Abstract
We describe a 4-year-old male child born to non-consanguineous Spanish parents with progressive encephalopathy (PE), microcephaly, and hypertonia. Whole exome sequencing revealed compound heterozygous BRAT1 mutations [c.1564G > A (p.Glu522Lys) and c.638dup (p.Val214Glyfs*189)]. Homozygous and compound heterozygous BRAT1 mutations have been described in patients with lethal neonatal rigidity and multifocal seizure syndrome (MIM# 614498). The seven previously described patients suffered from uncontrolled seizures, and all of those patients died in their first months of life. BRAT1 acts as a regulator of cellular proliferation and migration and is required for mitochondrial function. The loss of these functions may explain the cerebral atrophy observed in this case of PE. This case highlights the extraordinary potential of next generation technologies for the diagnosis of rare genetic diseases, including PE. Making a prompt diagnosis of PE is important for genetic counseling and disease management.
Keywords: Progressive encephalopathy, Whole exome sequencing, BRAT1, Lethal neonatal rigidity
1. Introduction
Progressive encephalopathy (PE) is rarely seen in general pediatric practice. Children may present with unexplained psychomotor retardation and signs of progressive CNS diseases.
The incidence of PE is unknown but has been estimated to be 0. 5–0.6 per 1000 live births.1,2 Stromme et al., [2007] calculated an overall incidence rate for PE of 6.43 per 100,000 person years in Oslo, Norway using local population data.1 PE is predominantly caused by inborn errors of metabolism or neurodegenerative diseases without an identifiable metabolic deficiency.1,3 Importantly, the risk of PE and other disabilities increases in consanguineous unions or in communities with a high incidence of known consanguinity.4–6 These data support the predominantly autosomal recessive inheritance pattern for described causes of PE.
In addition, mortality rates are increased significantly in patients with PE in comparison to the total population. Patient fatality varies from 17 to 37%3,7; this rate is higher in patients with neonatal onset and/or underlying metabolic diseases.7
Recently, homozygous or compound heterozygous BRAT1 mutations have been described as a new cause of severe PE with neonatal onset and high patient fatality.8–11 The seven earlier described cases suffered from refractory epilepsy, and all of them died in their first months of life. We describe a new patient with compound heterozygous BRAT1 mutations and PE but with some atypical characteristics; he is alive at the age of 4.5 years and he never presented seizures.
2. Case report
This 4-year-old male patient was the first child of non-consanguineous parents of Spanish origin. He was the product of a 38-week-pregnancy and born via uncomplicated C-section to a young primigravida mother. Caesarean was selected due to a podalic presentation. The Apgar scores were 9 and 9 at 1 and 5 min, respectively. The birth weight was 2700 gr (5th percentile), the length was 47 cm (5th percentile), and the OFD was 34 cm (25th percentile).
The patient was evaluated by us during the fourth year of life due to psychomotor retardation and progressive microcephaly. At that age, his weight was 14 kg (5th percentile), his height was 100 cm (30th percentile), and his OFD was 47 cm (<3rd percentile) (Fig. 1). Severe axial hypotonia, exaggerated deep-tendon reflexes and Babinski reflex were also observed. No abnormal movements or dysmorphic features were perceived during the physical examination.
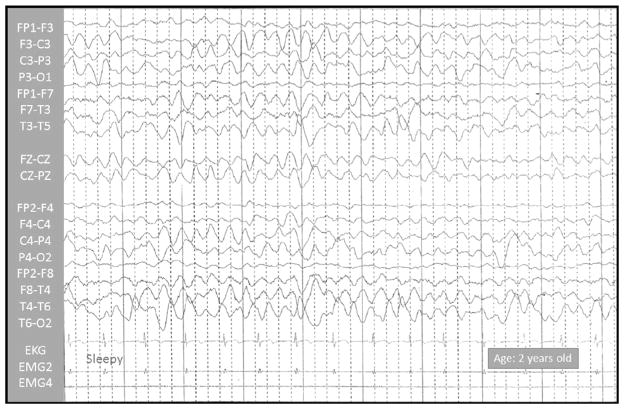
Fig. 1.
Sleep video-EEG test, performed on a 25-month-old boy. Normal EEG results; no epileptic paroxysms were registered.
