Abstract
Background:
Anterior cranial bone defects secondary to global war cranial defects pose a unique reconstructive challenge. The objective of this study was to evaluate the outcomes of alloplastic reconstructions of cranial bone with titanium mesh and fat graft after warfare-related cranial trauma.
Patients and Methods:
Thirty-five patients at the plastic and reconstructive surgery ward of our hospital underwent anterior cranioplasty with titanium mesh with or without fat grafts from lower abdominal wall. Inclusion criteria were anterior cranial bone defect due to warfare injuries, the mean age of these patients was 31 years (range, 23–48 years). Ninety-five percent were male, and 5% were female. Average follow-up was 12 months. Fat grafts were used to help obliterate endocranial dead spaces.
Results:
Twenty-five patients (71%) had more than 0.5 cm dead space under cranial defects, and we used fat graft under the titanium mesh. The majority groups of patients (80%) were injured as a result of previous explosive device blasts with or without neurosurgical procedures in the past. The average patient age was 31 years, and 95% of patients were male. The mean anterior cranial defect size was 6 cm × 8 cm, and there were no wound infection or flap necrosis after operations.
Conclusion:
We recommend this procedure (titanium mesh with or without fat graft) for warfare injured cranial defects in secondary anterior cranial reconstructions. Fat grafts eliminates dead space and reduce secondary complications.
Keywords: Cranioplasty, fat grafts, reconstructions, titanium mesh
INTRODUCTION
Anterior cranial bone defects secondary to global war cranial defects or decompression craniectomy pose a unique reconstructive challenge. The objective of this case series study was to evaluate the outcome of alloplastic reconstruction of cranial bone with titanium mesh and fat graft after warfare-related cranial trauma.
These group of patients ultimately need the reconstruction of the cranial defect for brain protection; esthetic restoration; and correction of the syndrome of the trephined, which includes the symptoms of dizziness, fatigability, cranial defect pain, depression, anxiety, and vibration sensitivity.[1]
These craniofacial injuries are unique because of the unusual Gram-negative bacteria (Acinetobacter baumannii) associated with war wounds and multiple concomitant injuries caused by the high-energy weapons used by the insurgents.[2,3]
Clinical outcome after bone replacement was determined by the adequacy of the recovered cranial defects with mesh and fat grafts to achieve satisfactory esthetic contour, the incidence of infection, and the need for revision surgery.
PATIENTS AND METHODS
From January of 2011 to December of 2014, 35 patients at the plastic and reconstructive surgery ward of our hospital underwent anterior cranioplasty with titanium mesh with or without fat grafts from lower abdominal wall. Inclusion criteria were anterior cranial bone defects due to warfare injuries (terrorist attack, explosion, gunshot injuries). Informed consent and ethical committee approval were taken for all patients, and advantage and disadvantage of cranioplasty with titanium mesh were explained to all patients.
At the time of the cranial reconstruction, the mean age of these patients was 31 years (range, 23–48 years). Ninety-five percent were males, and 5% were females. All patients had good condition before reconstructions, and they had no emergency situation. The time between primary injury and neurosurgical operation and our operation was varied between 6 months and 2 years. Duration of follow-up, defined as the interval between cranioplasty and last postoperative patient visit, was a minimum of 6 months to a maximum of 3 years, with an average of 12 months. For all cases, cranial computed tomography (CT)-scan with axial and coronal and three-dimensional views (bone and soft tissue windows) were performed before surgical planning. At the time of reconstructions, the scalp was opened with classic coronal incision and exposure of the cranial defects in the subperiosteal plane were undertaken. We generally used this approach for exposing cranial defects, but in some cases due to old traumatic scars in forehead, we changed incision site for the preservation of blood supply of flaps, the hexagonal titanium mesh proportional with defects (1.5–2 cm larger than defects margins) were then placed and secured with titanium 5 mm internal fixation self-screws.
If there was more than 0.5 cm dead space between mesh and dura in cranial defects, fat graft harvested from lower abdominal wall with mini panensteil incision as indicated was used to help obliterate endocranial dead space and before fixation of mesh these grafts were placed on cranial defects upon dura. The scalp incision was then closed in standard layered fashion with a subcutaneous closed drain (without suction) placed before closure, and drains were removed 24–48 h postoperation in all patients. Clinical outcome after anterior cranioplasty was determined by the adequacy of the prosthetic cranioplasty to achieve satisfactory aesthetic reconstruction, correction of deformity and contour, the incidence of infection, cerebrospinal fluid (CSF) leakage, exposure of mesh, and the need for revision surgery. Photographs were taken before and after reconstructions.
