Abstract
In this study, we show that depletion of Chk1 by small interfering RNA (siRNA) results in failure of reentry to the cell cycle after DNA replication has been stalled by exposure to hydroxyurea (HU). Casein kinase II (CKII) is degraded in these cells in a proteasome-dependent manner, resulting in decreased phosphorylation and PTEN levels. We show that phosphorylation of Chk1 at Ser317 but not at Ser345 is required for phosphorylation of PTEN at Thr383 by CKII, making cell cycle reentry after HU treatment possible. Like Chk1 depletion, loss of PTEN due to siRNA is followed by inability to return to the cell cycle following HU. In Chk1-siRNA cells, reintroduction of wild-type PTEN but not PTEN T383A restores the ability of the cell to reenter the G2-M phase of the cell cycle after stalled DNA replication. We conclude that, in response to stalled DNA replication, Chk1 is phosphorylated at Ser317 by ATR resulting in stabilization of CKII, which in turn leads to phosphorylation of PTEN at Thr383.
Introduction
DNA damage and replication checkpoints within the cell help maintain genetic stability by arresting cell cycle progression to allow time for repair. Checkpoint pathways efficiently regulate cellular processes, including DNA repair and replication, and cell cycle transitions. Abnormalities in these processes are a major factor in predisposition to cancer development (1, 2). Chk1 is critical for embryonic development and plays a crucial role in the DNA damage–induced checkpoint pathway (3). Chk1 not only is important for cell cycle signaling but also plays a critical role in homologous recombination repair (4). Chk1 has been implicated previously as a possible tumor suppressor disrupted in sporadic and also some hereditary cancers; therefore, investigation is currently ongoing into its therapeutic potential as a possible anticancer target (5).
ATM and ATR are members of the phosphoinositide 3-kinase–related protein kinase family, which are involved in regulating the DNA damage response (5, 6). They are integral to phosphorylation and activation of the cell cycle signaling pathway and are involved in the response to various types of damage including stalled replication and double-strand breaks. Previous models suggested that ATM and ATR function in separate pathways in the checkpoint response, with ATM activation occurring after ionizing radiation–induced double-strand breaks in a Chk2-dependent downstream pathway (7–9). Distinctively, ATR was shown to be required for phosphorylation of Chk1 at Ser317 and Ser345 after UV damage and replicative stress (10). Interestingly, most recently, both ATM and ATR have been shown to be important for Chk1 phosphorylation after ionizing radiation but independent of Chk2 (11). Although increasing evidence illustrates that alterations in cell cycle transition and regulation can facilitate tumorigenesis, a precise explanation for this cell cycle abrogation remains to be elucidated.
The tumor suppressor PTEN is frequently inactivated in several cancers including brain, prostate, and uterine cancer (12). PTEN contains a NH2-terminal phosphatase-like enzyme domain and a COOH-terminal regulatory domain (13). The latter region has been implicated to be important in the stability of the PTEN protein (14). Previous studies have shown that the PTEN COOH terminus is constitutively phosphorylated by the serine/threonine kinase casein kinase II (CKII). Evidence suggests that this phosphorylation is important in control of the biological activity of PTEN by regulating its stability via proteasome-mediated degradation (15).
In this study, we provide evidence that phosphorylation of both Chk1 and PTEN at specific sites is critical for successful recovery of the cell cycle after stalled DNA replication, thus linking the Chk1-PTEN proteins in an important cell cycle regulatory pathway. Specifically, phosphorylation of Chk1 by ATR at Ser317 is necessary for CKII-mediated phosphorylation of PTEN at Thr383 and important for cell cycle recovery in this setting. Our results help further clarify the roles of Chk1 and PTEN in the DNA damage response pathway and in the regulation of cell cycle transitions.
Materials and Methods
Antibodies
Primary antibodies used were rabbit polyclonal antibody to Chk1 and HA probe (Santa Cruz Biotechnology), phospho-PTEN S380/T382/T383, Chk1 phospho-Ser317 and phospho-Ser345 (Cell Signaling Technology), and ATR (Calbiochem). Mouse monoclonal antibodies used were to PTEN, CKII-α, cyclin B1, cyclin E, and tubulin (Santa Cruz Biotechnology).
Cell Culture
U2OS and mouse embryonic fibroblasts (MEF) cells were maintained in DMEM supplemented with 10% fetal bovine serum (Invitrogen). MEF cell lines were E1 transformed. ATRflox/− were generated by infecting with retrovirus pBabe-Cre (a gift from Dr. Tomo Shishido, Nara Institute of Science and Technology) for 48 h before analysis (5).
Cell Cycle Analysis and Flow Cytometry
Cells were treated with 1 mmol/L hydroxyurea (HU) for 18 h and subsequently washed with PBS three times. Fresh medium was then added to the cells and samples were collected every 24 h. At each time point, cells were trypsinized, washed with cold PBS, and collected by centrifugation then fixed with ice-cold 70% ethanol and precipitated overnight at 4°C. After fixation, cells were resuspended in room temperature PBS, treated with RNase A (50 mg/mL), and incubated for 30 min at 37°C followed by treatment with propidium iodide (50 µg/mL). Samples were analyzed using FACSCalibur (BD Biosciences). Data were processed with Verity ModFit version 5.2 Microsoft Windows software for cell cycle distribution analysis.
