Abstract
Objective
Type 1 diabetes (T1D) or celiac disease (CD) develops in at least 2% of the general population. Early detection of disease-specific autoimmunity and subsequent monitoring would be possible if screening tests were more widely available. Currently screening for islet autoimmunity is available only in a research setting and CD-specific autoimmunity screening is limited to those in high risk groups. This study assessed the feasibility of incorporating T1D and CD autoantibody screening into a pediatric practice.
Methods
Patient engagement strategies, blood collection preference, blood sample volume, rate of autoantibody detection in the general population, and parental satisfaction were assessed. Over 5 weeks, research staff recruited 200 patients aged 2–6 yr from two pediatric practices in the Denver area to be screened for six islet autoantibodies (IAs) and the transglutaminase antibody.
Results
Of the 765 parents approached, 200 (26%) completed the same day screening. Of the 565 subjects who did not complete the screening, 345 expressed interest, but were unable to make a participation decision. A finger stick, compared to a venous draw, was the preferred method of sample collection. Both methods yielded sufficient volume for autoantibody determination. IAs or the transglutaminase antibody were detected in 11 subjects. Parents expressed satisfaction with all aspects of participation.
Conclusion
The results of this study suggest that it is feasible to conduct this type of screening in a pediatric clinic. Such screening could lead to increased disease awareness and the possible benefits that can result from early detection.
Keywords: pediatric screening, islet autoantibody, childhood diabetes, celiac disease, feasibility
Introduction
By the age of 20, one out of 250 US youth will develop type 1 diabetes (T1D) (1). Approximately 30% will present in diabetic ketoacidosis (DKA) at the time of onset (2). A recent analysis of new cases in Colorado has shown this rate to be as high as 46% (3). Up to 120 children in the US may die as result of DKA at diagnosis each year (2); those surviving have poorer long-term glycemic control (4) and memory (5), compared with children diagnosed prior to DKA.
Reliable tests exist allowing for the early detection of islet autoimmunity preceding T1D (6). To date, such screening has been available only in the context of research studies. It may be considered that benefits of early detection of islet autoimmunity may have some limitations until an effective primary prevention is available. However, research studies that screen children for high genetic risk and the presence of islet autoantibodies (IAs) have shown to significantly reduce morbidity and prevent 80% of DKA, especially among children with no family history (7 – 9).
A majority of hospitalizations for DKA at diagnosis and associated life-threatening complications may be prevented by increasing disease awareness. Yet, previous prevention campaigns aimed to prevent DKA by increasing diabetes awareness among parents, teachers and health-care providers in Italy and Austria had mixed success (10, 11). With the recent increase in the occurrence of DKA at onset, innovative ways to prevent this potentially fatal and costly complication should be examined. Translation of early detection screening into general practice, in a way that would benefit population health, has been limited by both the cost of screening as well as the close monitoring of autoantibody-positive children. The integration of screening for islet autoimmunity with testing for another prevalent childhood disease, such as celiac disease (CD), could significantly reduce the cost. CD affects one in 100 US youth (12). Screening for CD using the tissue transglutaminase autoantibody (tTGA) is widely accepted, although still limited to high-risk groups, such as those with a first-degree relative or patients with T1D (13, 14). Routine screening of the general population for CD could prevent excess morbidity and cost associated with delayed diagnosis (15, 16).
The objective of this study was to assess the feasibility of screening for IAs and tTGA in 2 to 6 yr-old children who were patients of a general pediatric practice. Components assessed included: (i) provider interest and considerations for participation; (ii) parental interest in their child being tested for these autoimmune diseases; (iii) two methods for the minimal blood volume for autoantibody testing; (iv) ability to detect IA and tTGA in a general population; (v) parental satisfaction with participation; and (vi) identifying key considerations for integrating such screening into busy pediatric practices.
Methods
Practice assessment
The study was conducted by the Barbara Davis Center for Diabetes (BDC) research staff in collaboration with staff of two large pediatric practices in the metropolitan area of Denver, CO. The BDC staff met with the lead providers, practice administrators, and nurse supervisors to assess interest in participation and discuss the proposed research study plan. Both practices agreed to participate; however, the practice staff preferred not be directly engaged in the research as this would entail additional Institutional Review Board and Health Insurance Portability and Accountability Act training. This study protocol was approved by the Colorado Multiple Institutional Review Board.