RESULTS
From January of 2011 to December of 2014, 35 patients with old battle injured cranial defects in anterior cranium were referred to our reconstructive ward. Our goals were esthetic reconstruction of anterior cranium for preservation of brain and restoration of good cranial appearance. These patients underwent secondary cranioplasty procedures. Materials used for cranioplasty included alloplastic material (hexagonal titanium mesh and self-screws) with or without fat grafts, of these patients 25 patients (71%) had more than 0.5 cm dead space under cranial defects, and we used fat graft under the titanium mesh [Figure 1]. Fat grafts eliminates dead space and reduce secondary complications, and we had no infection or other sequel in these groups of reconstructions after fat grafting.
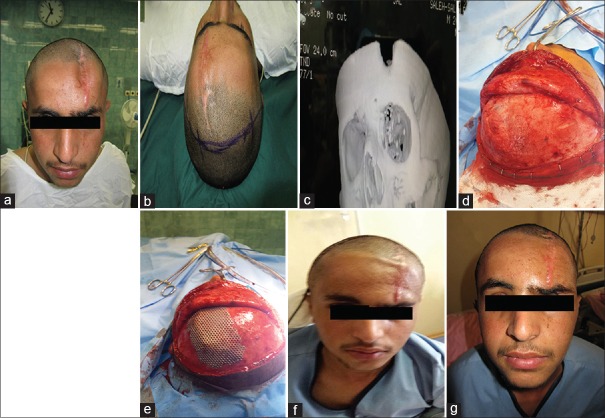
Figure 1.
A 20-year-old man with anterior cranial defect due to previous explosion injury and neurosurgical operation. (a) Before reconstruction, (b) incision line with coronal flap designing, (c) intraoperative view, (d) postoperative X-ray after reconstruction with titanium mesh and fat graft, and (e) long-term result after 6 months
The remaining patients were reconstructed only with titanium mesh [Figure 2] that most of them were in orbital roof area [Figure 3].
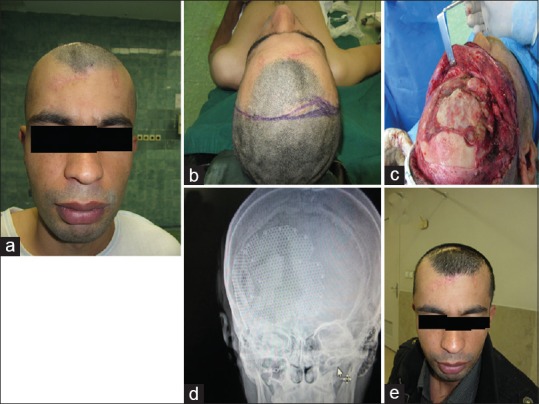
Figure 2.
A 23-year-old man with old gunshot injury and anterior cranial defect. (a) Before reconstruction, (b) coronal flap designing, (c) before reconstruction three-dimensional computed tomography scan, (d) intraoperative view, (e) intraoperative titanium mesh reconstruction, (f) early postoperation, and (g) delayed postoperation after 6 months
Figure 3.
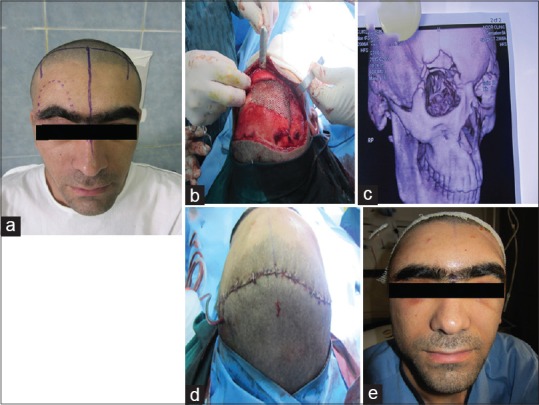
A 25-year-old man with orbital roof defect due to old explosion injury. (a) Before reconstruction, (b) three-dimensional computed tomography-scan view, (c) intraoperative titanium mesh reconstruction, (d and e) postoperation view
The majority groups of patients (80%) were injured as a result of previous explosive device blasts with or without neurosurgical procedures in the past. Other patients sustained injuries related to old gunshot wounds (20%). At the time of the cranial vault reconstruction, the average patient age was 31 years (range, 23–48 years) and 95% of patients were male. The mean anterior cranial defect size was 6 cm × 8 cm. Average follow-up was 12 months postoperation. There were no wound infection or flap necrosis after operation, temporal CSF leakage was seen in one case after repair of dura intraoperatively and it healed without any long-term complication. In two cases, dura were injured intraoperatively in the margin of cranial bone defect that was repaired by round prolen suture (5-0) after more exposure of dura with adjacent bone removal. Minimal exposure of mesh was seen in one case due to multiple traumatic scar in forehead that was repaired with a local flap without any need for mesh removal or long-term complication. Some characteristics of the patients are shown in Table 1.
