Abstract
Neurotoxicity from traditional chemotherapy and radiotherapy is widely recognized. The adverse effects of newer therapeutics such as biological and immunotherapeutic agents are less familiar and they are also associated with significant neurotoxicity in the central and peripheral nervous systems. This review addresses the main toxicities of cancer treatment by symptom with a focus on the newer therapeutics. Recognition of these patterns of toxicity is important as drug discontinuation or dose adjustment may prevent further neurologic injury. Also, knowledge of these toxicities helps to differentiate treatment-related symptoms from progression of cancer or its involvement of the nervous system.
Introduction
Neurotoxicity from cancer treatment has been widely recognized. Chemotherapy or radiotherapy may have significant effects on the central or peripheral nervous systems that can limit the course of treatment. With the development of biological and immunotherapeutic agents to treat cancer, there are new patterns of neurotoxicity that are less well-described. This article will review the main toxicities of cancer treatment with a focus on the newer therapeutics as their adverse effects are less familiar. We will approach the topic by symptom as this is how clinicians encounter patients.
Given the protection of the blood-brain, blood-cerebrospinal fluid (CSF), and blood-nerve barriers, and low reproductive rate of neurons, the nervous system should be relatively protected from chemotherapy and radiotherapy toxicities; however, neurotoxicity is second only to myelosuppression as the dose-limiting factor of cancer treatment1. The occurrence of nervous system toxicity depends on a variety of factors including the dose of treatment delivered, route of administration, interactions with other agents, the presence of underlying structural nervous system disease, and individual patient vulnerability, most of which are poorly understood2. Toxicity can occur by direct damage to neurons or glia, or indirectly by altering the surrounding microenvironment, such as localized vascular injury3. Typically there is no confirmatory test for the diagnosis, and the attribution of symptoms to treatment toxicity is largely a diagnosis of exclusion, but recognition is important as dose adjustment or discontinuation may prevent further injury.
Background
Traditional chemotherapy preferentially acts on dividing cells by inducing DNA damage and strand breakage, interfering with DNA repair and microtubule function. These mechanisms are nonspecific and can result in damage to normal cells. Because the nervous system was thought to be static and not to contain dividing cells, the expectation was that the nervous system would be spared injury. However, it is now clear that the central nervous system contains stem cells that replenish some neuron populations and that glia divide, albeit slowly4. Thus, the central nervous system is an unexpected target of drugs that affect dividing cells.
Biologic therapy includes treatments that use the immune system to recognize and fight cancer cells. Treatments include antibodies against receptors that are involved in cell growth, cell division, and angiogenesis, agents that upregulate the host immune system, and therapies that directly target specific mutations harbored by tumor cells. Because of their action on the host immune system, neurological toxicity can result from a heightened immune response and cross-reactivity with cells of the nervous system.
Radiation therapy (RT) targets dividing cells and can cause damage to neural structures directly or indirectly by causing vascular damage, endocrine disturbance, and fibrosis of neural structures. These effects are usually delayed by weeks to years, reflecting the slow rate of cell turnover in the nervous system. Adverse effects of radiation typically depend on the total dose, dose per fraction, total volume irradiated, and comorbid conditions5.
Central Nervous System
Headache
Headache is one of the most common complications of cancer treatment. Patients with a history of headache prior to treatment may be more susceptible; however all patients are at risk for developing headache. Headache may accompany administration of several chemotherapeutic agents (Table 1). The mechanisms of headache are largely unknown, but headache occurs more frequently with agents that penetrate the blood-brain barrier, particularly temozolomide, nelarabine, and either intrathecal (IT) or high-dose intravenous methotrexate.
Table 1.
Common Central Nervous System Complications of Chemotherapy
| Chemotherapy Associated with Headache | Chemotherapy Associated with Seizure |
|---|---|
| Asparaginase | Amsacrine |
| Etoposide | Asparaginase |
| Fludarabine | Busulfan (high-dose) |
| Hexamethylamine | Carmustine |
| Ixabepilone | Cisplatin |
| Mechlororethamine | Cytarabine |
| Methotrexate | Cyclosporine |
| Nelarabine | Dacarbazine |
| Retinoic acid | Etoposide |
| Tamoxifen | 5-fluorouracil |
| Temozolomide | Fludarabine |
| Intrathecal chemotherapy: Any drug | Gemcitabine |
| Ifosfamide | |
| Methotrexate | |
| Nelarabine | |
| Paclitaxel | |
| Vincristine | |
| Intrathecal Chemotherapy: Any drug |
Headache occurs with biologic treatments as well, such as with administration of rituximab, a human monoclonal antibody against CD20-positive cells that is used frequently to treat lymphoma, and rarely with trastuzumab, used commonly to treat HER-2 positive breast cancer. In a study of 420 patients receiving cetuximab for advanced colorectal cancer, 26% developed headache6 Headache is also seen with administration of chimeric antigen receptor (CAR) T-cells, a new immunotherapy for treatment of B-cell malignancies. The neurotoxicity associated with this treatment is likely related to the significantly elevated levels of inflammatory cytokines that accompany the transfer of these cells. Treatment with steroids may reduce these inflammatory changes, but also compromises the efficacy of the cells against the malignancy, so their use is often restricted. Interferons7 and interleukins are other immunotherapies that may be associated with headache.
Cancer treatment can also induce intracranial processes that result in headache. Headache may be a manifestation of aseptic meningitis, which is common after IT treatment with any chemotherapy or biological therapy, including steroids. Patients typically present within hours of drug administration with headache, nausea, neck stiffness, fever, and lethargy and are found to have a CSF pleocytosis with a negative CSF bacterial culture. The most common chemotherapeutic agents to cause aseptic meningitis after IT administration are cytarabine, methotrexate, thiotepa, and topotecan. Dexamethasone treatment should be given prophylactically with IT use of the liposomal preparation of cytarabine, Depocyte, as 40% of patients will develop significant aseptic meningitis with this agent.
Ipilimumab, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor used to treat metastatic melanoma, can also induce aseptic meningitis. By binding to the CTLA-4 receptor, inhibitory feedback is blocked, which leads to a highly activated T-cell response that can act against the patient’s own tissues in addition to the tumor, resulting in inflammation of several organ systems8. In the case of aseptic meningitis, diagnostic work up usually reveals an elevated CSF protein and mild pleocytosis as well as contrast enhancement of the meninges on magnetic resonance imaging (MRI) of the brain. Most patients improve with steroid treatment.
Retinoid treatment may also cause headache by inducing idiopathic intracranial hypertension (pseudotumor cerebri) with an elevated CSF opening pressure in the absence of a space occupying lesion in the brain. Headache may also occur in these patients even when intracranial pressure is not demonstrably elevated.
An acute toxicity of RT to the brain is headache secondary to disruption of the blood-brain barrier. This is most common with daily fractions greater than 300 cGy and in those patients with large intracranial lesions that have substantial associated edema. Glucocorticoids can both prevent and treat this problem.
Seizures
Seizures occur with a variety of intravenous chemotherapeutic agents (Table 1). For agents such as high-dose busulfan, the risk of seizure is so significant that anti-epileptic medication for seizure prophylaxis is recommended with drug administration.