Subjects
Those invited to participate in this study were healthy children between the ages of 2 and 6 years who were patients at the two pediatric clinics. The rationale for selecting this age range is based on a recent analysis from The Environmental Determinants of Diabetes in the Young (TEDDY) Study, an international observational study of genetically high-risk children, indicating that the incidence of certain insulin autoantibodies peaks at the age of 2 and remains constant in the subsequent 5 years (17). Furthermore, 2 – 6 years represents the first peak in the incidence of T1D, followed by a second peak in the teenage years (18). If the parent was able to understand and grant informed consent and the child was willing to provide a blood sample, they were eligible to participate in the screening. Because children with CD are at a greater genetic risk for developing T1D, they were invited to participate because they are not routinely tested for T1D autoantibodies as part of their CD care. However, children with T1D are routinely screened for CD as part of their standard diabetes care; therefore, they were not eligible to participate in the study.
Patients were categorized as ‘participants’ or ‘non-participants’. Participants were defined as those who were interested and completed the study. Two categories of non-participants were identified: (i) those interested, but unable to make the participation decision that day and (ii) those not interested in participating in the study. The reason for non-participation was documented when possible.
Protocol
To increase awareness and inform families of the research in a short time before the study began, the pediatric practices used social media such as Facebook, e-mail communication, and practice websites to announce the study. Over a 5 week period of time, parents were approached by the research staff while they were waiting for the appointment with their provider. The parents were provided a brief description of the study purpose, the choices in the method to obtain blood, and details regarding how the test results would be communicated. They were informed that the testing was free of charge and results would be shared with their provider upon request. Informed consent was obtained among those willing to participate. Additional information collected included the child’s name, date of birth, and family history of T1D and/or CD. The parent(s) was given a choice of either a finger stick or venipuncture to obtain the blood. Hand warmers were used for the finger stick prior to the blood collection. A benzocaine spray was offered when venipuncture was selected. Age appropriate distractions were utilized to ease the anxiety of the child. Once the collection was complete, the parent was asked to complete a five-question satisfaction survey about the screening procedure including blood collection, and the child was offered a prize and a sticker as a reward. The child’s reaction to the blood draw was observed and noted by the research assistant on a 5-point scale where 1 was ‘very bad’ and 5 was ‘great’.
Autoantibody screening
Autoantibody detection assays were conducted by radioimmunoassay for IAA, GADA, IA-2A, ZnT8A, and tTGA (6, 19 – 21). The results from this laboratory have previously shown to have high levels for sensitivity and specificity (6, 22). In addition, electrochemiluminescent (ECL) assays, demonstrating improved sensitivity for IAA and GADA over the standard radioassay, were used under the direction of Dr. Liping Yu at the BDC Clinical Immunology Laboratory in Aurora, CO (23, 24). The desired blood volume was 0.5 mL by venipuncture collected into an SST tube or the same volume by capillary finger stick into a microtainer. Whole blood samples were kept at room temperature until transport to the processing laboratory. Tubes or microtainers were spun to separate serum which was then transferred to cryovials, frozen, and stored at −80°C until autoantibody testing was conducted.
Parental satisfaction survey
A five-question satisfaction survey was administered upon completion of the blood sample. It queried parent’s satisfaction with (i) how the informed consent was reviewed including how the study was explained; (ii) how well their questions were answered; (iii) the way the research staff engaged their child; (iv) the process of the blood collection; and (v) the overall experience of participating in the study. Response choices were on a 4-point scale from 1 being ‘very dissatisfied’ to 4 being ‘very satisfied’.
Results notification and care referral
Parents were notified of negative screening results by mail. All positive results were initially communicated by phone, followed by a mailed letter. All result notification letters included the most common signs and symptoms of T1D and instructions to follow-up with the child’s pediatrician with any concerns. Furthermore, the letter emphasized the current results with a statement that this autoantibody status could change in the future. Children with tTGA-positive results were referred to their pediatrician for follow-up. Children with IA-positive results were offered follow-up with a pediatric endocrinologist at the BDC as part of this research study. This follow-up included obtaining a blood sample via venipuncture to confirm the initial test results, a random glucose measurement and measurement of hemoglobin A1C. Parents were given the option to continue with a follow-up protocol with study staff every 6 – 12 months. In addition, the children with two or more autoantibodies received glucometer teaching and education of signs and symptoms of T1D.