Table 1.
Some characteristic of patients with anterior secondary cranioplasty

DISCUSSION
The major accepted indications for cranioplasty are protective and cosmetic results for injured patients. Over the past few years due to the violence and terrorist attacks in Iraq, we have seen the largest volume of cranial bone defects referred to Iran for reconstructions. The pattern of warfare craniofacial injuries are different from other traumas, there are usually severe combined soft tissue and bone damages with brain injury and delayed cranioplasty in these patients are associated with escharotics soft tissues that are not easy expandable and vascular preservation during flap elevation is important in skin. The choice of reconstruction depends on several factors including age, size of defects, location of defects, patient's preference, and depth of defects.[4]
Cranioplasty has improved over the past two decades.[5,6,7,8] In reconstructive surgery, replacing missing structures with those most similar to them are fundamental dogma. In the case of cranioplasty, vascularized bone has the highest similarity to the native calvaria and is therefore our preferred reconstructive material when possible. Vascularized bone flaps for cranioplasty can be obtained from fibula[9] or rib.[8] These flaps have excellent strength, are readily integrated into surrounding tissues, and provide resistance to infection.[10]
Furthermore, because of the immediate presence of a blood supply, they are expected to undergo less resorption than would be experienced with nonvascularized bone grafts. Drawbacks to the use of vascularized bone flaps include donor-site morbidity, difficulty with contour, inadequate material, and long operative times.[10] In some severe injured cases, we have to use these flaps, of course the mortality of such patients are high due to combined brain injuries.
When vascularized bone cannot be used, nonvascularized bone grafts are considered next. They have the same virtues and limitations of vascularized bone flaps but may suffer from greater resorption.[11]
Autologous bone graft is still the criterion standard for reconstructing large cranial defects in pediatric patients.[12] Sources of bone include split cranium, rib, and iliac crest. Problems reported with rib grafts include greater resorption than cranial grafts, possible contour irregularity at the donor site, postoperative pain, pleuritic pain, and pneumothorax.[13,14,15,16]
In another study, cranial alloplast implants were used as the reconstruction construct of choice. They demonstrated that low rates of infection using alloplastic materials are possible in carefully selected patients using the criteria such as excellent soft-tissue coverage, no clinical evidence of infection, no biochemical evidence of infection, and no radiographic evidence of infection.[17] In addition, in another study reported a low risk of infection in bone and alloplastic reconstruction of the frontal area.[18] In this study, we used hexagonal titanium mesh with or without fat graft for reconstruction of anterior cranium in warfare injured patients without long-term sequel. We had no infection postoperation, in all cases at least 6 months since acute trauma.
The advantages of autologous bone over alloplastic materials have been explained in previous studies.[19,20,21,22,23,24] Alloplastic materials for cranioplasty were developed to avoid the donor-site morbidity due to autologous reconstruction and also because the quantity of autologous graft is restricted.[12] In this study, our choice for alloplastic material is titanium because of its availability, rigidity, and ease of molding. The atomic number of titanium is low. Thus, it does not form artifacts on either CT scan or magnetic resonance imaging.[25] In some studies, it is recommended that titanium as the method of selection for secondary cranioplasty.[26,27,28] In this study, we used this material in our patients with good results.
Injuries of trauma are best addressed with autologous bone or titanium, because early protection of intracranial contents and early restoration of structural support are important.[29,30,31]
When the size, defect geometry, or patient comorbidities preclude autologous reconstruction, titanium implants coupled with microvascular soft-tissue transfer is preferred.[5] The incidence of infection following cranioplasty in several studies reported in the range of 5% overall.[18] However, the incidence of infection due to cranioplasty in the frontal area has the highest incidence of complication.[18] Factors contributing to the complication per procedural after cranioplasty must be assessed thoroughly.[32] Customized poly ether ketone implants can also be valuable when reconstructing large areas with complicated contour.[10] As other studies, patients with preoperative infection were more likely to sustain complications.[11,33,34,35] Similarly, a meta-analysis study of infections following cranioplasty failed to find a significant difference between alloplastic and autologous reconstructions.[36] A final general consideration in cranioplasty planning is soft-tissue coverage. There were at least 6 months gap between acute trauma and reconstruction in our cases thereby reducing the infection rate and maturation of scar tissues.