Seizures may be non-convulsive in nature and may manifest as encephalopathy with non-convulsive status epilepticus demonstrated on electroencephalogram (EEG). Seizures may be related to the agents themselves, or as a result of environmental changes induced by therapy. For example, vincristine may induce a syndrome of inappropriate anti-diuretic hormone (SIADH) secretion that can lead to hyponatremia and subsequent seizure activity. Seizures are more common with IT administration of chemotherapy, particularly cytarabine, methotrexate, and rarely, topotecan.
Immunomodulatory agents such as interferon and interleukin-2 (IL-2) may cause seizure activity. Due to the cytokine storm induced by the transfer of CAR T-cells, seizures are common in patients receiving this treatment and can be difficult to control. Prophylactic anti-epileptic medication can be used to prevent onset of seizure activity; however, if seizures result, a combination of several anti-epileptic agents and steroids is typically required to control seizure activity. Other immunotherapy agents such as blinatumomab, a T-cell receptor antibody that reacts with normal CD3-positive T-cells and CD19-positive acute lymphocytic leukemia cells, causes seizures in 15–20% of treated patients1. The anti-angiogenic agent, thalidomide, rarely causes seizure activity.
The most common presenting symptom of posterior reversible encephalopathy syndrome (PRES) is seizure which will be described below in the encephalopathy section.
Encephalopathy
Acute altered mental status is the most common central nervous system toxicity after any one of a number of cancer treatments. Acute encephalopathy occurs commonly after agents with good penetration of the blood-brain barrier such as high-dose cytarabine or methotrexate, nitrosoureas, and procarbazine; however there are many other agents that can induce acute encephalopathy (Table 2). Encephalopathy varies in severity from drowsiness and inattentiveness to stupor. The encephalopathy usually improves with drug withdrawal but in some circumstances, it may require an antidote to reverse. For instance, the encephalopathy seen after administration of ifosfamide reverses with administration of methylene blue.
Table 2.
Treatments Associated with Encephalopathy
| Traditional Chemotherapy | L-Asparaginase |
| 5-azacytidine | |
| Cisplatin | |
| Cyclophosphamide (PRES) | |
| Cyclosporine (PRES) | |
| Cytarabine (high-dose) | |
| Etoposide | |
| Fludarabine | |
| 5-fluorouracil | |
| Gemcitabine (PRES) | |
| Hexamethylmelamine | |
| Ifosfamide (PRES or isolated encephalopathy) | |
| Methotrexate | |
| Mitomycin C | |
| Nelarabine | |
| Nitrosoureas | |
| Procarbazine | |
| Tamoxifen | |
| Thiotepa | |
| Vincristine (PRES or isolated encephalopathy) | |
| Small Molecule Inhibitors | Bortezomib |
| Anti-VEGF Agents | Bevacizumab (PRES) |
| Sorafenib (PRES) | |
| Sunitinib (PRES) | |
| Biologic Agents | Blinatumomab |
| CAR T-cell infusion | |
| Interferon-α | |
| Interleukin-2 | |
| Ipilimumab (PRES) | |
| Rituximab (PRES) | |
| Immunomodulatory Agents | Sirolimus (PRES) |
| Tacrolimus (PRES) |
Several of the small molecule inhibitors such as sunitinib and bortezomib (a proteasome inhibitor), and immunotherapeutic agents such as blinatumomab and CAR T-cell infusion are associated with acute changes in mental status that are usually reversible. At high doses, interferon-α (IFN-α), used to treat low grade lymphoma, chronic myeloid leukemia, multiple myeloma, melanoma, Kaposi sarcoma, and hairy cell leukemia can cause significant mental status changes. Patients become lethargic, confused, and develop hallucinations. Typically, these symptoms reverse with drug withdrawal, but they may progress to a chronic dementia or vegetative state7. At lower doses, patients may develop neuropsychiatric symptoms manifested by depression and cognitive slowing,9 which usually remit several weeks after discontinuing the medication. IL-2, sometimes used with interferon in the treatment of renal cell carcinoma and melanoma, may also induce similar dose-dependent neuropsychiatric symptoms in 30–50% of patients. These symptoms include depression, hallucinations, and cognitive changes. Symptoms usually resolve with cessation of treatment.10
Somnolence is a manifestation of acute encephalopathy and can be seen within hours of administration of thalidomide, resolving within two to three weeks of treatment, or as a delayed response, for example one week after administration of nelarabine.
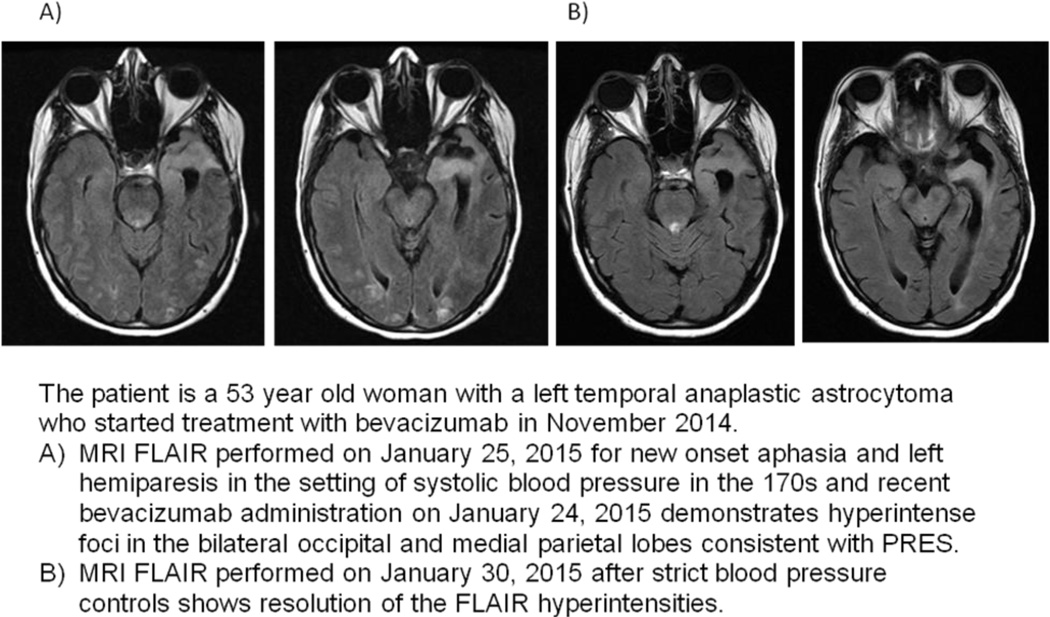
Several different types of cancer treatment can cause PRES, which is thought to be related to failure of autoregulation of cerebral blood pressure and local central nervous system inflammation. PRES is usually secondary to hypertension (sometimes treatment-induced); it is unclear if it can be induced directly by a chemotherapeutic agent or if the associated blood pressure elevation was missed in those who present with near normal blood pressure at symptom onset. Patients present with headache, confusion, visual changes, or seizures. The MRI has a typical appearance of bilateral white matter lesions seen on fluid attenuated inversion recovery (FLAIR) sequences with predominance in the posterior circulation (Figure 1). PRES is seen more commonly in patients receiving cyclosporine and cyclophosphamide and less commonly in patients receiving cisplatin, cytarabine, gemcitabine, ifosfamide, and vincristine. Immunomodulatory agents used to treat graft versus host disease in allogeneic stem cell transplant recipients, such as tacrolimus, are common etiologies of PRES. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), ipilimumab, sunitinib, and rituximab may also cause PRES in less than 1% of treated patients11. Hypertension from these agents may be volatile and any indication of increasing blood pressure requires tight blood pressure monitoring and control; it may also be that the patient has only mild elevation of blood pressure at presentation and the spikes in blood pressure were missed. Once PRES develops, discontinuation of the offending agent is essential and the clinical and radiographic changes of this syndrome are usually reversible unless cerebral infarction has occurred. Anti-epileptics are recommended if PRES is associated with seizure activity and treatment should be continued until radiographic changes resolve.
