Abstract
A case of metastatic renal cell carcinoma (RCC) to the mandible is reported. A 22-year-old man consulted us for hypoesthesia of the right lower lip. Panorama X-ray film showed a radiolucent lesion in the right mandibular body. A diagnosis of a metastatic tumor to the mandible from the right kidney was made after evaluation by computed tomography and bone scan with Tc99 methyl diphosphonate which also revealed multiple bone metastases. Histologically diagnosis was clear cell variant of RCC. Patient has been put on radiotherapy for symptomatic treatment and a molecularly targeted drug. The therapy effectively stopped the progressive growth of oral and other metastatic lesions. The quality of life is relatively well maintained with tolerable adverse effects. The patient is still on our follow-up with an improved quality of life.
Keywords: Clear cell variant of renal cell carcinoma, molecular targeted therapy, oral metastatic lesion
INTRODUCTION
Worldwide, renal cell carcinoma (RCC) is the 13th most common malignancy. Approximately, 270,000 cases of kidney cancer are diagnosed every year, and 116,000 patients die of their cancer.[1] However, metastatic tumors to the oral cavity are very uncommon and represent approximately 1% of oral neoplasms.[2] Renal carcinoma is the third most common infraclavicular neoplasm that metastasizes to the oral cavity, following that of lung and breast carcinoma.[3] RCC occurs predominantly in the sixth to eighth decade of life with a median age at diagnosis around 64 according to 2003–2007 National Cancer Institute's Surveillance, Epidemiology, and End Results cancer statistics review. In addition, it is very rare to find in blacks and Asians. It is extremely rare rather unusual to find this tumor <40 years of age. This tumor at a younger age has certain peculiarities that it is usually symptomatic and has a chromophobe histology.[4,5] RCC that has metastasized at the time of initial diagnosis has an extremely poor prognosis, and more than 80% of patients die within 1 year.[6] Clear cell variant of RCC often shows local invasion and distant metastasis. Clear cell RCC (ccRCC) is the most common histologic variant, accounting for 75–88% of RCCs in contemporary surgical series.[7,8] The prognosis is worse than that of other types of tumor[9,10] with a median survival time of 20 months for patients with zero risk factors, 10 months for intermediate risk, and 4 months for poor risk patients.[11] They have 5-year disease-specific survival rates of 50–69%, compared with 67–87% for papillary RCC and 78–87% for ccRCC.[12,13]
CASE REPORT
A 22-year-old man consulted our hospital on March 27, 2014, with a complaint of swelling of right lower face since 1½ month which was associated with hypoesthesia of the right lower lip for 2 months. His past history was unremarkable except for two fractures of his both forearms as a result of trauma some years back, treated by some local quack but they had healed, but now with an inability to supinate. Pertinent to mention also was the fact that he had recurrent urinary tract infection which responded to antibiotics prescribed by local medical practitioner. He also complained of mild pain in his ribs, shoulders, and back.
There was no history of fever, discharge, tooth decay, or rashes or eruptions. He had no adverse habits, bladder and bowel habits were normal, and sleep undisturbed.
His face was asymmetric with a bony hard swelling, roughly 3 cm × 4 cm with ill-defined borders, tender, nonmovable, nonfluctuant, noncompressible, and nonreducible in nature in the right body of the mandible. Overlying skin was freely movable; both cortices felt as expanded, [Figure 1a and b]. Submandibular lymph nodes in right side were palpable, tender, freely mobile, nonmatted but not increased in size. Hypoesthesia was observed in the right mental region. Associated with it was mobility of two molars 46, 47 with missing 48 but no toothache or decayed tooth.
Figure 1.
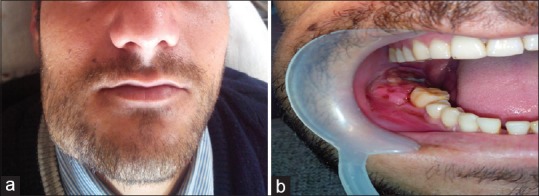
(a) Right mandibular body swelling. (b) Intraoral view of the lesion
Aspiration with an 18 gauge needle revealed blood venous but nonindicative of a vascular lesion. A fine-needle aspiration biopsy was ordered which revealed the following histology “high cellular smears comprised of discrete as well as clusters of osteoblasts with moderate to severe pleomorphism with prominent nucleoli and mitotic figures within osteoid matrix.” This raised the suspicion of a metastatic bone tumor. Hence, an incisional biopsy was planned to confirm.