An early intervention program was established in his first months of life, including motor, cognitive, speech development and social behavior approaches. In spite of this therapy, the patient exhibited a severe psychomotor delay during his first years of life. Head control was observed at 4 months, he sat unsupported at 30 months, he was able to stand with help at 42 months, and a social smile was observed at 12 months. Although the first bisyllabic babbling occurred at 12 months, language development was limited to some unmeaning sounds at the age of 4 years. His severe psychomotor retardation made impossible an appropriate cognitive evaluation.
The results of routine laboratory screening, including thyroid function, and neurometabolic tests were within normal range. Lactic and pyruvic acid were normal in serum and cerebrospinal fluid. Mitochondrial respiratory chain enzymatic activities on muscle tissue were normal at the age of 3 years old. Sleep and sleep-deprived video-EEG tests performed at the ages of 1, 2 and 4 years old, displayed normal results (Fig. 1). The eye and ear examinations yielded normal results. In addition, both visual and auditory evoked potentials were normal at the age of 3 years old.
Conventional genetic studies (karyotype and array comparative genomic hybridization analysis) revealed no abnormalities.
Two timed/spaced out 1.5 T MRI of the brain, which was performed at the ages of 19 and 48 months, showed moderate progressive cerebellar atrophy (Figs. 2–3).
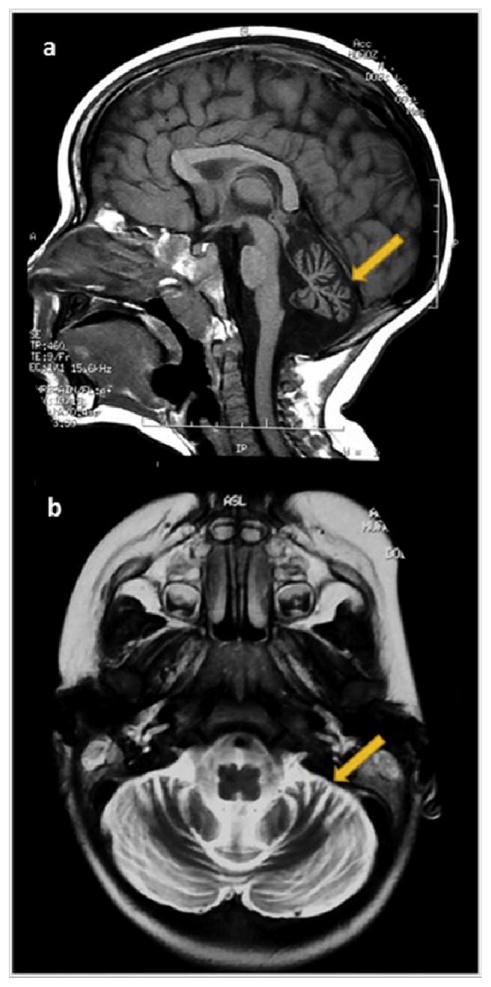
Fig. 2.
On the left side, MRI study performed on a 19-month-old boy (2a–2b). 2a. Midsagittal T1-weighted image exhibits a severe vermian diffuse atrophy (arrow) with fourth ventricle enlargement. The corpus callosum is normal, and the brainstem has a normal size. 2b. Axial T2-weighted image shows enlarged interfolia spaces (arrow) and moderately reduced volume of both cerebellar hemispheres.
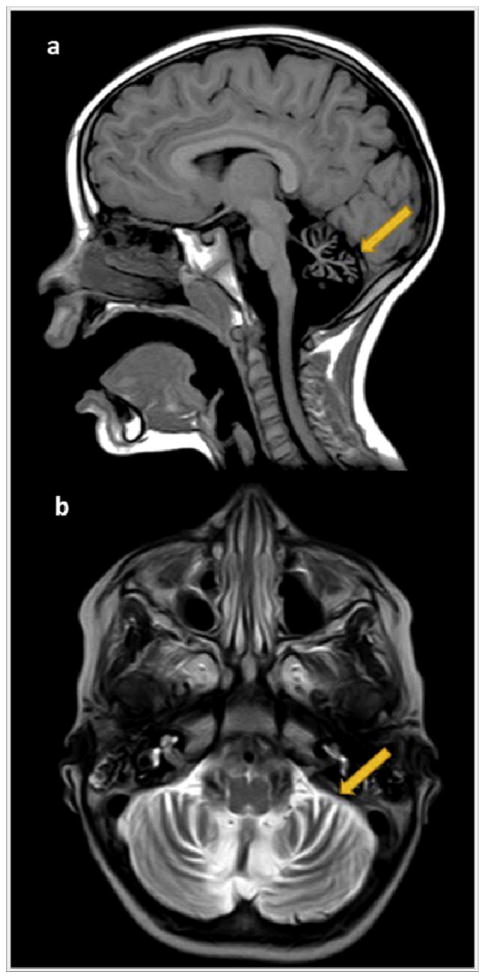
Fig. 3.