Figure 1. Posterior Reversible Encephalopathy Syndrome.
The patient is a 68-year-old woman with stage IV serous ovarian carcinoma treated with bevacizumab from 4/2012-9/2012. In 9/2012, she developed hypertension and mental status changes.
A. MRI Brain FLAIR sequence from 10/9/2012 shows confluent hyperintensities within the supratentorial white matter, predominantly within the posterior temporal and occipital lobes consistent with PRES.
B. MRI Brain FLAIR sequence from 4/4/2013 shows resolution of extensive white matter changes after discontinuation of bevacizumab treatment.
With large fractions of radiation to the brain (typically greater than 300 cGy), particularly in patients with elevated intracranial pressure, acute encephalopathy, headache, and lethargy can result. Acute delayed encephalopathy occurs weeks to months after cranial radiation (usually within three months of treatment), and is thought to be related to demyelination. Symptoms resolve over weeks to months and do not result in permanent disability. This syndrome does not predispose to late-delayed neurotoxicity, which is associated with irreversible cerebral injury such as the leukoencephalopathy that develops months to years after receiving treatment. Clinically, these patients present with slowly progressive cognitive dysfunction and personality change. Radiographically, there are diffuse periventricular white matter changes on MRI of the brain, sometimes with areas of focal enhancement, cerebral atrophy, and ventricular dilation. These changes may lead to severe weakness, dementia, coma, and death. Radiation to the brain, particularly whole brain RT, commonly leads to diffuse cerebral atrophy that usually begins within six months of completing treatment. Atrophy may be asymptomatic or present as memory loss, gait abnormalities, and incontinence, similar to the syndrome of normal pressure hydrocephalus. Patients may benefit from ventriculoperitoneal shunt placement although there is only partial reversal of symptoms, primarily improved bladder control and gait stability. Elderly patients who have received whole brain radiation are most susceptible to this syndrome.
Chemotherapy may also cause leukoencephalopathy on its own or can potentiate the toxicity associated with cranial RT, regardless of the sequence of the two modalities. This has been best studied with methotrexate; however, dementia may occur in patients receiving carmustine, cytarabine, fludarabine, thalidomide, tumor necrosis factor (TNF), or vincristine12. Any IT drug, including rituximab and topotecan, can lead to chronic encephalopathy. Rarely, capecitabine may cause multifocal leukoencephalopathy13.
In addition to overt cognitive changes, some cancer patients experience more subtle findings of cognitive dysfunction after receiving chemotherapy14. These changes are commonly referred to as “chemobrain” and many studies report subtle impairment in executive function, processing speed, and memory in patients receiving chemotherapy, particularly with high-dose regimens2. Deficits are more likely to be permanent if they are associated with structural changes in the brain seen on MRI.
Hippocampal neurogenesis may be affected by chemotherapy and is one potential cause of these mild cognitive changes15. The subgranular zone of the dentate gyrus in the hippocampus is one of the main regions of ongoing neurogenesis in the adult brain4. These hippocampal stem cells and progenitor cells contribute to neuron production in the hippocampus. With exposure to an enriched environment and exercise, there can be increases in neurogenesis in the region; however, with disruption of hippocampal neurogenesis, memory loss and cognitive deficits may result. Chemotherapy, RT, and inflammatory states may all interrupt hippocampal neurogenesis16, 17. RT inhibits the generation of new hippocampal granule cell neurons and causes changes in the surrounding microenvironment, producing a proinflammatory state leading to further interruption of neurogenesis18, 19. This is the basis for hippocampal-avoidance whole brain RT to treat multiple brain metastases without compromising cognition20, a technique currently under investigation in a randomized phase II trial.
A specific etiology of chronic mental status change is progressive multifocal leukoencephalopathy (PML), a fatal demyelinating disease caused by activation of latent JC virus. The risk of PML increases with administration of highly immunosuppressive agents such as fludarabine21. The risk is greater in elderly patients or those receiving treatment that induces chronic immunosuppression. The drugs usually associated with a risk of PML include agents that target B-cells such as rituximab (CD20 inhibitor), alemtuzumab (CD52 inhibitor), and brentuximab (CD30 inhibitor). In addition, the diseases for which these agents are used such as chronic lymphocytic leukemia are independently associated with PML and therefore, it may be the combination of longstanding disease and drug administration that may lead to the development of PML. There is no effective treatment for this condition other than efforts to reconstitute the immune system by withdrawal of the offending agents.
Cerebrovascular Disease
Cancer patients have an increased risk of stroke and other cerebrovascular disease due to the underlying hypercoagulability of malignancy as well as the treatments they receive. Chemotherapeutic agents such as L-asparaginase, cyclosporine, doxorubicin, estramustine, methotrexate, and platinum-based treatments all increase the risk of stroke. The risk of cisplatin-induced stroke increases if the drug is used in combination with other chemotherapeutic agents although the exact pathophysiology is unknown. High-dose methotrexate can induce a “stroke-like” syndrome of reversible and fluctuating neurological symptoms. MRI of the brain may demonstrate changes on both the diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) sequences that indicate ischemia, as well as slowing on EEG22. Clinical and radiographic findings are usually reversible and the patient is typically left without deficit.
In addition to arterial thrombosis, chemotherapeutic agents such as L-asparaginase increase the risk of venous sinus thrombosis, which may lead to stroke, intracranial hemorrhage, and seizures. Bevacizumab significantly increases the risk of cerebrovascular thrombosis as well as intracranial hemorrhage. Ipilimumab induces inflammation of the large blood vessels consistent with a giant cell arteritis which increases the risk for stroke.