In the meantime, an orthopantomogram, contrast-enhanced computed tomography (CECT) abdomen and a bone scan was ordered. Panorama X-ray film showed a diffuse radiolucent lesion of 4 cm × 5 cm in the right mandibular body. There was a loss of lamina dura around the roots of 46.47 with their circumferential resorption but not apical [Figures 2 and 3], and a 4 cm × 3 cm diffuse radiolucent lesion in the body of the mandible. On a total body scan with (Tc99 methyl diphosphonate), increased tracer uptake was seen in the right mandibular body, the upper thoracic T5 vertebrae, the right sternoclavicular joint, and the right 9th rib near angle. All these were leading to a metastatic bone disease.
Figure 2.
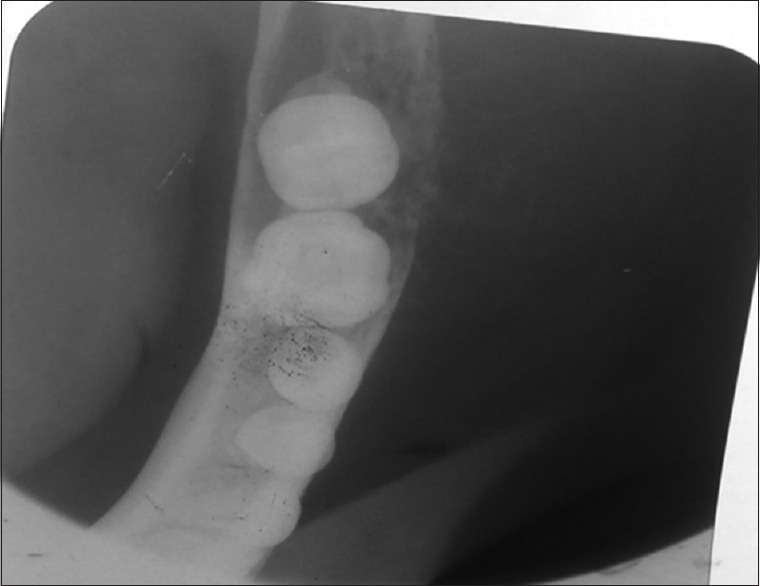
Occlusal view showing moth-eaten cortex
Figure 3.
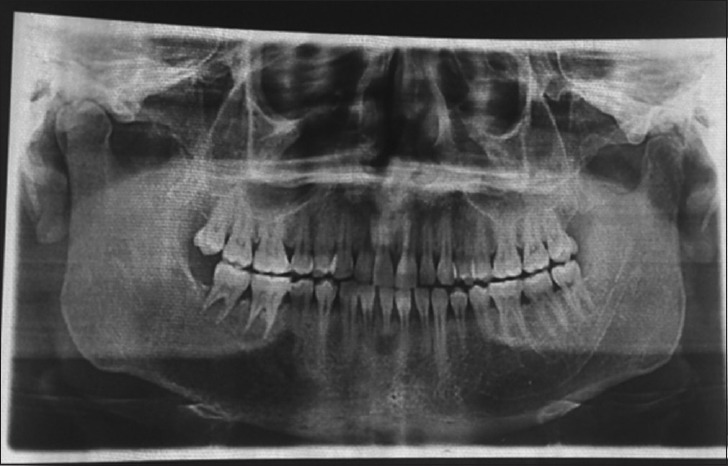
Orthopantomogram showing the right body rarefaction
After about 3 days of taking an incisional biopsy, the patient reported back with the right flank pain which turned the suspicion toward RCC. A ultrasound was ordered which was reported by the consultant radiologist as “a right renal complex cyst likely mitotic Bosniak type IV.”
Moreover, the CECT demonstrated a heterogeneous mass 4.5 cm × 3 cm at the upper pole of the right kidney with cystic changes [Figure 4a and b]. Multiple bone resorbing lesions in vertebrae, pelvis (acetabular and ischial region), right mandibular body, and left ramus and condylar region [Figure 5].
Figure 4.
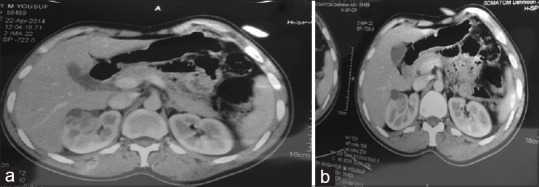
(a) Right kidney lesion. (b) Contrast-enhanced computed tomography showing the right kidney lesion
Figure 5.