Data analysis
Data were collected and managed using Redcap (Research Electronic Data Capture), a data management tool hosted at the University of Colorado CCTSI. SAS Version 9.3 was used for descriptive analyses of demographic characteristics and satisfaction survey results.
Results
A total of 765 parents of eligible children were approached and invited to participate in the screening study. Parents of 200 children (26%) consented and completed the same-day screening protocol. Figure 1 provides a diagrammatic representation of the study outcomes and the refusal reasons for the population approached for participation. Participation did not differ by sex or age of the child. Non-participants were more likely to have both parents making the participation decision (9%) than participants (2%). Most refusals indicated a lack of interest because of no family history of disease (29%) or for concern about the blood draw (28%). Reason for refusal did not differ by sex or age of the child.
Fig. 1.
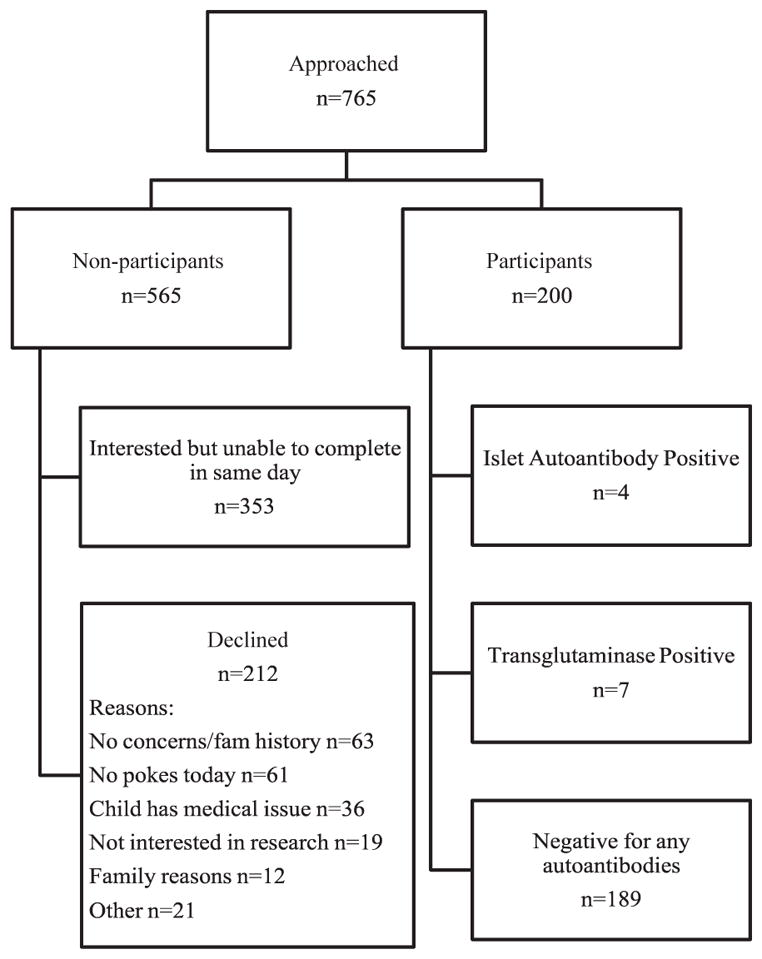
Outcomes of study population.
When the decision to refuse the study was made by mothers or both parents, the more common reason given was lack of family history for T1D and CD (41 and 29%, respectively). However, when the father refused, the primary reason was concern about the blood draw (38%).
Table 1 summarizes the outcome of the blood draw and autoantibody results. Parents selected a finger stick more often than venipuncture; the mean blood volume was greater from the venipuncture. The laboratory requested replacement venipuncture samples for two subjects with samples initially collected by finger stick due to hemolysis, which can lead to unreliable results. Both participants agreed to a second blood collection by venipuncture.
Table 1.