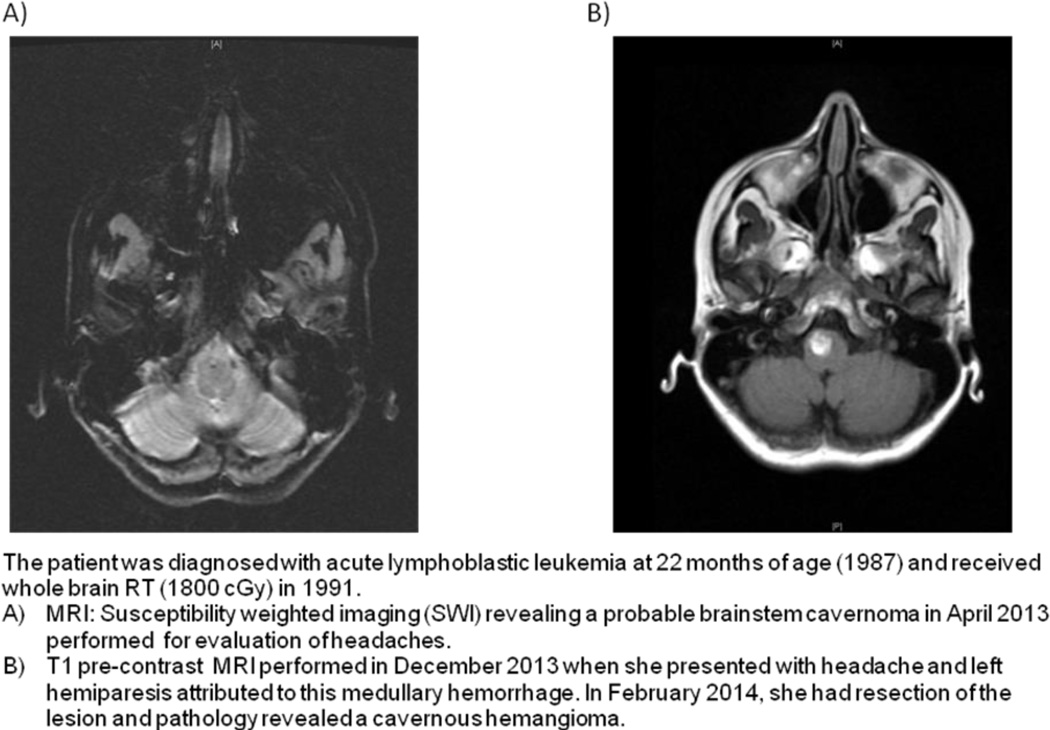
Radiation to the brain and neck can induce a delayed vasculopathy with accelerated atherosclerosis which increases the risk of stroke, but this increased risk manifests years after the RT23. Aside from acceleration of atherosclerosis and cerebrovascular disease, cavernomas may occur many years after cranial or spinal RT. These cavernomas have a higher risk of bleeding than those not associated with RT (Figure 2). In addition to hemorrhage, cerebral cavernomas may also cause seizures. Similarly, RT to the chest, neck, or spine may cause spinal cord telangiectasias that can result in spinal cord hemorrhage5. These patients usually present with the sudden onset of back pain and leg weakness that resolves over weeks to months.
Figure 2. Radiation-induced Cavernomas.
The patient is a 34-year-old woman with a history of medulloblastoma in 1989 who received craniospinal radiotherapy with a boost to the tumor bed and chemotherapy. MRI BRAIN SWI sequence from 9/22/2013 demonstrates numerous lesions throughout the posterior cranial fossa consistent with cavernomas.
Cerebellar dysfunction/Ataxia
Cerebellar dysfunction presents with nystagmus, dysarthria, confusion, scanning speech, and ataxia. The most common chemotherapy to induce a cerebellar syndrome is high-dose cytarabine, usually at cumulative doses greater than 36 g/m2. The effects are more pronounced in elderly patients and in those with pre-existing neurological conditions or renal dysfunction. Fortunately, most of the symptoms resolve within two weeks of discontinuing treatment, but some patients have permanent impairment secondary to loss of Purkinje cells in the cerebellum. Despite marked symptoms, imaging is usually normal; however there can be slowing seen on EEG. Other chemotherapeutic agents that can induce cerebellar dysfunction include capecitabine, 5-fluorouracil, hexamethylmelamine, nelarabine, oxaliplatin24, procarbazine, and vincristine. Blinatumomab can rarely induce a cerebellar syndrome, present in 2 of 95 patients in one cohort, which is reversible with discontinuation of the drug1.
Patients also report nonspecific dizziness with administration of cyclophosphamide, methotrexate, bortezomib, or rituximab. It is unknown whether this is a direct cerebellar or vestibular effect of the drug.
Movement Disorders
Abnormal movements may be seen with many of the supportive agents that accompany the administration of cancer treatments including anti-emetics. Metoclopramide, a common anti-emetic used to treat chemotherapy-induced nausea, is a dopamine receptor antagonist, with the majority of its effect at the D2 receptor. Normally, there is a balance of direct activity in the nigrostriatal pathway and indirect activity of the striatopallidal pathway within the basal ganglia to regulate movement. Inhibition of the D2 receptors in the striatopallidal projection can lead to imbalance of these inputs resulting in abnormal movements with chronic use. Acutely, patients may experience dystonic reactions that may require anti-cholinergic treatment. Within the first three months of chronic use, patients may experience akathesia (restlessness requiring the need for constant movement) and parkinsonism (tremor, bradykinesia, and rigidity). These side effects are usually reversible with discontinuation of the medication. Chronic treatment with metoclopramide may induce tardive dyskinesia, a syndrome of involuntary and repetitive movements, usually involving the lower face. Tardive dyskinesia usually manifests by tongue protrusion, lip smacking, lip pursing, and grimacing. Unfortunately, despite drug discontinuation, the condition may persist. The risk of developing tardive dyskinesia ranges from 1–10%25. For this reason other types of anti-emetics such as serotonin receptor antagonists, which have a much lower risk of causing tardive dyskinesia, are preferred. The serotonin receptor antagonists such as ondansetron may also cause acute dystonic reactions and extra-pyramidal symptoms, but less commonly than metoclopramide.
Tremor is another movement disorder that may be caused by a variety of chemotherapeutic agents, such as nelarabine and IFN- α, and may occur with steroid use. Vincristine may cause parkinsonism and athetosis.
Other Radiation Therapy Syndromes of the CNS
Radiation Necrosis
Most adverse effects that are specifically related to radiation usually result from direct radiotherapy to the CNS or are a consequence of CNS tissue being unavoidably included in a RT port. For instance, radiation necrosis usually occurs one to two or more years after RT to a primary or secondary brain tumor or may develop in a temporal lobe that was irradiated during treatment for a head and neck neoplasm26, 27. Symptoms including aphasia, hemiparesis, and seizures are dependent on the region of the brain involved and often suggest recurrent or new tumor in the brain. MRI shows extensive T2/FLAIR change and contrast enhancement that may mimic the original tumor or be confused with a new site of disease. Typically, lesions are hypometabolic on FDG-PET and are unlikely to have elevated plasma volume on MR perfusion imaging but these techniques do not always differentiate tumor from RT necrosis. Treatment includes corticosteroids, bevacizumab, and sometimes surgical resection, which can also provide definitive diagnosis28.
SMART Syndrome
Stroke-like migraine attacks after RT, known as SMART syndrome, occur within one to thirty plus years of brain radiation29. Patients may present with episodic focal neurological symptoms and seizures. While MRI may show focal enhancement at the time of the event, typically, these changes resolve as the syndrome is likely related to neuronal dysfunction rather than cerebral vascular compromise30. Repeated episodes may be prevented by drugs that are effective agents for migraine prophylaxis, such as valproic acid.
Secondary Malignancies
Prior radiation to the central or peripheral nervous system may cause secondary tumors including meningiomas, gliomas, malignant schwannomas, and sarcomas. These malignancies may occur even with low dose radiation, as they have been seen after treatment of tinea capitits with 100–200 cGy whole brain RT. Secondary tumors are more likely to occur after traditional RT; however, they may also develop after stereotactic radiosurgery31, 32. The delay is often many years after the RT, sometimes decades. The tumors are usually of a high grade variety, and full treatment of these secondary tumors is often compromised by the prior RT received.