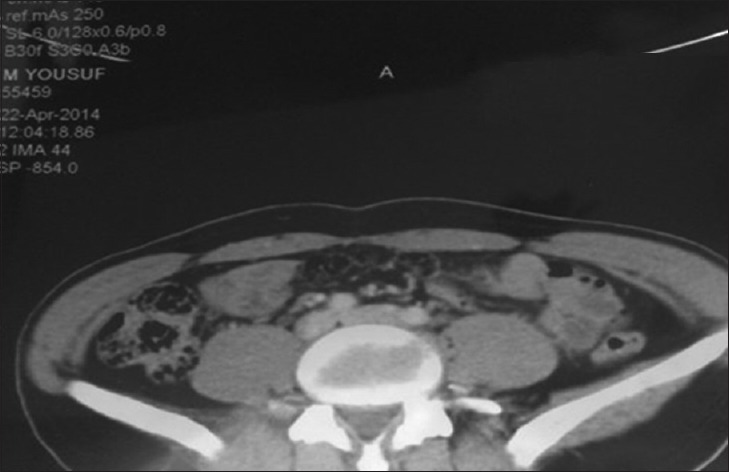
Vertebral body lesion
Hence, a diagnosis of RCC metastasizing to the mandible, sternoclavicular joint, rib, and vertebra and pelvis was established. It was confirmed by the histopathologocal examination (HPE) of the incisional biopsy which revealed a clear cell variant of the RCC [Figure 6].
Figure 6.
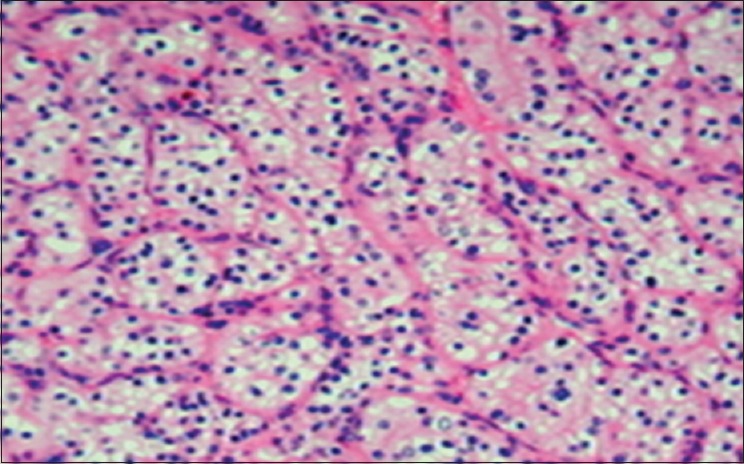
Histopathology of the mandibualr lesion showing clear cells
These findings led to the clinical diagnosis of a metastatic tumor of the mandible from the right kidney, although characteristic clinical symptoms of renal carcinoma such as abdominal pain, hematuria, and abdominal mass were not observed. The patient was referred to the Cardiovascular Surgery, Urology, and Radiology and Medical Oncology Departments for further evaluation. The patient then underwent radiation therapy for the tumor of the mandible, as it was progressively increasing and showing a tendency to bleed, and also the tender cervical vertebral lesions as a symptomatic treatment.
Since the patient had multiple unresectable lesions all over the body, it was decided to put the patient on molecular targeted therapy. His symptoms are improving and are still on follow-up.
DISCUSSION
Hereditary RCC syndromes are estimated to be a reason for 3–5% of RCC cases. To date, ten such syndromes have been described, all inherited with an autosomal dominant trait.
Tobacco smoking and obesity are the most consistently established causal factors, with a dose-dependent effect and strength of the association.[14] Moreover, male gender (Woldrich et al. 2008), hypertension (Navai and Wood 2012), and acquired renal cystic disease involve a marked risk (Maisonneuve et al. 1999). These tumors are relatively rare, among RCC cases 3.5–7.3% have been shown to occur in those younger than 40 years.[15]
The most common symptoms of metastatic disease are asthenia (30%), bone pain (26%), fever (17%), weight loss (15%), abdominal pain (15%), dyspnea (11%), neurological disturbances (10%), and cough (6%). However, 22% of patients with metastatic diseases are asymptomatic or atypical presentation.[16] As was our patient. Furthermore, in a Chinese study, 26% of patients were asymptomatic, 56% had local, and 19% had paraneoplastic symptoms as stated by Ou et al.[17]
Metastatic neoplasms to the oral cavity occur more often in patients over 60-year-old.[18,19] In approximately 20% of cases, these tumors were found before the primary tumors, and most commonly developed in the molar region of the mandible.[19,20,21,22,23,24] Good blood supply to the mandibular molar region is believed to contribute to hematogenous metastasis.[25] Hypoesthesia of the lower lip with no evidence of cyst-like lesion is a crucial indication of a somewhat sinister when osteomyelitis has been ruled out.[26] In the present case, primary or metastatic mandibular tumor was suspected from the progressive hypoesthesia of the lower lip. Nowadays, the diagnostic algorithm is to perform an abdominal ultrasound and then 2/3-phase helical computed tomography (CT) with 5 mm collimation to minimize partial volume artifacts.[27] In our case also, using several radiological examinations such as CT, scintigraphy, and positron emission tomography, the clinical diagnosis of the renal tumor with multiple metastases was obtained.