Immunoblot Analysis
Cells were lysed in EBC buffer [50 mmol/L Tris-HCl (pH 8.0), 120 mmol/L NaCl, 0.5% NP-40, 100 mmol/L NaF, 200 µmol/L sodium orthovanadate, 100 µg/mL phenyl-methylsulfonyl fluoride, 2 µg/mL leupeptin, 2 µg/mL aprotinin]. Protein concentrations in cell lysates were determined using the Bio-Rad protein assay kit. Whole-cell extract (40 µg) was loaded per lane and separated by 6% or 9% SDS-PAGE. Transfer to an Immobilon-P membrane (Millipore) was done using a semidry transfer method (Trans-Blot; Bio-Rad) in 25 mmol/L Tris, 192 mmol/L glycine, and 10% methanol for 1.5 h at 15 V. Membranes were blocked in 1% nonfat dried milk in PBS/0.05% Tween 20 and incubated with primary antibodies and horseradish peroxidase – conjugated secondary antibodies (Jackson Laboratories) followed by enhanced chemiluminescence detection.
Plasmids
A HA-tagged cDNA of wild-type (wt) Chk1 was cloned into pcDNA3 vector (Invitrogen). Chk1 S317A and Chk1 S345A mutants were generated by site-directed PCR mutagenesis from wt pcDNA3HA/Chk1 with primers of Ser317Ala (5′-GTGAAGTACTCCAGTGCTCAGCCAGAACCC-3′) and Ser345Ala (5′-CAAGGGATCAGCTTTGCCCAGCCCACATGT-3′), respectively. The small interfering RNA (siRNA)–resistant Chk1 silent mutation was generated using the following primer: 5′-GAGTAACTGAGGAAGCAGTAGCAGTGAAGA-3′. PTEN S380A, T382A, and T383A mutants were also generated using site-directed PCR mutagenesis. The primers used were as follows:
Ser380Ala: 5′-GATCATTATAGATATGCTGACACCACTGAC-3′;
Thr382Ala: 5′-TATAGATATTCTGACGCCACTGACTCTGAT-3′;
Thr383Ala: 5′-AGATATTCTGACACCGCTGACTCTGATCCA-3′.
Transfections were done using FuGENE (Roche Applied Science).
siRNA Assay
The Chk1-siRNA was generated by Dharmacon (1): 5′-AACTGAAGAAGCAGTCGCAGT-3′. PTEN-siRNA (Cell Signaling Technology) and ATR siRNA (Santa Cruz Biotechnology) were purchased commercially. Scrambled siRNA sequence is 5′-AAGATGATCGACGATCGCAGT-3′. Briefly, U2OS cells were seeded at 50% confluency the day before transfection and cells were transfected with Oligofectamine (Invitrogen). Samples were collected after 48 h.
Results
Chk1 Is Required for Recovery and Reentry to the Cell Cycle following HU-Induced Arrest
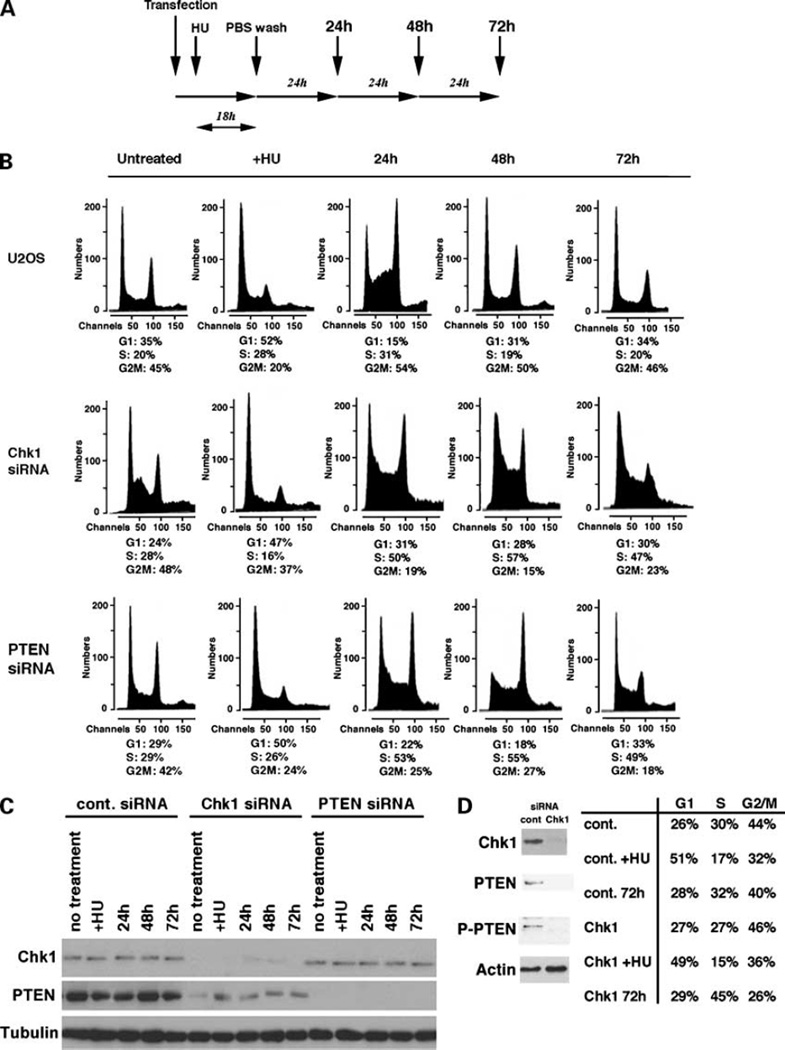
It has been shown previously that loss of Chk1 expression results in the inability of cells to arrest at G2-M after DNA damage and leads to apoptosis (3). To further elucidate the role of the Chk1 pathway in the checkpoint response, in particular after stalled DNA replication, we down-regulated levels of Chk1 in U2OS cells using siRNA specific for the Chk1 gene followed by HU treatment. As a control, U2OS cells were transfected with scrambled siRNA. Cells were treated with HU for 18 h and washed with PBS to allow recovery from stalled DNA replication and reentry into the cell cycle. Cell cycle profiles were determined by fluorescence-activated cell sorting analysis every 24 h (Fig. 1A). After treatment with HU, both control and Chk1-siRNA cells showed significant accumulation in the late G1 phase (52% and 47%, respectively; Fig. 1B). After HU was removed, control U2OS cells entered into the S and G2-M phases in 24 h and showed cell cycle profiles similar to those of untreated control U2OS cells in 48 h. DNA contents of Chk1-siRNA cells at each time point were carefully aligned to those of untreated control U2OS cells (Fig. 1B, top left). We found that, at 24 h after HU treatment, numbers of cells in the S phase were significantly higher (51%) than those of control cells (31%) and that such cell cycle arrest in the S phase was