Characteristics of Blood Draw and Autoantibody Test Results
| Characteristics of Blood Draw | Total Screened N=200 |
|---|---|
|
| |
| Participant Blood Collection Choice n (%) | |
| Fingerstick | 113 (56.5) |
| Venipuncture | 87 (43.5) |
|
| |
| Serum volume-mean (sd) | |
| Overall | 0.50 ml (0.23) |
| Fingerstick | 0.37 ml (0.18) |
| Venipuncture | 0.68 ml (0.14) |
|
| |
| Description of the Autoantibody Positive Children | |
|
| |
| Islet Autoantibody Positive n (%) | 4 (2.0) |
| Single (*) | 3 |
| Multiple (+) | 1 |
| Gender of Positive | |
| Male | 2 (50.0) |
| Female | 2 (50.0) |
| Age of IA Positive-Mean years (sd) | 5.3 (1.2) |
|
| |
| Transglutaminase Autoantibody Positive | 7 (3.5) |
| Gender of Positive n (%) | |
| Male | 3 (43.0) |
| Female | 4 (57.0) |
| Age of tTGA Positive-Mean years (sd) | 5.4 (1.4) |
Single Islet Autoantibodies Detected: IA-2, ECL-GAD, ECL-IAA
Multiple Islet Autoantibodies Detected: GADA, IAA, ZnT8A, ECL-GAD, ECL-IAA
Staff assessed the child’s reaction to the blood collection: 79% of children had a ‘great’ and 11% had a ‘good’ reaction. ‘Neutral’, ‘bad’, and ‘very bad’ reaction was reported in, respectively, 7.5, 1.5, and 0.5% of the cases. The distribution was similar by sex and age. Family history of T1D or CD was reported by 36 (18%) of the participants; 18 indicated a relative with T1D (2 first-degree relatives) and 18 indicated a relative with CD (7 first-degree relatives).
Four (2%) of the children were positive for IAs (Table 1); three children tested positive for only one islet autoantibody and one was positive for multiple autoantibodies (IAA, GADA, and ZnT8A). Of the three who tested positive for one autoantibody, one subject was confirmed and remains positive for the same autoantibody (IA-2A). The subject with multiple autoantibodies has been persistently positive for the same autoantibodies on two subsequent draws. All subjects who were IA positive at the initial draw are being followed every 6 – 12 months for changes in autoantibody status. To date, all random glucose levels and HbA1c levels have been within a normal range. None of the subjects who were IA positive reported relatives with either T1D or CD.
Seven (3.5%) of the children were tTGA positive; none of these children were positive for IA. One had been previously diagnosed with CD (confirmed by biopsy) and another one, a first-degree relative of a person with CD, had a positive biopsy following the screening. To date, the other six subjects have not had a biopsy, but one subject started a gluten-free diet at the recommendation of their gastroenterologist.
Parental satisfaction survey results by responding parent are summarized in Table 2. Participants were overwhelmingly satisfied with all aspects of the study and with the overall experience. There was no difference between mothers, fathers, or participants where both parents answered the survey with regard to overall satisfaction. The satisfaction did not differ by the type of blood draw the family chose. Additional comments provided by parents fell into two groups – being appreciative of the chance to participate in the screening study and the value of the research staff’s knowledge and experience.
Table 2.
Satisfaction with Feasibility Study Participation for All Participants and by Responding Parent
| How Satisfied were you with: | Total N=201 | Mothers N=166 | Fathers N=31 | Both N= 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VS | S | D | VS | S | D | VS | S | D | VS | S | D | |
|
| ||||||||||||
| Q1. The review of the informed consent - how the study was explained? | 89% | 11% | -- | 87% | 13% | -- | 97% | 3% | -- | 100% | -- | -- |
|
| ||||||||||||
| Q2. The way my questions were answered? | 93% | 7% | -- | 91% | 9% | -- | 100% | -- | -- | 100% | -- | -- |
|
| ||||||||||||
| Q3. The way the research staff engaged my child? | 92% | 8% | -- | 97% | 3% | -- | 100% | -- | -- | 100% | -- | -- |
|
| ||||||||||||
| Q4. The process for collecting the blood? | 90% | 9% | 1% | 90% | 9% | 1% | 87% | 13% | -- | 100% | -- | -- |
|
| ||||||||||||
| Q5. Your overall experience with this study? | 93% | 7% | -- | 97% | 3% | -- | 94% | 6% | -- | 100% | -- | -- |
VS: Very Satisfied; S: Satisfied; D: Dissatisfied
Discussion
This pilot study provided strong evidence for both provider and parental interest in implementing screening for autoimmunity related to T1D and CD in a general pediatric population of 2 – 6 yr olds. In addition, it demonstrated feasibility of sample collection by either finger stick or venipuncture and the ability to reliably detect autoantibodies in this population.