On the right side, MRI study performed at 48 months old (3a–3b). 3a. Midsagittal T1-weighted image evidences a more severe vermian diffuse atrophy (arrow) with enlargement of the supravermian cistern and a wide fourth ventricle. The corpus callosum and the brainstem remain normal. 3b. Axial T2-weighted image shows severe symmetric atrophy of the cerebellar hemispheres (arrow).
Whole exome sequencing revealed two mutations in the BRAT1 gene (BRCA1-Associated ATM Activator 1) at chromosome 7 positions g.2579211C > T, c.1564G > A; p.Glu522Lys and g. 2583388C > CT, c.638dupA, p.Val214Glyfs*189 [NM_152743]. Compound heterozygosity of the identified mutations was confirmed, as both variants were identified in heterozygosity in the mother and father, consistent with autosomal recessive inheritance.
The BRAT1 p.Glu522Lys mutation occurred at a highly conserved amino acid in the HEAT (this acronym comes from four cytoplasmic proteins: Huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A), and the yeast kinase TOR1) protein domain. Many HEAT repeat-containing proteins have been noted to be involved in intracellular transport processes. The BRAT1 p.Glu522Lys mutation was predicted to be damaging by sorting intolerant from tolerant (SIFT), PolyPhen2, MutationTaster, and LRT. The second variant, BRAT1 p.Val214Serfs*189, was predicted to change the amino acid sequence after valine 214 by producing a stop codon that prematurely truncates the protein 188 positions downstream. An allelic frequency of 0.005% has been reported by the exome aggregation consortium in the European (non-Finnish) population.12 However, the pathogenicity of this variant has been reported previously, as this variant has been identified as a homozygous frameshift mutation associated with lethal neonatal rigidity and multifocal seizure syndrome (MIM# 614498) in 3 patients.8
3. Method: genetic study
Exome sequencing was performed using genomic DNA isolated (MagnaPure, Roche) from whole blood from the proband and his parents. Libraries were prepared using the Ion AmpliSeq™ Exome Kit (Life Technologies) and quantified by qPCR. The enriched libraries were prepared using Ion Chef™ and sequenced on a PI™ Chip in the Ion Proton™ System (Life Technologies) to provide at least 20X coverage of >90% of amplicons. Signal processing, base calling, alignment and variant calling were performed on a Proton™ Torrent Server using the Torrent Suite™ Software. Variants were annotated using Ion Reporter™ Software, and pedigree analysis was performed using the Genetic Disease Screen (GDS) trio workflow. Variant filtering and prioritization were performed using an in-house software program and a local database. Candidate variants were visualized using IGV (Integrative Genomics Viewer). Candidate variants were evaluated based on stringent assessments at both the gene and variant levels, taking into consideration both the patient’s phenotype and the inheritance pattern. Variants were classified according to the guidelines of the American College of Medical Genetics and Genomics (ACMG). A board-certified molecular clinical geneticist evaluated each variant and classified it as pathogenic, likely pathogenic, or variant of uncertain significance. The geneticist then decided which, if any, variants should be reported. In every case, causal variants were discussed with the referring physician and/or a clinical geneticist. Identified variants were confirmed by Sanger sequencing.