observed until 72 h after HU treatment. These results show that cells deficient in Chk1 cannot enter the G2-M phase after DNA has been stalled by HU treatment. Defects in the G2-M checkpoint have also been observed in PTEN−/− cells obtained from PTEN mutant mice (16), and Chk1 was found to be sequestered to the cytoplasm in these cells, suggesting the functional interaction between these proteins. We investigated whether PTEN is involved in cell cycle recovery and progression after HU treatment. PTEN expression was reduced by transfecting U2OS cells with PTEN-siRNA and cells were treated with HU. Cells were then similarly washed and restimulated with fresh medium as described previously for Chk1-siRNA experiments. Interestingly after propidium iodide staining and flow cytometry analysis, the cell cycle profile of PTEN-siRNA cells indicated that they were arrested at the G1 phase after HU treatment, like control and Chk1-siRNA cells. However, on washing and addition of fresh medium, cells were unable to recover and reenter the normal cell cycle progression as observed in control cells. Thus, most cells remained in the S phase of the cell cycle and did not reenter the normal cell cycle progression. These profiles were closely similar to those exhibited in Chk1-siRNA cells following the same HU treatment (Fig. 1B). Of note, Chk1-siRNA cells showed increased early S phase in the absence of HU treatment, suggesting that levels of Chk1 is important to regulate G1-S transition. Decreased levels of Chk1 and PTEN in siRNA-transfected cells were confirmed by immunoblot analysis (Fig. 1C). In control cells, levels of both Chk1 and PTEN were sustained for 72 h after HU treatment. Levels of Chk1 and PTEN were reduced when cells were transfected with specific siRNA for these proteins, respectively. Interestingly, levels of PTEN were markedly decreased in Chk1-siRNA cells in comparison with control U2OS cells (Fig. 1C). We performed the similar experiments using MCF7 cells. Briefly, cells were transfected with control or Chk1-siRNA and treated with HU. Cells were then washed and cell cycle reentry was studied after 72 h. As indicated (Fig. 1D), knockdown of Chk1 resulted in less phosphorylation of PTEN, and inhibition of cell cycle reentry as observed in U2OS cells.
Figure 1.
Loss of Chk1 and PTEN abrogates recovery from stalled DNA replication. A, schematic presentation of the experiments. Human osteosarcoma U2OS cells were transfected with scrambled siRNA (control, 10 µmol/L) or siRNA specific for Chk1 (20 µmol/L) and PTEN (20 µmol/L). Next day, HU was added to the cell culture medium (100 µmol/L). After 18 h, cells were washed with PBS three times, and fresh medium was supplied. Cell cycle profiles were analyzed every 24 h for the next 3 d. B, U2OS cells transfected with scrambled siRNA were used as controls for fluorescence-activated cell sorting analysis to determine the alignment of G1, S, and G2-M phases. The fraction of cells in each cell cycle is shown for each sample. Profiles of +HU are the results of fluorescence-activated cell sorting analysis after treatment with HU for 18 h. C, immunoblot analysis of Chk1 and PTEN for each time point. Tubulin was used as a loading control. D, MCF7 cells were transfected with control or Chk1-siRNA as described above and treated with HU similarly. Cells were then washed with PBS and fluorescence-activated cell sorting analysis was done after 72 h. Each cell cycle population was determined as U2OS cells. Levels of Chk1, PTEN, and phospho-PTEN (P-PTEN) are also indicated.