Cranial Neuropathy
A variety of drugs can induce specific cranial neuropathies; the causes may be idiosyncratic or part of a more diffuse peripheral neuropathy from a drug such as vincristine. RT can also cause cranial nerve injury when the nerve is included as part of a therapeutic port (Table 3).
Table 3.
Cranial Neuropathies
| Symptom | Causes | Notes |
|---|---|---|
| Anosmia | Any chemotherapy RT |
Dysgeusia |
| Visual Loss | Carmustine Cisplatin Cytarabine Fludarabine Oxaliplatin Tamoxifen Bevacizumab RT |
Intra-arterial administration Reversible Retinal deposits Optic neuropathy Dry eye syndrome, cataract, glaucoma, retinopathy, optic neuropathy (begins 7–26 months after RT, painless, monocular or bilateral blindness, papilledema and retinal hemorrhage may be present) |
| Extraocular Palsy | Cytarabine Vincristine Interferon-α |
|
| Ptosis | Vincristine | |
| Facial Weakness | Vincristine Vemurafenib |
Isolated case reports of peripheral facial nerve palsy, sometimes bilateral |
| Hearing Loss | Carmustine Cisplatin Vincristine Oxaliplatin RT (to head or ear) |
Intra-arterial administration Hair cell damage70, usually doses greater than 60 mg/m2, typically irreversible. Possible prophylactic treatments include amifostine, vitamin E71, sodium thiosulfate, and intratympanic steroids, but there is limited evidence to support their use. RT-induced otitis media causes conductive hearing loss, perhaps requiring myringotomy. RT-induced endarteritis produces vascular damage within the cochlea or CNVIII that causes late delayed sensorineural hearing loss |
| Vestibular Dysfunction | Cisplatin | |
| Hoarseness | Cytarabine Vincristine Bevacizumab |
|
| Dysarthria | Irinotecan Methotrexate |
Usually transient, but can occur with subsequent infusions |
| Bulbar dysfunction | Cytarabine RT (to the neck) |
Late delayed effect (over 10 years), myokymia on electromyography, may involve recurrent laryngeal, vagal, hypoglossal nerves as well as sympathetic fibers |
| Jaw tightening | Oxaliplatin Vincristine |
Jaw pain |
Spinal Cord Syndromes
Myelopathy usually causes lower extremity weakness, sensory change, and alterations in bowel and bladder control; back pain may or may not be a feature. These symptoms occur after IT administration of any chemotherapeutic agent such as methotrexate, cytarabine, or thiotepa. Symptoms can start within minutes, but may also have their onset delayed by up to two weeks. The risk of myelopathy is higher in patients who have received prior spinal irradiation. Myelopathy from intravenous chemotherapy is less common, but can occur with cisplatin. Cisplatin and oxaliplatin may cause transient demyelination of the cervical spinal cord with normal imaging. Patients with cisplatin-induced demyelination may experience transient electric shock-like sensations radiating down the spine or into the limbs with flexion of the neck, known as Lhermitte phenomenon33. Lhermitte phenomenon may also appear after radiation to the neck as an early side effect. In both circumstances, the symptom is temporary and does not predict permanent spinal cord injury.
Radiation-induced myelopathy occurs as a late-delayed effect of treatment and usually starts as a painless Brown Séquard syndrome of crossed motor and sensory deficits below the site of injury (ipsilateral weakness and proprioceptive deficits and contralateral pain and temperature sensory loss) with progression to paraparesis and even quadriparesis over the weeks to months following onset. MRI findings are usually nonspecific, however, in the early stages of radiation-induced myelopathy, the spinal cord may appear swollen and enhance with gadolinium, and at later stages the cord is atrophic. Unfortunately, there is no effective treatment for radiation-induced myelopathy although steroid administration may delay progression. Reports of hyperbaric oxygen and anticoagulation have not been validated and it is unknown if bevacizumab may be helpful as it has been reported useful for cerebral radiation necrosis28.
Ipilimumab may induce inflammation of the spinal cord resulting in an acute transverse myelitis. Patients present with sensory, motor, and bowel and bladder changes below a spinal cord level, usually in the thoracic cord. MRI spine may demonstrate T2 signal change at the affected spinal cord level and CSF shows a pleocytosis and elevated protein without evidence of malignant cells, infection, or other inflammatory processes. In one patient, symptoms started a week after treatment. When treatment with ipilimumab was withheld and high-dose steroids were administered, the patient improved within two weeks’ time34.
Peripheral Nervous System Complications
Plexopathy
There are two major plexuses of peripheral nerves that may be affected by cancer treatment; the brachial plexus that innervates the arm and involves spinal roots C5-T1, and the lumbosacral plexus that innervates the leg and involves spinal roots L1–L5. Both of these regions are susceptible to the effects of treatment. A plexopathy manifests by a combination of weakness, sensory impairment, and reflex abnormalities with or without pain involving multiple nerve roots innervating the affected limb. This syndrome is rarely induced by cytarabine, IFN-α, and IL-2 but is more commonly seen as either an early-delayed or a late-delayed effect of RT which is typically painless35. Brachial plexopathy is common after radiation to the chest wall for treatment of breast or lung cancer, while lumbosacral plexopathy can be seen after RT to the pelvic region. Radiation-induced plexopathy is usually associated with total doses greater than or equal to 5000 cGy, but can be seen with lower doses as well. Clinical diagnosis can be supported by electrodiagnostic testing which may demonstrate segmental slowing on nerve conduction studies, fasciculations and myokymic discharges on electromyography (EMG) which are specific for the condition but present in only about 60% of patients36. CT or MRI may show diffuse loss of tissue planes and occasionally fibrosis which can be confused with tumor if sufficiently bulky, but PET imaging characteristically shows hypometabolism. The brachial plexus is more commonly affected than the lumbosacral plexus. Delayed damage to these plexuses may be slowly progressive over many years and is thought to be related to damage of the Schwann cells. Unfortunately late injury is usually permanent and disabling.
Peripheral Neuropathy
Peripheral neuropathy is the most common neurological complication of cancer treatment and can be a dose-limiting toxicity, particularly in elderly patients. Many traditional chemotherapies cause peripheral neuropathy (Table 4)37–55. All of these drug-induced neuropathies can be enhanced by pre-existing or new peripheral neuropathy from a non-cancer-related cause, such as diabetes or alcohol, or may be amplified by the sequential or combined use of neurotoxic agents, such as vinorelbine following a platinum-based treatment. Several neuroprotective strategies including calcium and magnesium supplementation, and administration of glutathione, glutamine, acetyl-L-carnitine, and erythropoietin have been suggested to prevent chemotherapy-induced peripheral neuropathy without compromising the treatment effect; however, clinical data supporting these agents are negative or weak56, 57. There is limited evidence to suggest that glutamine may help prevent neuropathy associated with paclitaxel and oxaliplatin58. More recently, specific genetic polymorphisms inherent to the patient may enhance or reduce vulnerability to chemotherapy-induced peripheral neuropathy59.
Table 4.