4. Discussion
We describe an infrequent case of PE caused by compound heterozygous BRAT1 mutations with some distinct manifestations. Previously reported patients had died in the first months of life, and all of those patients suffered from severe intractable seizures (Table 1). The first report described two unrelated Amish infants with lethal, multifocal seizures, hypertonia, microcephaly, apnea, and bradycardia.8 After this report, five other cases were described. Saunders et al., [2012] described a new case from Mexico with lethal, seizures, microcephaly, dysmorphic features, congenital heart defects, and severe dyspnea; the presence of several tracts of homozygosity in aCGH analysis suggested shared parentage.10 Japanese siblings with compound heterozygous mutations in BRAT1 were described recently; those siblings showed some dysmorphic features, arrested head growth, uncontrolled seizures, and hypertonia. Brain MRI showed cerebral and cerebellar atrophy in both cases.9 One new report described two siblings born to consanguineous Arab-Muslim parents who have mutations in the same gene, with myoclonic seizures, progressive microcephaly, hypertonia, and apnea-bradycardia.11 Our case also had microcephaly and hypertonia; however, he was still alive at the age of 4.5 years and no seizures were observed by parents, therapists or clinicians; no paroxysmal events were recorded during the sleep and sleep-deprived video-EEG either.
Table 1.
Clinical features, brain MRI findings and BRAT1 mutations.
| Puffenberg EG et al.8 N = 2 | Saunders CJ et al.10 N = 1 | Saitsu H et al.9 N = 2 | Straussberg R et al.11 N = 2 | Our case N = 1 | |
|---|---|---|---|---|---|
| Clinical Features | |||||
| Dysmorphic features | − | + | + | − | − |
| Microcephaly | + (at birth) | + (at birth) | + (postnatal) | + (postnatal) | + (postnatal) |
| Rigidity/Hypertonia | + | + | + | + | + |
| Seizures | + | + | + | + | − |
| Dysautonomia (apnea, bradycardia) | + | + | − | + | − |
| Brain MRI | |||||
| Cerebral atrophy | + (mild hypoplasia of frontal lobes) | − | + | − | − |
| Cerebellar atrophy | − | − | + | − | + |
| Died | Before age 4 months | Age 5 months | Ages 1 year and 9 months (1st case) and 3 months (2nd case) | Before age 6 months | Alive at 52 months |
| BRAT1 mutation | Homozygous c.638_639insA | Homozygous c.453_454insATCTTCTC | Compound heterozygous c.176T > C and c.962_963del | Homozygous c.1173delG | Compound heterozygous c.1564G > A and c.638dup |
| Population | Amish | Mexican | Japanese Siblings | Arab-Muslim Siblings | Spanish |
The BRAT1 gene encodes the BRCA1-associated ATM activation-1 protein (BRAT1).13 This protein interacts with the BRCT domain of the tumor suppressor gene BRCA1 and binds to ATM1 and DNA-PKs, suggesting a role of BRAT1 in DNA damage responses.13–15 BRAT1 is also involved in cellular growth processes, including cell proliferation and tumorigenicity, and required for mitochondrial functions.16 The loss of BRAT1 expression due to homozygous or compound heterozygous BRAT1 mutations may decrease cell proliferation and migration, affect mitochondrial homeostasis,16 and cause neuronal atrophy.8,10 The lower severity observed in our patient might be related to his genotype or the absence of epilepsy. Aggressive ictal and electrical epileptogenic activity during brain maturation are believed to contribute to progressive cognitive deterioration or regression.17
Genomic advances have transformed clinical practice, and these cases demonstrate that whole exome sequencing (WES) has the potential to change clinical practice for genetic diseases. Making a correct and prompt diagnosis is important for genetic counseling, prenatal diagnostic options, carrier testing, and the prevention of disease. In some genetic disorders, the identification of causative mutations may have the potential to lead to new therapies or to change the management of acutely ill infants.10,18
In summary, we describe an infrequent case of PE, hypertonia, progressive microcephaly, and severe psychomotor retardation with compound heterozygous BRAT1 mutations. Our case demonstrates that this gene is related to PE, even without epilepsy. Until now, scientific references have probably reported the most severe cases. As other colleagues have suggested, BRAT1 should be added to the list of genes that are related to PE.11 Finally, we should insist on the necessity of genetic studies in patients with PE. Additional investigations will be worthwhile to determine the prevalence and contribution of these genes mutations to PE. Despite the clinical and etiology diversity of PE, genetic studies may be decisive for genetic counseling and management.
Acknowledgments
Part of this work was supported by R01CA79892, R01CA90631, and 5P30CA16056 from the National Institutes of Health, USA (T.O.).
Footnotes
Conflict of interest
None.