Chk1 Expression Is Required for Phosphorylation of PTEN by CKII
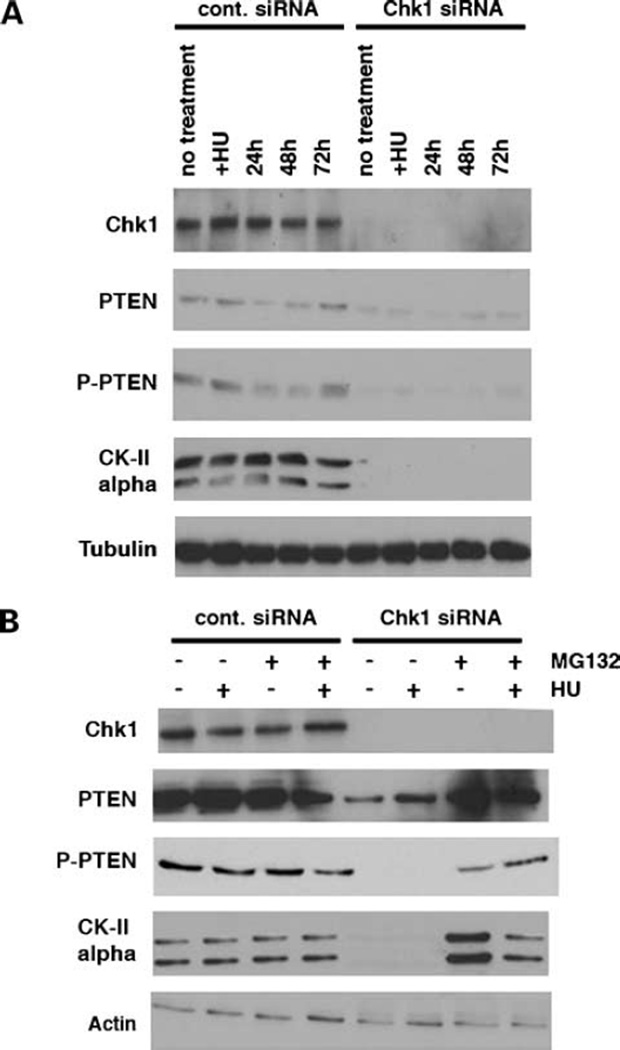
Previous studies suggested that PTEN protein stability is directly correlated to its phosphorylation status as phosphorylation-deficient PTEN mutants showed decreased stability and underwent rapid proteasomal degradation in comparison with wt PTEN (15). Therefore, we next examined PTEN phosphorylation status in the absence of Chk1 expression (Fig. 2). Cells were transfected with Chk1-siRNA and treated with HU as before. Immunoblot analysis using a phosphospecific antibody of PTEN recognizing phosphorylated Ser380/Thr382/Thr383 revealed that, in U2OS control cells, PTEN is constitutively phosphorylated and this phosphorylation is not affected by HU treatment and subsequent cell cycle progression. Interestingly, loss of Chk1 expression resulted in loss of basal levels of PTEN phosphorylation at its COOH terminus containing these residues illustrated with this antibody (Fig. 2A). CKII is known to be responsible for phosphorylation of the COOH terminus of PTEN at residues including Ser370, Ser380, Thr382, Thr383, and Ser385 (15). Therefore, we determined whether loss of Chk1 was abrogating CKII-mediated phosphorylation of PTEN at these sites. Using an antibody specific to the α-subunit of CKII, we determined that loss of Chk1 expression resulted in loss of expression of the CKII α-catalytic subunit. To further investigate this loss of the CKII α-subunit expression, we used the proteasome inhibitor MG132. U2OS cells were treated with MG132 after transfection of control or Chk1-siRNA. In Chk1-siRNA cells, MG132 completely stabilized the catalytic subunit of CKII in the presence and also absence of HU treatment (Fig. 2B). Consequently, on addition of MG132 in Chk1-siRNA cells, both total levels and phosphorylation of PTEN were also restored. These data strongly indicate that proteasome-mediated protein degradation of the CKII α-subunit in the absence of Chk1 is involved in regulating the steady-state level of PTEN phosphorylation and may play a role in cell cycle recovery and reentry following stalled DNA replication in HU-treated cells.
Figure 2.
Loss of Chk1 decreases levels of CKII by proteasome-mediated protein degradation. A, U2OS cells transfected with control or Chk1-siRNA were immunoblotted with CKII, phospho-PTEN, and PTEN antibodies. Depletion of Chk1 results in decreased levels of CKII. B, levels of CKII are restored in the presence of proteasome inhibitor, MG132, in Chk1-siRNA cells. After transfection of Chk1-siRNA, cells were maintained with MG132 (50 µmol/L). After treatment of cells with HU for 18 h, cell lysates were immunoblotted with indicated antibodies.