Previous studies have determined that children experience islet and celiac autoimmunity months to years before they become symptomatic; yet currently, the screening is limited to those involved in research or in those with high risk for disease (25 – 27). A screening program where early detection and close monitoring is the intervention to prevent serious outcomes at diagnosis requires a full consideration of the extent and cost of these outcomes. The multicenter SEARCH for Diabetes in Youth Study estimated in 2002 – 2004 that 29.4% of newly diagnosed patients with T1D had DKA at diagnosis (2). The rates of DKA in Colorado youth have increased from 29.9% in 1998 to 46.2% in 2012 (3). There are no reliable current onset mortality data for the US youth; the estimates range from 0.06 to 0.6% (3, 28). With the rates of DKA increasing, evaluation of the cost of treatment is an important consideration as to the value of early screening. Since the feasibility of screening for T1D and CD is established, the next step would be to design a model screening protocol based on this experience that could be integrated into well-check visits at pediatric primary care clinics. The discussion below integrates the lessons learned from the field with current research to assess the feasibility of taking this screening approach from pilot to general practice.
Provider interest
The Colorado Health Access Survey reports that 89% of children in Denver were reported to have a usual source of care ranging from private practice to community and public health clinics (29). This data supported our proposal to conduct the study in a pediatric clinic setting. The providers we approached appreciated the opportunity to participate in a study that was addressing important pediatric health issues and were active participants in how to best conduct the study in those practice settings. Future screening program development should consider approaches tailored to specific types of primary care settings to maximize practice and patient participation.
Parental interest and participation
Of the 765 children aged 2 – 6 yr that were approached, 565 (72%) parents expressed interest in the screening. The in-person recruitment by professional research assistants well trained in diabetes research was an important factor in rapidly accomplishing the recruitment goal and the acceptability of the protocol. This observation is consistent with studies showing parents are more content in the research process with confident and courteous research staff (30). Parents are more likely to respond to in-person recruitment compared with mailings and phone calls (31). Among the 565 interested parents, 36% were able to complete the sample-day sample collection for this study. A higher rate of participation might have been obtained had interested parents had options for future screening. In addition, staff reported that a significant number of parents with children >6 yr old were interested in having their child tested. Indeed, if resources were available in the future, one recommendation could be to widen the age range for the screening.
The recruitment approach requiring the participation decision be made at the time of that visit may have played a part in the incompletion of the study among this group of interested parents. The study staff did not formally assess whether or not parents were aware of the study announcement on the Facebook page or the website e-mail. Despite the efforts to inform patients about the study in advance via social media and e-mail, most parents, both participants and non-participants, learned of the study in the waiting room while waiting to see their provider. People who have past experience in research or have a sense of knowledge with science or medicine are more confident in the decision-making process on whether to participate in a research study (32). Those with less experience or information may be less prepared to make a same-day decision to participate. Further, informational efforts and more flexibility around timing of this decision could improve participation.
It has been shown that parents are more motivated and recruitment rates are higher when their provider is directly involved in the research (32, 33). While the practices were able to show their support for the research being conducted in their offices, study participation may have been greater if the providers had been truly engaged in the research. Even as a research protocol, assessing mechanisms for integrating such screening options into routine practice will be important.
Reasons for not participating
The two most common reasons for not participating were concerns related to the blood draw and not being interested because there was not a family history of T1D or CD. This is consistent with studies that show children and their parents are more likely to agree to studies not involving a blood sample and often refuse studies that involve extra injections beyond the routine care (33, 34). The fact that eligible participants with no family history of T1D or CD were less likely to participate is a finding consistent with the notion that parental perception of the study’s importance is the main reason they agree or decline participation (35). This pattern is also consistent with participation rates among genetically at-risk populations, where those without a family history of T1D are less likely to participate than those with an affected first-degree relative (36, 37). This does suggest that a future screening program development should expend effort to thoroughly educate the general public about risk of these conditions.