References
- 1.Stromme P, Kanavin OJ, Abdelnoor M, et al. Incidence rates of progressive childhood encephalopathy in Oslo, Norway: a population based study. BMC Pediatr. 2007;7:25. doi: 10.1186/1471-2431-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surtees R. Understanding neurodegenerative disorders. Curr Paediatr. 2002;12(13):191–8. [Google Scholar]
- 3.Tomas Vila M, Vitoria Minana I, Gomez Gosalvez F, et al. Epidemiology of progressive intellectual and neurological deterioration in childhood. A multicentre study in the Community of Valencia. An Pediatr. 2013 May;78(5):303–7. doi: 10.1016/j.anpedi.2012.08.012. Epidemiologia del deterioro intelectual y neurologico progresivo en la infancia. Estudio multicentrico en la Comunidad Valenciana. [DOI] [PubMed] [Google Scholar]
- 4.Nelson J, Smith M, Bittles AH. Consanguineous marriage and its clinical consequences in migrants to Australia. Clin Genet. 1997 Sep;52(3):142–6. doi: 10.1111/j.1399-0004.1997.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 5.Weller M, Tanieri M, Pereira JC, et al. Consanguineous unions and the burden of disability: a population-based study in communities of Northeastern Brazil. Am J Hum Biol official J Hum Biol Counc. 2012 Nov-Dec;24(6):835–40. doi: 10.1002/ajhb.22328. [DOI] [PubMed] [Google Scholar]
- 6.Stromme P, Suren P, Kanavin OJ, et al. Parental consanguinity is associated with a seven-fold increased risk of progressive encephalopathy: a cohort study from Oslo, Norway. Eur J Paediatr neurology EJPN official J Eur Paediatr Neurology Soc. 2010 Mar;14(2):138–45. doi: 10.1016/j.ejpn.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Stromme P, Magnus P, Kanavin OJ, et al. Mortality in childhood progressive encephalopathy from 1985 to 2004 in Oslo, Norway: a population-based study. Acta Paediatr. 2008 Jan;97(1):35–40. doi: 10.1111/j.1651-2227.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 8.Puffenberger EG, Jinks RN, Sougnez C, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7(1):e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitsu H, Yamashita S, Tanaka Y, et al. Compound heterozygous BRAT1 mutations cause familial Ohtahara syndrome with hypertonia and microcephaly. J Hum Genet. 2014 Dec;59(12):687–90. doi: 10.1038/jhg.2014.91. [DOI] [PubMed] [Google Scholar]
- 10.Saunders CJ, Miller NA, Soden SE, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012 Oct 3;4(154):154ra35. doi: 10.1126/scitranslmed.3004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straussberg R, Ganelin-Cohen E, Goldberg-Stern H, et al. Lethal neonatal rigidity and multifocal seizure syndrome–report of another family with a BRAT1 mutation. Eur J Paediatr neurology EJPN official J Eur Paediatr Neurology Soc. 2015 Mar;19(2):240–2. doi: 10.1016/j.ejpn.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Exome Aggregation Consortium ExAC. ExAC Browser Beta. 2015 [updated July]. Available from: http://exac.broadinstitute.org.
- 13.Aglipay JA, Martin SA, Tawara H, Lee SW, Ouchi T. ATM activation by ionizing radiation requires BRCA1-associated BAAT1. J Biol Chem. 2006 Apr 7;281(14):9710–8. doi: 10.1074/jbc.M510332200. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi M, Ouchi T. Regulation of ATM/DNA-PKcs phosphorylation by BRCA1-associated BAAT1. Genes & cancer. 2010 Dec;1(12):1211–4. doi: 10.1177/1947601911404222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So EY, Ouchi T. Functional interaction of BRCA1/ATM-associated BAAT1 with the DNA-PK catalytic subunit. Exp Ther Med. 2011 May;2(3):443–7. doi: 10.3892/etm.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So EY, Ouchi T. BRAT1 deficiency causes increased glucose metabolism and mitochondrial malfunction. BMC cancer. 2014;14:548. doi: 10.1186/1471-2407-14-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S, Al Baradie R. Epileptic encephalopathies: an overview. Epilepsy Res Treat. 2012;2012:403592. doi: 10.1155/2012/403592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willig LK, Petrikin JE, Smith LD, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015 May;3(5):377–87. doi: 10.1016/S2213-2600(15)00139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]