Phosphorylation of Chk1 at Ser317 Is Necessary for PTEN Phosphorylation and Cell Cycle Recovery and Progression after HU Treatment
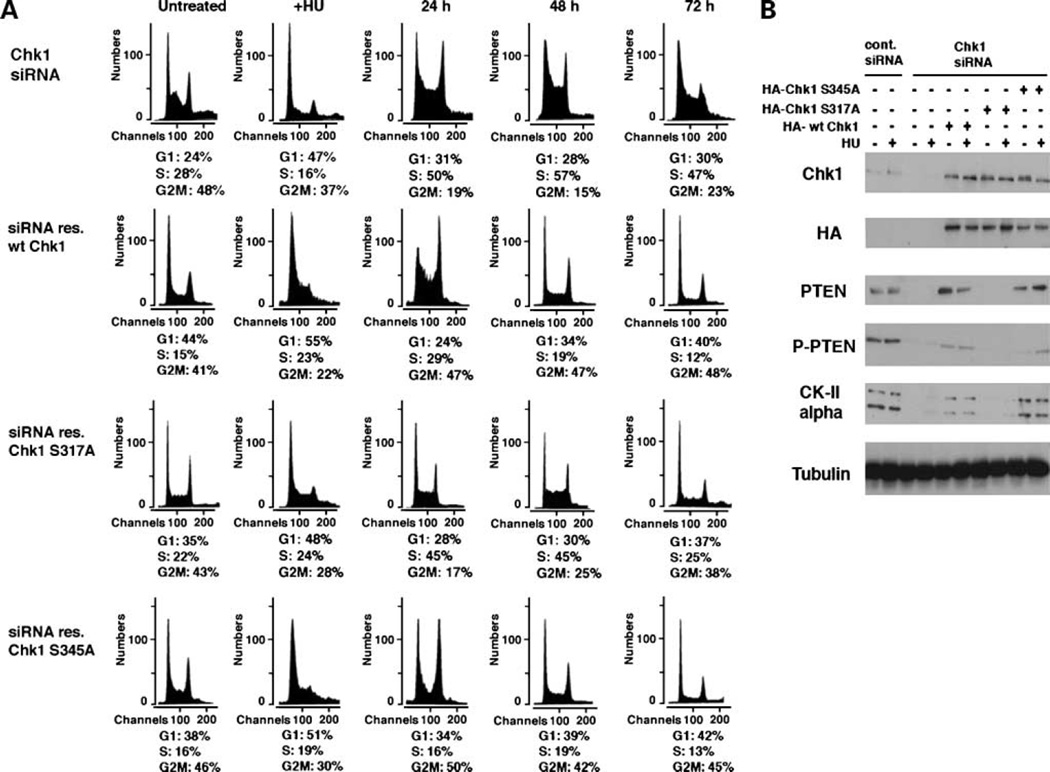
Studies have clearly shown that Chk1 is phosphorylated after HU treatment at Ser317 and Ser345 by ATR (10). Therefore, we next examined whether this abrogation in cell cycle recovery involves Chk1 phosphorylation after HU. We generated Chk1 phosphorylation mutants for Ser317 and Ser345 by substituting alanine for serine using site-directed mutagenesis. siRNA-resistant forms of wt and phospho-mutant Chk1 were then generated by introducing silent mutations in the siRNA target region. U2OS cells were transfected with Chk1-siRNA for 24 h followed by transfection with each of the HA-tagged Chk1 mutants and incubation for a further 48 h. Chk1-siRNA repressed expression of endogenous Chk1 but not exogenous siRNA-resistant Chk1, findings confirmed by immunoblot analysis with anti-Chk1 and anti-HA antibodies (Fig. 3B). Cells were HU treated, washed, and incubated in fresh medium as stated above and the cell cycle profile carefully aligned to that of untreated U2OS cells used in Fig. 1B. These results illustrate that, after HU-induced G1 arrest of Chk1-depleted cells, reintroduction of either wt Chk1 or Chk1 S345A enabled cells to reenter the S and subsequent G2-M phase of the cell cycle as seen in control cells in Fig. 1B. In contrast, reintroduction of Chk1 S317A in Chk1-depleted cells resulted in accumulation of the S-phase cells whose reentry into the cell cycle was significantly delayed 48 to 72 h after HU treatment. Of note, although reintroduction of wt Chk1 and Chk1 S345A into Chk1-siRNA cells recovered expression of the CKII α-catalytic subunit, expression of Chk1 S317A could not restore expression of the CKII catalytic subunit in the absence of HU treatment (Fig. 3B). Consequently, phosphorylation of PTEN was also restored in wt Chk1-expressing and Chk1 S345A-expressing cells; however, expression of Chk1 S317A did not recover PTEN phosphorylation in these conditions. Taken together, these results strongly suggest that phosphorylation of Chk1 at Ser317 is specifically required for CKII-mediated phosphorylation of PTEN at its COOH terminus and subsequent recovery and reentry into the cell cycle following HU-induced arrest.
Figure 3.
Phosphorylation of Chk1 at Ser317 is required for reentry to the cell cycle after stalled DNA replication. A, U2OS cells transfected with Chk1-siRNA (Fig. 1B) were further transfected with wt or phosphorylation-deficient mutants (S317A and S345A) of Chk1 cDNA, which is resistant to siRNA-mediated depletion (see Materials and Methods). Gates for the fraction of each cell cycle determined in control U2OS cells in Fig. 1B were used to study the progression of the cell cycle. B, cell lysates from each time point were immunoblotted for the indicated proteins.
