 0.05), but differed significantly from negative control. The mean microhardness of CPP-ACPF and negative control group differed insignificantly (P > 0.05).
Table 2.
Pair-wise comparison of mean surface microhardness using post hoc Tukey
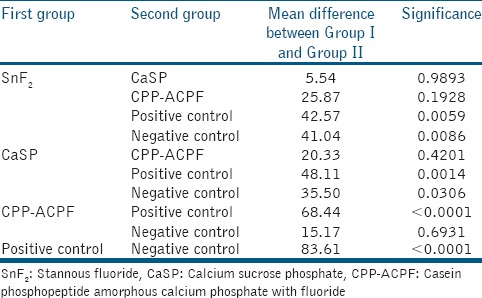
In summary, this study reveals that the overall difference of mean microhardness across groups was mainly due to positive and negative control groups, while mean values for the three experimental groups were insignificantly different from each other.
DISCUSSION
Early enamel caries can histologically be described as a subsurface carious lesion of enamel. Prominent feature being a subsurface demineralized zone with intact and unscathed enamel surface. Even though surface is intact, the mineral content is deficient. Hence, there is a lower microhardness of early enamel caries as opposed to sound enamel.[2]
During demineralization, Ca2+, OH−, PO42−, F−, CO3−, Na+ and Mg2+ get displaced from the enamel surface to the exterior. More the acidic environment, greater is the outward flow of ions. However, mineral content of surface is higher than the body of the lesion.[2]
In the present study, the specimens were stored in the demineralizing solution for 48 h at 37°C in Universal Incubator simulating oral conditions. This resulted in a subsurface demineralization with an intact surface replicating an early enamel lesion.[2]
Owing to the importance of surface intactness, SMH measurement was a suitable and rapid method for this experimental design. Therefore, in the present study, the microhardness values for each specimen were measured.[2]
In the present study, the mean microhardness of positive control group was significantly higher than all other groups marking the accuracy of my study. Among materials, SnF2 has the highest microhardness value (239.78 ± 23.4 MPa), which is in accordance with previous studies by Hove et al. reason can be due to the formation of fluoride metal (tin-fluoride) complexes. Tin-fluoride complexes reduce demineralization by increasing the time of fluoride retention on the enamel. McCann showed that tin-fluoride surface complex is resistant to strong acids.[6,7,8,9] Moreover, pH of the material is acidic and causes mild demineralization of the enamel surface. This enhances the depth of penetration of fluoride ions.[7]
CaSP is a combination of calcium salts of sucrose phosphate esters, blended with inorganic calcium. It soon breaks down into calcium ions, phosphate ions, and sucrose phosphate ions into saliva. It is composed of 10–12% calcium and 8–10% phosphorous (by weight). CaSP permits creation of aqueous solution consisting of very high concentration of calcium and phosphate without occurrence of precipitation. It acts as an ideal carrier for calcium and phosphate. In this study, CaSP has shown improved SMH with mean value of 234.24 ± 19.05 MPa. The mechanism of action is by adsorption of sucrose phosphate ions rapidly on the enamel surface, reducing the rate of acid dissolution of hydroxyapatite and quick remineralization by calcium and phosphate ions by common ion effect.[5,10]
CPP-ACPF is a supersaturated solution of amorphous and crystalline calcium phosphate phases. The remineralizing capacity is directly proportional to the levels of CPP-stabilized free calcium and phosphate ions. It is stabilized by CPP in a way that spontaneous precipitation of calcium phosphate is restrained.[11] When CPP-ACPF is applied, the sticky CPP binds readily to enamel, biofilm and soft tissues releasing the calcium and phosphate ions. The free calcium and phosphate ions enter the enamel rods and reform the apatite crystals.[3,12]
In the present study, CPP-ACPF got the least mean microhardness of 213.91 ± 31.81 MPa. It is suggested that CPP-ACP molecules need an acidic exposure to be activated and it should separate ACP from the casein. The samples were not acid challenged for activation and they were challenged to demineralization cycle only once. When it was necessary for activation they were washed by artificial saliva. This might be the reason for least microhardness value of CPP-ACPF.[13,14]
Another possible reason for least values obtained may be due to the short treatment duration. Therefore, it is necessary to have a longer period of application to be able to detect deposition of calcium and phosphate ions in the demineralized lesion.