In studies in secondary cranioplasty due to warfare injuries when adequate tissue cannot be found in local flaps, they advocate free tissue transfer, particularly anterolateral thigh, latissimus dorsi, or parascapular flaps.[37] In some difficult cases, combined free flap and titanium mesh may be necessary for scalp and cranium reconstructions.
CONCLUSION
With regard to low morbidity of cranioplasty with titanium mesh with fat graft, we recommend this procedure for warfare injured cranial defects in secondary anterior cranial reconstructions. Fat grafts eliminate dead space and reduce secondary complications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488–512. doi: 10.1097/00000658-193910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy JG. Introduction to plastic surgery. Vol. 1. Philadelphia: Saunders; 1990. pp. 10–1. [Google Scholar]
- 3.Kumar AR, Grewal NS, Chung TL, Bradley JP. Lessons from the modern battlefield: Successful upper extremity injury reconstruction in the subacute period. J Trauma. 2009;67:752–7. doi: 10.1097/TA.0b013e3181808115. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimi A, Nejadsarvari N. Experience with cervicofacial flap in cheek reconstruction. J Craniofac Surg. 2013;24:e372–4. doi: 10.1097/SCS.0b013e3182902f88. [DOI] [PubMed] [Google Scholar]
- 5.St-Hilaire H, Mithani SK, Taylor J, Simmons OP, Singh N, Rodriguez ED. Restoring the failed cranioplasty: Nonanatomical titanium mesh with perforator flap. Plast Reconstr Surg. 2009;123:1813–7. doi: 10.1097/PRS.0b013e3181a65bce. [DOI] [PubMed] [Google Scholar]
- 6.Eppley BL, Kilgo M, Coleman JJ., 3rd Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: Indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002;109:864–71. doi: 10.1097/00006534-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lai JB, Sittitavornwong S, Waite PD. Computer-assisted designed and computer-assisted manufactured polyetheretherketone prosthesis for complex fronto-orbito-temporal defect. J Oral Maxillofac Surg. 2011;69:1175–80. doi: 10.1016/j.joms.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Seitz IA, Adler N, Odessey E, Reid RR, Gottlieb LJ. Latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap reconstruction in composite defects of the scalp: Case series and review of literature. J Reconstr Microsurg. 2009;25:559–67. doi: 10.1055/s-0029-1236834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluebond-Langner R, Zamani A, Rodriguez ED. Frontal bandeau reconstruction with a fibula flap in a patient with Freeman-Sheldon syndrome. J Craniofac Surg. 2009;20:256–8. doi: 10.1097/SCS.0b013e31818436d7. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Khalifian S, Flores JM, Bellamy J, Manson PN, Rodriguez ED, et al. Clinical outcomes in cranioplasty: Risk factors and choice of reconstructive material. Plast Reconstr Surg. 2014;133:864–73. doi: 10.1097/PRS.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 11.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–53. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rogers GF, Greene AK, Mulliken JB, Proctor MR, Ridgway EB. Exchange cranioplasty using autologous calvarial particulate bone graft effectively repairs large cranial defects. Plast Reconstr Surg. 2011;127:1631–42. doi: 10.1097/PRS.0b013e31821084f0. [DOI] [PubMed] [Google Scholar]
- 13.Zins JE, Whitaker LA. Membranous versus endochondral bone: Implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72:778–85. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100:163–8. doi: 10.3171/ped.2004.100.2.0163. [DOI] [PubMed] [Google Scholar]
- 15.Netscher DT, Stal S, Shenaq S. Management of residual cranial vault deformities. Clin Plast Surg. 1992;19:301–13. [PubMed] [Google Scholar]
- 16.Pochon JP, Klöti J. Cranioplasty for acquired skull defects in children – A comparison between autologous material and methylmethacrylate 1974-1990. Eur J Pediatr Surg. 1991;1:199–201. doi: 10.1055/s-2008-1042487. [DOI] [PubMed] [Google Scholar]
- 17.Kumar AR, Bradley JP, Harshbarger R, Stevens F, Bell R, Moores L, et al. Warfare-related craniectomy defect reconstruction: Early success using custom alloplast implants. Plast Reconstr Surg. 2011;127:1279–87. doi: 10.1097/PRS.0b013e318205f47c. [DOI] [PubMed] [Google Scholar]
- 18.Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: Risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888–904. [PubMed] [Google Scholar]