PTEN Phosphorylation at Thr383 Is Necessary to Recover Cell Cycle Progression after HU Treatment
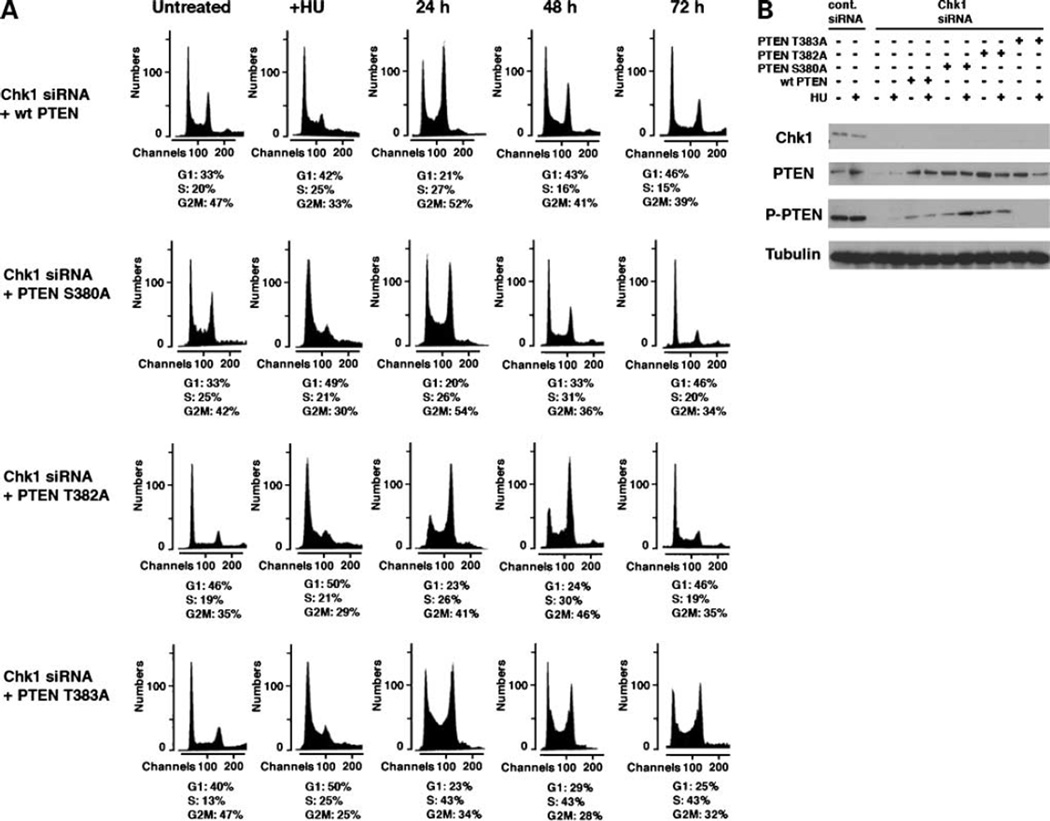
We next investigated whether PTEN phosphorylation was necessary for recovery and reentry to the cell cycle after HU treatment. The COOH-terminal region of PTEN is constitutively phosphorylated on serine and threonine residues by CKII (15). These residues include Ser380, Thr382, and Thr383, which together are recognized by the phosphorylated-PTEN antibody used in this study. To investigate whether any of these phosphorylated residues are differentially important in cell cycle progression after HU treatment, we generated PTEN mutants deficient in phosphorylation at each of these sites using site-directed mutagenesis. We generated Ser380 Ala (S380A), Thr382 Ala (T382A), and Thr383 Ala (T383A) mutants of PTEN. Chk1-siRNA cells were transfected with either wt PTEN, S380A, T382A or T383A PTEN constructs, and cell cycle profile and progression were observed (Figs. 1B and 3A). Remarkably, wt PTEN restored the cell’s ability to recover from arrest after HU and subsequently reenter the cell cycle in the absence of Chk1 expression (Fig. 4A). Similarly, PTEN S380A and T382A recovered cell cycle progression after HU in Chk1-siRNA cells. In contrast, expression of PTEN T383A could not restore cell cycle reenter after HU treatment. The cell cycle profile was similar to that of Chk1-siRNA cells alone. Reexpression of PTEN in Chk1-siRNA cells was confirmed by immunoblot analysis (Fig. 4B). These results indicate that phosphorylation of PTEN Thr383 is important for cell cycle recovery after HU and subsequent reentry to the cell cycle after arrest. We suggest that Chk1 Ser317 phosphorylation is required for the CKII catalytic activity necessary for phosphorylation of PTEN at Thr383, resulting in cell cycle reentry.
Figure 4.
Reexpression of PTEN restores reentry to the cell cycle of Chk1-siRNA cells. A, U2OS cells transfected with Chk1-siRNA (Fig. 1B) were further transfected with wt or phosphorylation-deficient mutants (S380A, T382A, and T383A) of PTEN. Gates for the fraction of each cell cycle determined in control U2OS cells in Fig. 1B were used to study the progression of the cell cycle of these cells. B, cell lysates from each time point used for the fluorescence-activated cell sorting analysis were studied for immunoblots of Chk1 and PTEN.