Histologically, ccRCCs is a renal cortical tumor typically characterized by malignant epithelial cells with clear cytoplasm and a compact alveolar (nested) or acinar growth pattern interspersed with intricate, arborizing vasculature. A variable proportion of cells with granular eosinophilic cytoplasm may be present.
Clinically, ccRCCs present more often with high stage and grade and are more likely to develop distant metastases than papillary or chromophobe tumors. These non-clear cell histologies carry a favorable prognosis after nephrectomy for a localized tumor. The cystic variant of ccRCC seems to be an indolent tumor.[28,29]
The prognosis of patients with metastatic neoplasms in the oral region is poor.[20,21] Most patients die within 1 year since metastatic tumors in the oral region are often preceded or accompanied by multiple metastatic lesions at other sites and organs.[19] Renal clear cell carcinoma, with metastasis at the initial diagnosis, shows an extremely poor prognosis with the median survival time of 6.3 months[9,10,11] The NCCN guideline[30] also indicates radiation therapy for bone metastases in some instances for palliation of symptoms such as pain or cord compression.
M1 placed our patient directly in stage IV category according to the tumor, nodes, and metastases staging.[31] Based on the most widely used Memorial Sloan Kettering Cancer Center model,[32] our patient was classified as having good prognosis as his Karnofsky performance was >70, serum lactate dehydrogenase less than 1.5 times the upper limit of normal (ULN), hemoglobin level was within normal limits, corrected serum calcium level was less than ULN, and time from diagnosis to therapy less than 1 year. Therefore, the expected survival period according to the NCCN guidelines was thought to be about 30 months in this case.[30]
In the present case also, same guidelines were followed, and radiation therapy was performed as surgical measures could not be done. This decreased the size of the lesions and also provided the patient with symptomatic relief. The patient was put on molecular targeted therapy and is still on our follow-up.
CONCLUSION
A case of metastatic renal clear cell carcinoma to the mandible is reported. The age of presentation of the patient as well as the presenting complaint was both unusual. The bottom line being swelling with hypoesthesia should never be underestimated, it can be fatal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Maestre-Rodríguez O, González-García R, Mateo-Arias J, Moreno-García C, Serrano-Gil H, Villanueva-Alcojol L, et al. Metastasis of renal clear-cell carcinoma to the oral mucosa, an atypical location. Med Oral Patol Oral Cir Bucal. 2009;14:e601–4. doi: 10.4317/medoral.14.e601. [DOI] [PubMed] [Google Scholar]
- 3.Pritchyk KM, Schiff BA, Newkirk KA, Krowiak E, Deeb ZE. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112:1598–602. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Ordonez MA, Iasonos A, Secin FP, Guillonneau B, Russo P, et al. Renal cell carcinoma in young and old patients – Is there a difference? J Urol. 2008;180:1262–6. doi: 10.1016/j.juro.2008.06.037. Siemer S, Hack M, Lehmann J, Becker F, Stöckle M Outcome of renal tumors in young adults. J Urol 2006;175:1240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook A, Lorenzo AJ, Salle JL, Bakhshi M, Cartwright LM, Bagi D, et al. Pediatric renal cell carcinoma: Single institution 25-year case series and initial experience with partial nephrectomy. J Urol. 2006;175:1456–60. doi: 10.1016/S0022-5347(05)00671-3. [DOI] [PubMed] [Google Scholar]
- 6.Montie JE, Stewart BH, Straffon RA, Banowsky LH, Hewitt CB, Montague DK. The role of adjunctive nephrectomy in patients with metastatic renal cell carcinoma. J Urol. 1977;117:272–5. doi: 10.1016/s0022-5347(17)58429-3. [DOI] [PubMed] [Google Scholar]
- 7.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J Clin Oncol. 2005;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Schachter LR, Cookson MS, Chang SS, Smith JA, Jr, Dietrich MS, Jayaram G, et al. Second prize: Frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: What to tell our patients. J Endourol. 2007;21:819–23. doi: 10.1089/end.2006.9937. [DOI] [PubMed] [Google Scholar]