- 19.Lykins CL, Friedman CD, Costantino PD, Horioglu R. Hydroxyapatite cement in craniofacial skeletal reconstruction and its effects on the developing craniofacial skeleton. Arch Otolaryngol Head Neck Surg. 1998;124:153–9. doi: 10.1001/archotol.124.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Kirschner RE, Karmacharya J, Ong G, Gordon AD, Hunenko O, Losee JE, et al. Repair of the immature craniofacial skeleton with a calcium phosphate cement: Quantitative assessment of craniofacial growth. Ann Plast Surg. 2002;49:33–8. doi: 10.1097/00000637-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Losee JE, Karmacharya J, Gannon FH, Slemp AE, Ong G, Hunenko O, et al. Reconstruction of the immature craniofacial skeleton with a carbonated calcium phosphate bone cement: Interaction with bioresorbable mesh. J Craniofac Surg. 2003;14:117–24. doi: 10.1097/00001665-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Smartt JM, Jr, Karmacharya J, Gannon FH, Ong G, Jackson O, Bartlett SP, et al. Repair of the immature and mature craniofacial skeleton with a carbonated calcium phosphate cement: Assessment of biocompatibility, osteoconductivity, and remodeling capacity. Plast Reconstr Surg. 2005;115:1642–50. doi: 10.1097/01.prs.0000161466.74294.1e. [DOI] [PubMed] [Google Scholar]
- 23.Elshahat A, Shermak MA, Inoue N, Chao EY, Manson P. The use of Novabone and Norian in cranioplasty: A comparative study. J Craniofac Surg. 2004;15:483–9. doi: 10.1097/00001665-200405000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Baker SB, Weinzweig J, Kirschner RE, Bartlett SP. Applications of a new carbonated calcium phosphate bone cement: Early experience in pediatric and adult craniofacial reconstruction. Plast Reconstr Surg. 2002;109:1789–96. doi: 10.1097/00006534-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chandler CL, Uttley D, Archer DJ, MacVicar D. Imaging after titanium cranioplasty. Br J Neurosurg. 1994;8:409–14. doi: 10.3109/02688699408995107. [DOI] [PubMed] [Google Scholar]
- 26.Blake GB, MacFarlane MR, Hinton JW. Titanium in reconstructive surgery of the skull and face. Br J Plast Surg. 1990;43:528–35. doi: 10.1016/0007-1226(90)90115-g. [DOI] [PubMed] [Google Scholar]
- 27.Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26:E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 28.Eufinger H, Wehmöller M. Individual prefabricated titanium implants in reconstructive craniofacial surgery: Clinical and technical aspects of the first 22 cases. Plast Reconstr Surg. 1998;102:300–8. doi: 10.1097/00006534-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez ED, Stanwix MG, Nam AJ, St Hilaire H, Simmons OP, Christy MR, et al. Twenty-six-year experience treating frontal sinus fractures: A novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122:1850–66. doi: 10.1097/PRS.0b013e31818d58ba. [DOI] [PubMed] [Google Scholar]
- 30.Tantawi D, Armonda R, Valerio I, Kumar AR. Management of decompressive craniectomy defects: Modern military treatment strategies. J Craniofac Surg. 2012;23(7 Suppl 1):2042–5. doi: 10.1097/SCS.0b013e318258ba36. [DOI] [PubMed] [Google Scholar]
- 31.Al-Tamimi YZ, Sinha P, Trivedi M, Robson C, Al-Musawi TA, Hossain N, et al. Comparison of acrylic and titanium cranioplasty. Br J Neurosurg. 2012;26:510–3. doi: 10.3109/02688697.2011.633640. [DOI] [PubMed] [Google Scholar]
- 32.Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, et al. Complications following cranioplasty: Incidence and predictors in 348 cases. J Neurosurg. 2015;123:182–8. doi: 10.3171/2014.9.JNS14405. doi: 10.3171/2014.9.JNS14405. [DOI] [PubMed] [Google Scholar]
- 33.Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008;15:1115–9. doi: 10.1016/j.jocn.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008;122:195e–208e. doi: 10.1097/PRS.0b013e3181858eee. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez ED, Stanwix MG, Nam AJ, St Hilaire H, Simmons OP, Manson PN. Definitive treatment of persistent frontal sinus infections: Elimination of dead space and sinonasal communication. Plast Reconstr Surg. 2009;123:957–67. doi: 10.1097/PRS.0b013e318199f4cd. [DOI] [PubMed] [Google Scholar]
- 36.Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011;68:1124–9. doi: 10.1227/NEU.0b013e31820a5470. [DOI] [PubMed] [Google Scholar]
- 37.Giessler GA, Cornelius CP, Suominen S, Borsche A, Fieger AJ, Schmidt AB, et al. Primary and secondary procedures in functional and aesthetic reconstruction of noma-associated complex central facial defects. Plast Reconstr Surg. 2007;120:134–43. doi: 10.1097/01.prs.0000263657.49956.8d. [DOI] [PubMed] [Google Scholar]