ATR Is Required for Phosphorylation of Chk1 at Ser317 after HU and for Subsequent Downstream CKII-Mediated PTEN Phosphorylation
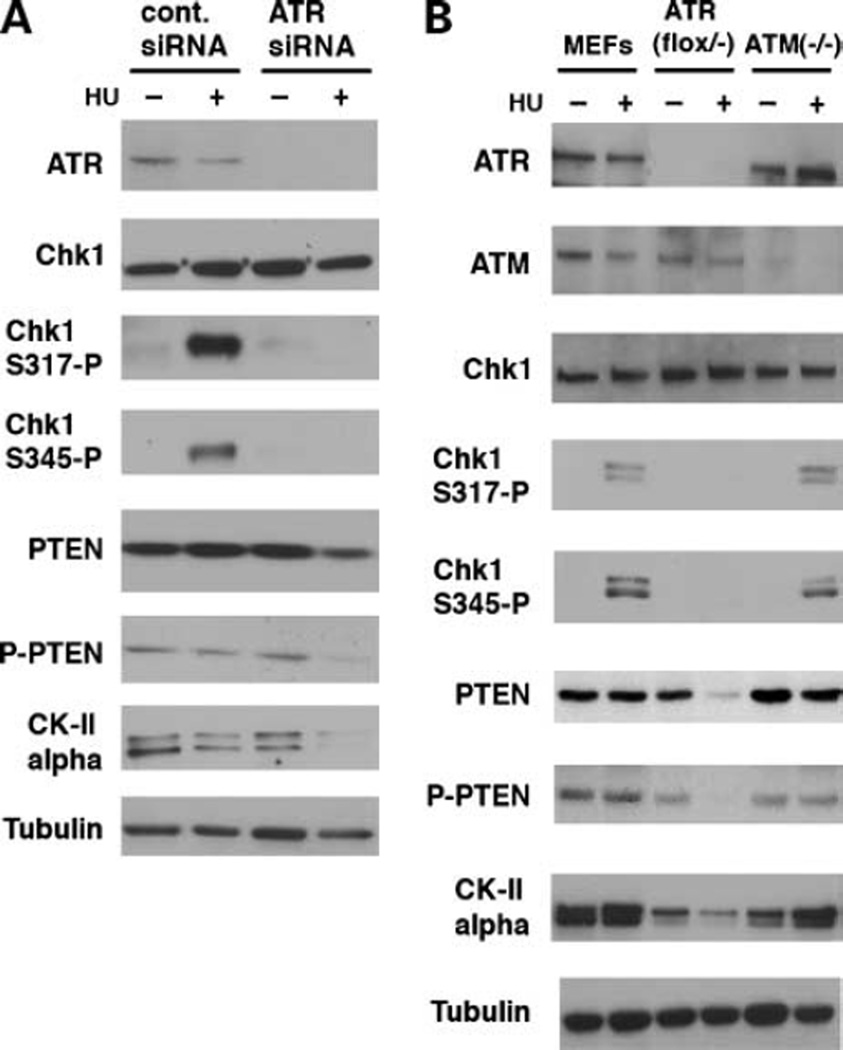
It is widely known that ATR is responsible for phosphorylation of Chk1 at Ser317 and Ser345 after HU-treatment (10). We investigated whether ATR was required for the CKII catalytic activity necessary for PTEN phosphorylation at Thr383 observed in this study. In cells in which ATR is knocked down by siRNA, CKII α-subunit levels were similar to those of control U2OS cells without HU treatment, but they markedly decreased after HU treatment, although they were not affected in control cells. Phosphorylation of Chk1 Ser317 and Ser345 were not detected in ATR-depleted cells. Levels of phospho-PTEN were also decreased in ATR-siRNA cells after HU treatment, although levels of Chk1 did not change in control and ATR-siRNA cells. These results support the idea that ATR-mediated phosphorylation of Chk1 is necessary for phosphorylation of PTEN when cells are treated with HU (Fig. 5).
Figure 5.
ATR-mediated phosphorylation of Chk1 at Ser317 is required for reentry to the cell cycle. A, U2OS cells were transfected with control or ATR-specific siRNA. HU was added to cell culture medium on the next day as in the previous experiments. After 18 h, cell lysates were immunoblotted for the indicated antibodies. HU treatment strongly induces phosphorylation of Chk1 at both Ser317 and Ser345 in control cells but not in ATR-siRNA cells. In the absence of Chk1 phosphorylation, levels of CKII were dramatically reduced. Consequently, phosphorylation and total levels of PTEN were reduced in these cells. B, ATR-mediated phosphorylation of Chk1 is critical for basal levels of CKII and PTEN after HU treatment. ATRflox/− and ATM−/− were generated as described previously (5). ATRflox/− were infected with retrovirus pBabe-Cre 48 h before analysis. wt MEFs, ATRflox/− MEFs, and ATM−/− MEFs were treated with HU. Levels of CKII were lower in ATRflox/− MEFs compared with normal and ATM−/− MEFs, which were further reduced after HU treatment. Both Ser317 and Ser345 of Chk1 are normally phosphorylated in ATM−/− MEFs after HU treatment.
To further confirm this observation, we used MEFs obtained from ATR- and ATM-mutant mice (5, 17). Control, ATRflox/−, and ATM−/− MEFs were HU treated as described above. Phosphorylation of Chk1 Ser 317 and Ser345 was similarly detected in both control and ATM−/− MEFs, indicating that Chk1 phosphorylation after HU treatment is independent of ATM expression. However, consistent with the results obtained in ATR-siRNA cells, Chk1 was not phosphorylated at both sites after HU treatment and consequently PTEN phosphorylation was abrogated. Basal levels of CKII were slightly lower in ATRflox/− MEFs, treated with pBabe-Cre, than control MEFs, consequently reducing the phosphorylation and total levels of PTEN in ATRflox/− MEFs. When these cells were treated with HU, CKII was further decreased, leading to more reduced phosphorylation and total levels of PTEN. Taken together, these results show that the level of Chk1 is responsible for constitutive levels of CKII and PTEN in ATR-independent manner in the absence of HU. When cells are treated with HU, ATR-dependent phosphorylation of Chk1 Ser317 is necessary for regulation of CKII/PTEN.