Treatment-induced Peripheral Neuropathy
| Class | Agent | Modalities Affected |
Notes | Outcome |
|---|---|---|---|---|
|
Platinum-based agents |
Carboplatin Cisplatin Oxaliplatin |
Sensory Sensory Autonomic Sensory |
Infrequent neuropathy, incidence increased when combined with paclitaxel (reaches 20% incidence) Symptoms may progress despite cessation of treatment Acute dysesthesias (particularly of the face, mouth, and throat), persistent sensory neuropathy |
May progress after discontinuation. Sensory ataxia common May be permanent Acute changes resolve within days, there may be a subacute neuropathy for approximately 3 months |
| Vinca alkaloids | Vinblastine Vincristine Vindesine Vinorelbine |
Sensory Motor Autonomic |
Predominantly affects legs, possible weakness, decreased/absent reflexes, constipation, orthostatic hypotension. Vincristine is the most neurotoxic. |
Symptoms resolve within 3 months, but may be permanent. A dose-limiting toxicity, particularly in older patients |
| Taxanes | Cabazitaxel Docetaxel Nabpaclitaxel Paclitaxel |
Sensory Motor |
Feet more affected than hands, painful paresthesias mild weakness, myalgias |
Symptoms improve after treatment discontinuation, but may persist after 1 year |
| Alkylators | Ifosfamide Procarbazine |
Sensory | Gradual onset of paresthesias in feet with loss of deep tendon reflexes |
Slow recovery after treatment Usually mild and rarely problematic |
| Anti-metabolites | Cytarabine Gemcitabine Nelarabine |
Sensory Sensory, autonomic Sensory, Motor |
Rare Rare |
|
| Epothilones | Ixabepilone Eribulin |
Sensory Motor Autonomic |
Painful paresthesias, 10– 16% weakness, rare autonomic changes |
Resolves with drug discontinuation |
| Hormonal agents | Anastrozole Exemestane Letrozole |
Sensory | Carpal tunnel syndrome |
Bortezomib, a proteasome inhibitor, can induce peripheral neuropathy in 60–75% of patients receiving twice weekly therapy and this neuropathy is severe with grade 3 or 4 toxicity in approximately 15% of patients60, 61. The risk of neuropathy is decreased if the medication is administered via a subcutaneous rather than an intravenous route, if the drug is given weekly rather than twice weekly, and if the total dose is reduced. The patients who tend to be most affected are those who have an underlying baseline neuropathy which may be secondary to prior cancer treatment or the disease itself, as bortezomib is used for multiple myeloma. The neuropathy occurs from damage to the dorsal root ganglia, and less commonly affects the axons. Patients typically present with painful, mild-moderate distal sensory neuropathy and occasionally they develop mild weakness in the legs (approximately 10% of patients) or autonomic symptoms such as constipation and orthostatic hypotension. Patients are particularly susceptible to pressure-point neuropathies which can be super-imposed upon the diffuse neuronal injury. Diagnostic workup reveals either demyelinating or mixed axonal-demyelinating neuropathy on nerve conduction studies, an elevated CSF protein, and sometimes enhancement of the nerve roots on MRI. Some patients appear to respond to glucocorticoids or intravenous immunoglobulin, and some derive symptom improvement with topical menthol or agents for neuropathic pain such as duloxetine62. Symptoms usually resolve within 3 months of discontinuing treatment; however, many patients are left with permanent sensory and motor deficits. Carfilzomib, a second generation proteasome inhibitor, causes a much less severe neuropathy than bortezomib.
The anti-angiogenesis agents thalidomide, lenalidomide, and pomalidomide, also cause a predominantly sensory peripheral neuropathy. Neuropathy is most common in patients receiving thalidomide and is typically related to the duration, rather than the total dose, of treatment63. Patients complain of painful dysesthesias and sensory loss, but may also develop weakness, muscle cramps, fasciculations, and tremor. Autonomic manifestations include constipation and rarely, impotence and bradycardia. At typical doses, 50% of patients taking thalidomide develop peripheral neuropathy (10% with severe symptoms), with an increased incidence in older patients64. Thalidomide-associated neuropathy is a common reason for dose reduction. With cessation of treatment, symptoms are at least partially reversible, but may persist for over one year63. Lenalidomide is a second generation, potent anti-angiogenic agent which is much less neurotoxic than thalidomide. Likewise, pomalidomide and bevacizumab rarely cause mild neuropathy.
Peripheral Neuropathy from Biologic Agents
Peripheral neuropathy is a common side effect of biologic agents and immunotherapies. Brentuximab, an antibody against the CD30 marker found on B and T lymphocytes, used to treat anaplastic large cell and refractory Hodgkin lymphoma, commonly causes a peripheral neuropathy by disturbing axonal transport. Peripheral neuropathy occurs in 36–53% of patients, and is severe in 10–14% of patients65. The neuropathy is predominantly sensory with only 11% of patients experiencing motor symptoms. The median time of onset is 27 weeks into treatment and in 50% of patients, the symptoms improve and reverse completely within 3 months of treatment discontinuation; the remaining 50% of patients take longer to recover. Because the neuropathy is related to cumulative dose, treatment can be continued at a lower dose once the neuropathy improves to grade 1. Ado-trastuzumab, used to treat HER-2 positive breast cancer in patients refractory to trastuzumab, can also cause a predominantly sensory neuropathy.
With respect to immunotherapy- related neuropathy, ipilimumab can induce autonomic neuropathy. IFN-α can cause a distal sensory neuropathy that manifests with subjective pain and paresthesias and objective loss of pain and temperature sensation on examination43. IL-2 can cause a more focal neuropathy of the median nerve which presents as a carpal tunnel syndrome with pain and sensory changes in the first three digits and palm of the hand.
Inflammatory Demyelinating Polyneuropathy
Ipilimumab may cause an acute inflammatory demyelinating polyneuropathy consistent with Guillain-Barre syndrome which is thought to be triggered by cross-reactivity of the stimulated T-cells against Schwann cells that have antigens similar to those found on melanoma cells. Symptoms are identical to those seen with idiopathic Guillain-Barre with progressive sensory symptoms, ascending weakness, and respiratory insufficiency. CSF reveals elevated protein concentration without a pleocytosis (albuminocytologic dissociation) and electrophysiologic studies show delayed nerve conduction velocities, increased distal motor latencies, and prolonged H-reflexes. Treatment should include glucocorticoids to counteract the T-cell response in addition to standard intravenous immunoglobulin or plasmapheresis8.
In addition to an acute inflammatory demyelinating polyneuropathy, ipilimumab rarely causes a chronic inflammatory demyelinating polyneuropathy (CIDP). Symptoms begin within one week of treatment and progress over weeks to months with sensory, motor, and autonomic changes and electrodiagnostic studies consistent with CIDP. With cessation of treatment and plasmapheresis, neurological symptoms usually resolve in a matter of weeks34.
Neuro-muscular Junction
Few cancer treatments can induce a myasthenic-type syndrome affecting the neuromuscular junction; these include cisplatin, IFN-α, IL-266, and ipilimumab34. Symptoms include ptosis, diplopia, weakness, and respiratory difficulty and typically worsen with fatigue or repetitive activity. If the syndrome is treatment-induced, antibodies against the acetylcholine receptor will be negative. Electrophysiologic examination demonstrates decremental responses with repetitive nerve stimulation identical to myasthenia gravis. Treatment can include discontinuing the offending agent, treating symptomatically with pyridostigmine, and initiating immunomodulatory treatment with plasmapheresis or intravenous immunoglobulin.