Blood collection methods
Given the choice, parents preferred the finger stick to a venipuncture as a method of blood collection. Regardless of method, parents expressed a high degree of satisfaction with the blood collection procedure. Observations from the field experience noted that parents often commented on the length of time it took to obtain the blood via the finger stick; parents who chose the venipuncture were equally surprised at how quickly the procedure was completed. Experienced research staff easily distracted the children and the positive reinforcement they consistently provided was well accepted by the children, perhaps this would be less common in a busy clinical practice.
With the 200 samples collected by either method, blood volume was sufficient to determine the panel of the IA and tTGA autoantibodies for this pilot protocol. All samples were able to be reliably analyzed with the exception of the two samples initially collected by finger stick that needed to be redrawn by venipuncture due to hemolysis.
Detection of autoantibodies
T1D autoantibody screening has generally been conducted in populations determined to be at increased risk for disease because of family history or identification of high-risk genetic markers (38, 39). The results of this study demonstrated the prevalence of T1D-related autoantibodies to be 2% in 2 to 6 yr-old children. Positive autoantibody findings were consistent with prevalence reported from another general population study (40).
The prevalence of CD worldwide has been estimated at approximately 1% (41) and a recent report using NHANES data from 2009 to 2010 estimated the prevalence of CD in USA at 0.71% of the total population, with most cases undiagnosed (42). The detection of tTGA has long been demonstrated to be a reliable indicator of CD among symptomatic individuals (43 – 45). Among asymptomatic individuals, the prevalence of tTGA positivity is 12% in a genetically high-risk population (27). In a low-risk population, tTGA prevalence is 0.75% [95% confidence interval (CI): 0.5 – 1.1) (46). In this general population pilot study where there is no special selection criteria with regard to risk for CD, the prevalence of tTGA positivity was 3.5 % (93% CI: 1.0 – 6.0). This is within the range expected based on these previous studies.
The purpose of this pilot study was the first step in determining if provider and parents were interested in this type of screening. It is important to note that a one-time screening of children between the ages 2 and 6 is not sufficient to identify all the potential children who could become autoantibody positive for T1D or CD in the future. Should a more comprehensive screening program be developed, it would require individual risk assessment and include repeat measures on an evidence-based schedule.
Participant satisfaction
The high degree of satisfaction with study participation demonstrated the value of an experienced and educated staff that could explain the importance of the screening. Parents were also satisfied because their child’s experience was positive. These findings underscore the importance of an interactive informed consent process and training of staff. In a practice setting, insuring that those involved with screening are well versed in explaining its purpose and answering questions would be important. Future recommendations would be to provide a short written explanation of the highlights and benefits of what the screening entails followed by further discussion with the provider. If the screening was presented and recommended by the pediatrician or provider, it is thought that parents would feel more comfortable asking questions, be more willing to complete the screening at the visit, and satisfied with the process.
In this case, translation of findings from research to clinical practice has the potential to increase disease awareness and permit more children to receive the benefits of early detection that have been demonstrated in research settings (7, 8, 27). Persons with IA positivity followed closely in research and who subsequently develop T1D have less acute and severe presentation, fewer hospital admissions, are more informed and prepared when diagnosis occurs and may have longer preservation of pancreatic function compared with those cases arising in the general population without the benefit of close observation and education (7, 8). In addition, identification of tTGA can provide direction for diagnosis and treatment of CD which would likely result in the prevention of extensive intestinal damage and other health risks or complications due to the lack of nutrient uptake associated with this damage (47, 48). Integrating such observation and education into clinical practice would be a straightforward and cost-efficient intervention for patients screened as tTGA positive.
Assessing the feasibility of early detection screening for T1D and CD in two busy pediatric practices provided valuable insight for how best to translate these research observations into a screening effort for the general population. The experience and results of this pilot study suggest that the next step of scaling-up such a screening approach for implementation within the practice setting is warranted.