- 9.Tomera KM, Farrow GM, Lieber MM. Sarcomatoid renal carcinoma. J Urol. 1983;130:657–9. doi: 10.1016/s0022-5347(17)51388-9. [DOI] [PubMed] [Google Scholar]
- 10.Bertoni F, Ferri C, Benati A, Bacchini P, Corrado F. Sarcomatoid carcinoma of the kidney. J Urol. 1987;137:25–8. doi: 10.1016/s0022-5347(17)43860-2. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 12.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, Einarsson GV. Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: A retrospective nation-wide study of 629 patients. Eur Urol. 2005;48:593–600. doi: 10.1016/j.eururo.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Dhote R, Thiounn N, Debré B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am. 2004;31:237–47. doi: 10.1016/j.ucl.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, Leibovich BC, et al. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 2005;173:1893–6. doi: 10.1097/01.ju.0000158157.57981.80. [DOI] [PubMed] [Google Scholar]
- 16.Citterio G, Bertuzzi A, Tresoldi M, Galli L, Di Lucca G, Scaglietti U, et al. Prognostic factors for survival in metastatic renal cell carcinoma: Retrospective analysis from 109 consecutive patients. Eur Urol. 1997;31:286–91. doi: 10.1159/000474469. [DOI] [PubMed] [Google Scholar]
- 17.Ou YC, Yang CR, Ho HC, Cheng CL, Kao YL, Su CK, et al. The symptoms of renal cell carcinoma related to patients’ survival. J Chin Med Assoc. 2003;66:537–43. [PubMed] [Google Scholar]
- 18.Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: Analysis of 157 cases. J Oral Pathol Med. 1993;22:385–90. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M, Miyata M, Okabe K, Sakashita H. A case series of 9 tumors metastatic to the oral and maxillofacial region. J Oral Maxillofac Surg. 2002;60:942–4. doi: 10.1053/joms.2002.33868. [DOI] [PubMed] [Google Scholar]
- 20.Meyer I, Shklar G. Malignant tumors metastatic to mouth and jaws. Oral Surg Oral Med Oral Pathol. 1965;20:350–62. doi: 10.1016/0030-4220(65)90167-2. [DOI] [PubMed] [Google Scholar]
- 21.Astacio JN, Alfaro C. Oral mucosa metastasis from gastric adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1969;28:859–61. doi: 10.1016/0030-4220(69)90339-9. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura Y, Yakata H, Kawasaki T, Nakajima T. Metastatic tumours of the mouth and jaws. A review of the Japanese literature. J Maxillofac Surg. 1982;10:253–8. doi: 10.1016/s0301-0503(82)80050-7. [DOI] [PubMed] [Google Scholar]
- 23.Zachariades N. Neoplasms metastatic to the mouth, jaws and surrounding tissues. J Craniomaxillofac Surg. 1989;17:283–90. doi: 10.1016/s1010-5182(89)80098-8. [DOI] [PubMed] [Google Scholar]
- 24.Zohar Y, Ben-Tovim R, Gal R, Laurian N. Metastatic carcinoma of oral soft tissue. Head Neck Surg. 1985;7:484–6. doi: 10.1002/hed.2890070609. [DOI] [PubMed] [Google Scholar]
- 25.Roser SM, Nicholas TR, Hirose FM. Metastatic chondrosarcoma to the maxilla: Review of the literature and report of case. J Oral Surg. 1976;34:1012–5. [PubMed] [Google Scholar]
- 26.Glaser C, Lang S, Pruckmayer M, Millesi W, Rasse M, Marosi C, et al. Clinical manifestations and diagnostic approach to metastatic cancer of the mandible. Int J Oral Maxillofac Surg. 1997;26:365–8. doi: 10.1016/s0901-5027(97)80798-9. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich A, Ravery V. European Society of Oncological Urology. Preoperative imaging in renal cell cancer. World J Urol. 2004;22:307–15. doi: 10.1007/s00345-004-0411-2. [DOI] [PubMed] [Google Scholar]
- 28.Beck SD, Patel MI, Snyder ME, Kattan MW, Motzer RJ, Reuter VE, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11:71–7. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 29.Valdair F. Muglia and Adilson Prando. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48:166–74. doi: 10.1590/0100-3984.2013.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH. Practice guidelines committee of the American urological association. Guidelines for management of clinical T1 renal masses. J Urol. 2009;182:1271–9. doi: 10.1016/j.juro.2009.07.004. NCCN Clinical Practice Guidelines in Oncology (version 2.2014) Kidney Cancer. [DOI] [PubMed] [Google Scholar]
- 31.AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. pp. 479–89. [Google Scholar]
- 32.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]