Muscle Symptoms
Myalgias are a side effect of both newer and older cancer treatments. Myalgias are common in patients treated with cisplatin, but also with several biological agents such as brentuximab and rituximab. Muscle cramps occur in 20–50% of patients receiving imatinib, the BCR-ABL, c-kit tyrosine kinase inhibitor. Rarely do patients develop elevated creatine kinase levels, muscle edema, or rhabdomyolysis2. These muscle cramps usually respond to supplementation with calcium, magnesium, or quinine.
Gemcitabine may cause an inflammatory myopathy manifested by painful, focal weakness, typically of proximal muscles that resolves with discontinuation of the drug; it also preferentially affects muscles contained in previously irradiated areas and in unusual locations such as the abdominal wall67. Ipilimumab, along with causing inflammation of other organ systems, causes an inflammatory myopathy that may involve the facial muscles, pharyngeal muscles, or muscles of the extremities. IL-2 may also cause myositis. All inflammatory myopathies may elevate creatinine kinase and respond to steroids.
While most myopathies affect the proximal muscles of the limbs, “Dropped Head Syndrome” is caused by isolated weakness of the neck extensors. The syndrome has been observed after exposure to selumetinib, a small molecule inhibitor of MEK [MAPK (mitogen-activated protein kinase) ERK (extracellular signal related kinase) kinase], which is used to treat melanoma and other solid tumors. Selumetinib causes a focal non-inflammatory myopathy that is characterized by neck pain and neck extensor weakness68. Diagnostic workup reveals elevated creatine kinase as well abnormal uptake on FDG-PET imaging. Because this is a non-inflammatory process, steroids are not helpful in treatment of this syndrome and can actually exacerbate myopathic symptoms. With discontinuation of the drug, the myopathy usually resolves. A similar syndrome occurs years after mantle radiation for treatment of Hodgkin lymphoma. Clinically, patients develop reduced cervical range of motion, weakness of neck flexion and extension, and pain in the neck. EMG demonstrates amyotrophy of the neck and shoulder muscles which is due to radiation injury to the cervical nerves. No treatment reverses this problem and it can be slowly progressive.
Motor Neuron Syndrome
Radiation treatment, typically to the pelvic region or craniospinal RT, may cause a rare motor neuron syndrome from three months to twenty-three years after treatment69. Patients present with the subacute onset of flaccid leg weakness that affects both proximal and distal muscles and is accompanied by atrophy, fasciculations, and areflexia. Typically, patients have preserved sensory and autonomic function. MR imaging can be normal or show enhancement of the spinal nerve roots. Nerve conduction studies show normal sensory and motor conduction velocities, but there may be variable denervation and myokymia seen on EMG. Symptoms may be progressive or may stabilize over the course of several months to years.
Conclusions
As cancer treatments continue to develop, the mechanisms of neurotoxicity also evolve. Both traditional and newer cancer treatments cause significant neurological toxicity in the central and peripheral nervous systems which may limit the course of treatment. Recognition of these symptoms as treatment-induced is important as drug discontinuation or dose-adjustment may prevent further neurological injury. Also, knowledge of these toxicities helps to differentiate treatment-related symptoms from progression of cancer into the nervous system.
Key points.
-
-
Cancer treatments are associated with significant neurotoxicity
-
-
Treatments affect both the central and peripheral nervous systems
-
-
Biological and immunological therapies have different mechanisms of toxicity
-
-
Neurotoxicity is a diagnosis of exclusion
-
-
Recognition of neurotoxicity is important to prevent further neurological injury and to distinguish from nervous system involvement by disease
Footnotes
The authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this article.
References
- 1.Magge RS, DeAngelis LM. The double-edged sword: Neurotoxicity of chemotherapy. Blood Rev. 2014 doi: 10.1016/j.blre.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soffietti R, Trevisan E, Ruda R. Neurologic complications of chemotherapy and other newer and experimental approaches. Handb Clin Neurol. 2014;121:1199–1218. doi: 10.1016/B978-0-7020-4088-7.00080-8. [DOI] [PubMed] [Google Scholar]
- 3.Plotkin SR, Wen PY. Neurologic complications of cancer therapy. Neurol Clin. 2003;21:279–318. doi: 10.1016/s0733-8619(02)00034-8. x. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Rogers LR. Neurologic complications of radiation. Continuum (Minneap Minn) 2012;18:343–354. doi: 10.1212/01.CON.0000413662.35174.a8. [DOI] [PubMed] [Google Scholar]
- 6.Pfeiffer P, et al. Cetuximab and irinotecan as third line therapy in patients with advanced colorectal cancer after failure of irinotecan, oxaliplatin and 5-fluorouracil. Acta Oncol. 2007;46:697–701. doi: 10.1080/02841860601009455. [DOI] [PubMed] [Google Scholar]
- 7.Meyers CA, Scheibel RS, Forman AD. Persistent neurotoxicity of systemically administered interferon-alpha. Neurology. 1991;41:672–676. doi: 10.1212/wnl.41.5.672. [DOI] [PubMed] [Google Scholar]
- 8.Bot I, Blank CU, Boogerd W, Brandsma D. Neurological immune-related adverse events of ipilimumab. Pract Neurol. 2013;13:278–280. doi: 10.1136/practneurol-2012-000447. [DOI] [PubMed] [Google Scholar]
- 9.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63:761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 10.Denicoff KD, et al. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- 11.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. discussion 980–2. [DOI] [PubMed] [Google Scholar]
- 12.Myers JS. Chemotherapy-related cognitive impairment. Clin J Oncol Nurs. 2009;13:413–421. doi: 10.1188/09.CJON.413-421. [DOI] [PubMed] [Google Scholar]
- 13.Tipples K, Kolluri RB, Raouf S. Encephalopathy secondary to capecitabine chemotherapy: a case report and discussion. J Oncol Pharm Pract. 2009;15:237–239. doi: 10.1177/1078155209102511. [DOI] [PubMed] [Google Scholar]
- 14.Waber DP, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007;25:4914–4921. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 15.Christie LA, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 16.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers JS. The possible role of cytokines in chemotherapy-induced cognitive deficits. Adv Exp Med Biol. 2010;678:119–123. doi: 10.1007/978-1-4419-6306-2_15. [DOI] [PubMed] [Google Scholar]
- 18.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev. 2008;14:238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 19.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 20.Gondi V, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidarsson B, Mosher DF, Salamat MS, Isaksson HJ, Onundarson PT. Progressive multifocal leukoencephalopathy after fludarabine therapy for low-grade lymphoproliferative disease. Am J Hematol. 2002;70:51–54. doi: 10.1002/ajh.10085. [DOI] [PubMed] [Google Scholar]
- 22.Haykin ME, Gorman M, van Hoff J, Fulbright RK, Baehring JM. Diffusion-weighted MRI correlates of subacute methotrexate-related neurotoxicity. J Neurooncol. 2006;76:153–157. doi: 10.1007/s11060-005-4569-2. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor MM, Mayberg MR. Effects of radiation on cerebral vasculature: a review. Neurosurgery. 2000;46:138–149. doi: 10.1093/neurosurgery/46.1.138. discussion 150–1. [DOI] [PubMed] [Google Scholar]