Acknowledgments
This research was supported by the National Institutes of Health grants DK32493, DK32083, DK050979, and DK57516, and by the Juvenile Diabetes Research Foundation grant 17-2013-535. The abstract of this study was presented as a poster presentation at the 75th Scientific Session of the American Diabetes Association, Boston, MA, 5 June 2015 to 9 June 2015. The authors would like to thank Dr. Daniel Feiten, Dr. Martha Middlemist, the partners and staff, and most importantly, the children, and parents of Greenwood Pediatrics and Pediatrics 5280 for their support and participation.
Footnotes
Author contributions
M.R. developed the research design and recruited the participating pediatric practices. P. G. and K. B. recruited the subjects and collected data. Y. L. directed the laboratory analysis. P. G., K. B., K. W., and J. B. drafted the manuscript. K. W. analyzed the data. J. N., M.R., and Y. L. contributed, reviewed, and edited the manuscript. P. G and J. B finalized the manuscript. P. G is the guarantor of this work. With full access to the data and a personal role in the conduct of the study, she takes responsibility for the contents of this article.
References
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121:e1258–e1266. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 3.Rewers A, Dong A, Slover RH, Klingensmith GJ, Rewers M. Prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in youth in Colorado, 1998–2012. JAMA. 2015;313:1570–1572. doi: 10.1001/jama.2015.1414. [DOI] [PubMed] [Google Scholar]
- 4.Fredheim S, Johannesen J, Johansen A, et al. Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia. 2013;56:995–1003. doi: 10.1007/s00125-013-2850-z. [DOI] [PubMed] [Google Scholar]
- 5.Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156:109–114. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JM, Goehrig S, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27:1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 8.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34:2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triolo TM, Chase HP, Barker JM, Group DPTS. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32:769–773. doi: 10.2337/dc08-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care. 1999;22:7–9. doi: 10.2337/diacare.22.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Fritsch M, Schober E, Rami-Merhar B, et al. Diabetic ketoacidosis at diagnosis in Austrian children: a population-based analysis, 1989–2011. J Pediatr. 2013;163:1484–1488. e1481. doi: 10.1016/j.jpeds.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Pediatr Gastroenterol Nutr. 2014;59(Suppl 1):S7–S9. doi: 10.1097/01.mpg.0000450393.23156.59. [DOI] [PubMed] [Google Scholar]
- 13.Kordonouri O, Klingensmith G, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines. Other complications and diabetes-associated conditions in children and adolescents. Pediatr Diabetes. 2014;2014(15 Suppl 20):270–278. doi: 10.1111/pedi.12183. [DOI] [PubMed] [Google Scholar]
- 14.Adlercreutz EH, Svensson J, Hansen D, et al. Prevalence of celiac disease autoimmunity in children with type 1 diabetes: regional variations across the Oresund strait between Denmark and southernmost Sweden. Pediatr Diabetes. doi: 10.1111/pedi.12200. Epub ahead of print 2014 Aug 18. [DOI] [PubMed] [Google Scholar]
- 15.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 16.Warncke K, Liptay S, Frohlich-Reiterer E, et al. Vascular risk factors in children, adolescents, and young adults with type 1 diabetes complicated by celiac disease: results from the DPV initiative. Pediatr Diabetes. doi: 10.1111/pedi.12261. Epub ahead of print 2015 Feb 12. [DOI] [PubMed] [Google Scholar]
- 17.Krischer JP, Lynch KF, Schatz DA, et al. The six-year incidence of diabetes associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–987. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–148. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Yu L, Tiberti C, et al. A report on the International Transglutaminase Autoantibody Workshop for celiac disease. Am J Gastroenterol. 2009;104:154–163. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61:179–186. doi: 10.2337/db11-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao D, Guyer KM, Dong F, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62:4174–4178. doi: 10.2337/db13-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15:118–126. doi: 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rewers M, Norris JM, Eisenbarth GS, et al. Beta-cell autoantibodies in infants and toddlers without IDDM relatives: diabetes autoimmunity study in the young (DAISY) J Autoimmun. 1996;9:405–410. doi: 10.1006/jaut.1996.0055. [DOI] [PubMed] [Google Scholar]