Discussion
In this study, we show the functional interaction between Chk1 and PTEN. First, in the absence of stalled DNA replication, basal levels of PTEN expression are determined by Chk1. When Chk1 is depleted, phosphorylation and total levels of PTEN are also decreased. Depletion of Chk1 also caused accumulation of cells in S phase without HU treatment. It was shown that Chk1 is activated only between S and M phases (18), suggesting that Chk1 regulates G1-S transition catalytic activity independent manner. Second, phosphorylation of PTEN at Thr383 requires ATR-mediated phosphorylation of Chk1 at Ser317 by HU treatment. Both phosphorylation steps are necessary for reentry to the cell cycle after release from stalled DNA due to HU treatment.
CKII is composed of two subunits, the catalytic α-subunit and the regulatory β-subunit (19). It has been shown previously that the CKII regulatory β-subunit can associate with, and also activate, Chk1 kinase activity (20), although the CKII α-subunit does not bind to Chk1 (data not shown). It has also been shown that the regulatory β-subunit, but not the α-subunit, is phosphorylated by Chk1 (21). Our data that Chk1 phosphorylation is required for expression and CKII catalytic activity after stalled DNA replication suggest that the CKII β-subunit determines stability of the α-subunit through Chk1.
Mechanism of reentry to the cell cycle after the DNA checkpoint is less characterized compared with that for growth arrest and/or apoptosis. In this work, we have established a model showing the importance of phosphorylation of Chk1 and PTEN in regulation of the cell cycle after stalled DNA replication. Because the complete loss of Chk1 in mice is lethal but is tolerable in a p53-deficient chicken tumor cell line, it is predicted that alterations in Chk1 or Chk1 hypomorphic mutations occurring during cancer progression may result in increased genetic instability (3). Although Chk1 associated cancers are not common, they have been shown in colon, lung, stomach, and endometrium. Thus, tumor-predominant expression of an alternative Chk1 isoform was described in small cell lung cancer, lacking the XI subdomain of the Chk1 catalytic domain, which has been suggested to be important for substrate selectivity (22). In addition, it was reported that frameshift mutations in the polyadenine tract of the Chk1 gene were found in colon and endometrial cancers exhibiting microsatellite instability (23). These mutations result in loss of the COOH-terminal end of the catalytic domain and loss of the entire SQ-rich regulatory domain in the Chk1 protein that contains multiple ATM/ATR phosphorylation sites including Ser317. However, the exact functional effect of these mutations in cancer cells is yet to be determined. These results suggest that abrogation of PTEN phosphorylation and the normal cell cycle transition due to loss of Ser317 phosphorylation as described in this study may further clarify the role of Chk1 in cancer development.
We propose that Chk1 is not only an important signal transducer in the cell cycle checkpoint pathway but also integral in PTEN phosphorylation and stabilization through CKII in the absence of DNA stress.
Acknowledgments
We thank Eric Brown (University of Pennsylvania) for kindly providing the ATRflox/− and ATM−/− MEFs and all members of the Ouchi laboratory.
Grant support: NIH grants CA79892 and CA90631.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Khanna KK, Jackson SP. DNA double-strand breaks: signalling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q, Guntuku S, Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen CS, Hansen LT, Dziegielewski J, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 5.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 7.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- 8.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 9.Meichionna R, Chen XB, Blasina A, McGowan CH. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat Cell Biol. 2000;2:762–765. doi: 10.1038/35036406. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers JS, Cortez D. Rapid Activation of ATR by ionizing radiation requires ATM and Mre-11. J Biol Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 13.Lee JO, Yang H, Georgescu MM, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 14.Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Aci U S A. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 16.Puc J, Keniry M, Li HS, et al. Lack of pten sequesters chk1 and initiates genome instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in at m−/− mice. Mol Cell Biol. 1999;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko Y, Watanabe N, Morisaki H, et al. Cell cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18:3673–3681. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 19.Niefind K, Guerra B, Ermakowa I, Issinger OG. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 2001;20:5320–5331. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra B, Issinger OG, Wang JYJ. Modulation of human checkpoint kinase Chk1 by the regulatory b-subunit of protein kinase CK2. Oncogene. 2003;22:4933–4942. doi: 10.1038/sj.onc.1206721. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen LP, Larsen MR, Hojrup P, Issinger OG, Guerra B. Phosphorylation of the regulatory β-subunit of protein kinase CK2 by checkpoint kinase Chk1: identification of the in vitro CK2 β phosphorylation site. FEBS. 2001;569:217–223. doi: 10.1016/j.febslet.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 22.Haruki N, Saito H, Tatematsu Y, Konishi H, Harano T, Masuda A. Histological type-selective tumor predominant expression of a novel chk1 isoform and infrequent in vivo somatic chk2 mutation in small cell lung cancer. Cancer Res. 2000;60:4689–4692. [PubMed] [Google Scholar]
- 23.Bertoni F, Codegoni AM, Furlan D, Tibiletti MG, Capella C, Broggini M. Chk1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Genes Chromosomes Cancer. 1999;26:176–180. [PubMed] [Google Scholar]