- 24.Land SR, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 25.Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11–19. doi: 10.1111/j.1365-2036.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 26.Fink J, Born D, Chamberlain MC. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12:276–285. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, et al. Radiation induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: a review of new avenues in its management. Radiat Oncol. 2011;6:128. doi: 10.1186/1748-717X-6-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin VA, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerklaan JP, et al. SMART syndrome: a late reversible complication after radiation therapy for brain tumours. J Neurol. 2011;258:1098–1104. doi: 10.1007/s00415-010-5892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farid K, et al. Normal cerebrovascular reactivity in Stroke-like Migraine Attacks after Radiation Therapy syndrome. Clin Nucl Med. 2010;35:583–585. doi: 10.1097/RLU.0b013e3181e4db6f. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramaniam A, et al. Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: case report and review of the literature. Neuro Oncol. 2007;9:447–453. doi: 10.1215/15228517-2007-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan J, Yen CP, Steiner L. Gamma knife surgery-induced meningioma. Report of two cases and review of the literature. J Neurosurg. 2006;105:325–329. doi: 10.3171/jns.2006.105.2.325. [DOI] [PubMed] [Google Scholar]
- 33.Taieb S, Trillet-Lenoir V, Rambaud L, Descos L, Freyer G. Lhermitte sign and urinary retention: atypical presentation of oxaliplatin neurotoxicity in four patients. Cancer. 2002;94:2434–2440. doi: 10.1002/cncr.10500. [DOI] [PubMed] [Google Scholar]
- 34.Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen BC, Verma A, Brandon AH, Fiedler JA. Radiation-induced brachial plexopathy: MR and clinical findings. AJNR Am J Neuroradiol. 1996;17:1932–1936. [PMC free article] [PubMed] [Google Scholar]
- 36.Esteban A, Traba A. Fasciculation-myokymic activity and prolonged nerve conduction block. A physiopathological relationship in radiation-induced brachial plexopathy. Electroencephalogr Clin Neurophysiol. 1993;89:382–391. doi: 10.1016/0168-5597(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 37.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008;8 doi: 10.1038/nrc2167-c1. 1p following 71; author reply 1p following 71. [DOI] [PubMed] [Google Scholar]
- 39.Cavaletti G, Alberti P, Frigeni B, Piatti M, Susani E. Chemotherapy-induced neuropathy. Curr Treat Options Neurol. 2011;13:180–190. doi: 10.1007/s11940-010-0108-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 41.Park SB, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 42.Walsh TJ, Clark AW, Parhad IM, Green WR. Neurotoxic effects of cisplatin therapy. Arch Neurol. 1982;39:719–720. doi: 10.1001/archneur.1982.00510230045013. [DOI] [PubMed] [Google Scholar]
- 43.Toyooka K, Fujimura H. Iatrogenic neuropathies. Curr Opin Neurol. 2009;22:475–479. doi: 10.1097/WCO.0b013e32832fbc52. [DOI] [PubMed] [Google Scholar]
- 44.Sul JK, Deangelis LM. Neurologic complications of cancer chemotherapy. Semin Oncol. 2006;33:324–332. doi: 10.1053/j.seminoncol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40:872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Mayer EL. Early and late long-term effects of adjuvant chemotherapy. Am Soc Clin Oncol Educ Book. 2013:9–14. doi: 10.14694/EdBook_AM.2013.33.9. [DOI] [PubMed] [Google Scholar]
- 47.Hansen SW. Autonomic neuropathy after treatment with cisplatin, vinblastine, and bleomycin for germ cell cancer. BMJ. 1990;300:511–512. doi: 10.1136/bmj.300.6723.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassier PA, et al. Gemcitabine and oxaliplatin combination chemotherapy for metastatic well-differentiated neuroendocrine carcinomas: a single-center experience. Cancer. 2009;115:3392–3399. doi: 10.1002/cncr.24384. [DOI] [PubMed] [Google Scholar]
- 49.Cavaletti G, et al. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer. 2010;46:479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- 51.Chen WW, Wang F, Xu RH. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis. PLoS One. 2013;8:e68974. doi: 10.1371/journal.pone.0068974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dormann AJ, Grunewald T, Wigginghaus B, Huchzermeyer H. Gemcitabine-associated autonomic neuropathy. Lancet. 1998;351:644. doi: 10.1016/S0140-6736(05)78426-9. [DOI] [PubMed] [Google Scholar]
- 53.Dropcho EJ. Neurotoxicity of cancer chemotherapy. Semin Neurol. 2010;30:273–286. doi: 10.1055/s-0030-1255217. [DOI] [PubMed] [Google Scholar]
- 54.Gaurav K, Goel RK, Shukla M, Pandey M. Glutamine: A novel approach to chemotherapy-induced toxicity. Indian J Med Paediatr Oncol. 2012;33:13–20. doi: 10.4103/0971-5851.96962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giglio P, Gilbert MR. Neurologic complications of cancer and its treatment. Curr Oncol Rep. 2010;12:50–59. doi: 10.1007/s11912-009-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beijers AJ, Jongen JL, Vreugdenhil G. Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med. 2012;70:18–25. [PubMed] [Google Scholar]
- 57.Loprinzi CL, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance) J Clin Oncol. 2014;32:997–1005. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2008;42:1481–1485. doi: 10.1345/aph.1L179. [DOI] [PubMed] [Google Scholar]
- 59.Diouf B, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. Jama. 2015;313:815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badros A, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110:1042–1049. doi: 10.1002/cncr.22921. [DOI] [PubMed] [Google Scholar]
- 61.Richardson PG, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 62.Seretny M, Colvin L, Fallon M. Therapy for chemotherapy-induced peripheral neuropathy. Jama. 2013;310:537–538. doi: 10.1001/jama.2013.7902. [DOI] [PubMed] [Google Scholar]
- 63.Mileshkin L, et al. Development of neuropathy in patients with myeloma treated with thalidomide: patterns of occurrence and the role of electrophysiologic monitoring. J Clin Oncol. 2006;24:4507–4514. doi: 10.1200/JCO.2006.05.6689. [DOI] [PubMed] [Google Scholar]
- 64.Mazumder A, Jagannath S. Thalidomide and lenalidomide in multiple myeloma. Best Pract Res Clin Haematol. 2006;19:769–780. doi: 10.1016/j.beha.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 66.Bora I, et al. Myasthenia gravis following IFN-alpha-2a treatment. Eur Neurol. 1997;38:68. doi: 10.1159/000112905. [DOI] [PubMed] [Google Scholar]
- 67.Pentsova E, et al. Gemcitabine induced myositis in patients with pancreatic cancer: case reports and topic review. J Neurooncol. 2012;106:15–21. doi: 10.1007/s11060-011-0672-8. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Schwartz GK, DeAngelis LM, Kaley T, Carvajal RD. Dropped head syndrome: report of three cases during treatment with a MEK inhibitor. Neurology. 2012;79:1929–1931. doi: 10.1212/WNL.0b013e318271f87e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowen J, Gregory R, Squier M, Donaghy M. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy? Brain. 1996;119(Pt 5):1429–1439. doi: 10.1093/brain/119.5.1429. [DOI] [PubMed] [Google Scholar]
- 70.Travis LB, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Argyriou AA, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer. 2006;14:1134–1140. doi: 10.1007/s00520-006-0072-3. [DOI] [PubMed] [Google Scholar]