- 27.Liu E, Lee HS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–49. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childhood Diabetes Research Committee Ministry of Health and Welfare-Japan, Polish Diabetes Research Group-Poznan, The Netherlands Institute for Preventative Health Care-Leiden, Diabetes Research Center of Pittsburg, Pennsylvania. How frequently do children die at the onset of insulin-dependent diabetes mellitus? Analyses of registry data from Japan, Poland, the Netherlands and Allegheny County, Pennsylvania. Diab Nutr Metab. 1990;3:57–62. [Google Scholar]
- 29.Colorado Health Institute. Analysis of the Colorado Health Access 2013 Survey. 2014. [Google Scholar]
- 30.Jollye S. An exploratory study to determine how parents decide whether to enroll their infants into neonatal clinical trials. J Neonatal Nurs. 2009;15:18–24. [Google Scholar]
- 31.Mazzocco M, Harum KH, Myers G, Reiss A. Children’s participation in genetic prevalence research: influences on enrollment and reports of parent satisfaction. J Appl Soc Psychol. 1999;29:2308–2327. [Google Scholar]
- 32.Chantler TE, Lees A, Moxon ER, Mant D, Pollard AJ, Fiztpatrick R. The role familiarity with science and medicine plays in parents’ decision making about enrolling a child in vaccine research. Qual Health Res. 2007;17:311–322. doi: 10.1177/1049732306298561. [DOI] [PubMed] [Google Scholar]
- 33.Langley JM, Halperin SA, Mills EL, Eastwood B. Parental willingness to enter a child in a controlled vaccine trial. Clin Invest Med. 1998;21:12–16. [PubMed] [Google Scholar]
- 34.Bernhardt BA, Tambor ES, Fraser G, Wissow LS, Geller G. Parents’ and children’s attitudes toward the enrollment of minors in genetic susceptibility research: implications for informed consent. Am J Med Genet A. 2003;116A:315–323. doi: 10.1002/ajmg.a.10040. [DOI] [PubMed] [Google Scholar]
- 35.Tait AR, Voepel-Lewis T, Siewert M, Malviya S. Factors that influence parents’ decisions to consent to their child’s participation in clinical anesthesia research. Anesth Analg. 1998;86:50–53. doi: 10.1097/00000539-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Baxter J, Vehik K, Johnson SB, Lernmark B, Roth R, Simell T. Differences in recruitment and early retention among ethnic minority participants in a large pediatric cohort: the TEDDY Study. Contemp Clin Trials. 2012;33:633–640. doi: 10.1016/j.cct.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lernmark B, Johnson SB, Vehik K, et al. Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemp Clin Trials. 2011;32:517–523. doi: 10.1016/j.cct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 40.Maclaren NK, Lan MS, Schatz D, Malone J, Notkins AL, Krischer J. Multiple autoantibodies as predictors of type 1 diabetes in a general population. Diabetologia. 2003;46:873–874. doi: 10.1007/s00125-003-1123-7. [DOI] [PubMed] [Google Scholar]
- 41.Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Semin Immunopathol. 2012;34:473–478. doi: 10.1007/s00281-012-0311-2. [DOI] [PubMed] [Google Scholar]
- 42.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–1544. doi: 10.1038/ajg.2012.219. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 43.Carroccio A, Di Prima L, Falci C, et al. Predictive value of serological tests in the diagnosis of celiac disease. Ann Ital Med Int. 2002;17:102–107. [PubMed] [Google Scholar]
- 44.Reeves GE, Squance ML, Duggan AE, et al. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol. 2006;18:493–501. doi: 10.1097/00042737-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Singh P, Kurray L, Agnihotri A, et al. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J Clin Gastroenterol. 2014;49:212–217. doi: 10.1097/MCG.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 46.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Manzanares A, Tenias JM, Lucendo AJ. Bone mineral density directly correlates with duodenal Marsh stage in newly diagnosed adult celiac patients. Scand J Gastroenterol. 2012;47:927–936. doi: 10.3109/00365521.2012.688217. [DOI] [PubMed] [Google Scholar]
- 48.Theethira TG, Dennis M, Leffler DA. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev Gastroenterol Hepatol. 2014;8:123–129. doi: 10.1586/17474124.2014.876360. [DOI] [PubMed] [Google Scholar]