Seven-day remineralization failed to remineralize artificial enamel caries completely. It is one of the drawbacks observed in the study. Hence, the period of application for complete remineralization cannot be determined for all the remineralizing agents used. Although surface remineralization was confirmed, enamel subsurface remineralization was not evaluated. Within the limitations of this in vitro study, one can infer that remineralization takes place with the use of SnF2, CPP-ACPF and CaSP. However, complete remineralization did not occur within the time span of 7 days.
CONCLUSION
All remineralizing agents showed improved surface remineralization. However, complete remineralization did not occur within 7 days. SnF2 showed the highest potential for remineralization followed by CaSP and CPP-ACPF. Having said that, SnF2 shows gingival irritation and has metallic taste. To overcome these drawbacks, CaSP can be used as an effective tool for remineralization of early enamel caries as it is economical and has shown improved microhardness than that of CPP-ACPF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Patil N, Choudhari S, Kulkarni S, Joshi SR. Comparative evaluation of remineralizing potential of three agents on artificially demineralized human enamel: An in vitro study. J Conserv Dent. 2013;16:116–20. doi: 10.4103/0972-0707.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayarajan J, Janardhanam P, Jayakumar P. Deepika. Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization – An in vitro study using scanning electron microscope and DIAGNOdent. Indian J Dent Res. 2011;22:77–82. doi: 10.4103/0970-9290.80001. [DOI] [PubMed] [Google Scholar]
- 4.Sargod SS, Bhat SS, Hegde S, Karunakaran R. Remineralization potential using calcium sucrose phosphate (EnaFix) on artificial carious lesion: A polaroid microscopic study. Indian J Appl Res. 2015;5:421–3. [Google Scholar]
- 5.Shetty S, Hegde MN, Bopanna TP. Enamel remineralization assessment after treatment with three different remineralizing agents using surface microhardness: An in vitro study. J Conserv Dent. 2014;17:49–52. doi: 10.4103/0972-0707.124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCann HG. The effect of fluoride complex formation on fluoride uptake and retention in human enamel. Arch Oral Biol. 1969;14:521–31. doi: 10.1016/0003-9969(69)90145-9. [DOI] [PubMed] [Google Scholar]
- 7.Hove LH, Holme B, Young A, Tveit AB. The protective effect of TiF4, SnF2 and NaF against erosion-like lesions in situ. Caries Res. 2008;42:68–72. doi: 10.1159/000112816. [DOI] [PubMed] [Google Scholar]
- 8.Young A, Thrane PS, Saxegaard E, Jonski G, Rölla G. Effect of stannous fluoride toothpaste on erosion-like lesions: An in vivo study. Eur J Oral Sci. 2006;114:180–3. doi: 10.1111/j.1600-0722.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 9.Huysmans MC, Jager DH, Ruben JL, Unk DE, Klijn CP, Vieira AM. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45:518–23. doi: 10.1159/000331391. [DOI] [PubMed] [Google Scholar]
- 10.Harris R, Schamschula RG, Beveridge J, Gregory G. The cariostatic effect of calcium sucrose phosphate in a group of children aged 5-17 years. Part IV. Aust Dent J. 1968;14:42–9. doi: 10.1111/j.1834-7819.1969.tb03331.x. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 12.Somasundaram P, Vimala N, Mandke LG. Protective potential of casein phosphopeptide amorphous calcium phosphate containing paste on enamel surfaces. J Conserv Dent. 2013;16:152–6. doi: 10.4103/0972-0707.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta R, Nandlal B, Prashanth S. Comparative evaluation of remineralization potential of casein phosphopeptide-amorphous calcium phosphate and casein phosphopeptide-amorphous calcium phosphate fluoride on artificial enamel white spot lesion: An in vitro light fluorescence study. Indian J Dent Res. 2013;24:681–9. doi: 10.4103/0970-9290.127610. [DOI] [PubMed] [Google Scholar]
- 14.Hegde MN, Moany A. Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J Conserv Dent. 2012;15:61–7. doi: 10.4103/0972-0707.92609. [DOI] [PMC free article] [PubMed] [Google Scholar]


