Abstract
Objective:
To evaluate, through radiographic and histological analysis, the tissue reaction induced by a biomaterial based on deproteinized bovine bone matrix (DBBM) in the muscle of sheep.
Materials and Methods:
Sixteen sheep were used. The animals underwent surgery to insert polyethylene tubes containing the biomaterial in the muscle of the lower back (ectopic site) and were euthanized after 3 and 6 months. Each sheep received three tubes: Group 1 - sham group (negative control - tube without biomaterial), Group 2 - particulate autogenous bone (positive control), and Group 3 - DBBM biomaterial (GenOx Inorg). The material removed was evaluated by radiographic, macroscopic, and microscopic analysis, descriptively.
Results:
Macroscopic analysis showed that Group 3 had a greater tissue volume maintenance. Microscopic analysis indicated that Group 1 had a higher concentration of dense, thin collagen fibers (3 and 6 months); in Group 2, there was a decrease in the inflammatory process and the deposition of dense, thin collagen fibers (3 and 6 months); in Group 3, the presence of a dense connective tissue was noted, in which the DBBM particles (3 months) were found. On the periphery of these particles, a deposition of basophilic material was found, indicating the formation of mineral particles and the formation of tissues with osteoid characteristics (6 months).
Conclusion:
Based on the results obtained, it can be concluded that the biomaterial based on DBBM led to the formation of tissue with similar characteristics to an osteoid matrix in a postoperative period of 6 months. However, none of the groups evaluated showed ectopic bone neoformation.
Keywords: Bone matrix, ectopic graft, sheep
INTRODUCTION
In recent years, with the aim of restoring and preserving the morphology of alveolar bone, new materials, and techniques for bone grafting have been developed.[1,2,3] The biological rationale for the use of bone grafting is based on three healing mechanisms: Osteogenesis, osteoinduction, and osteoconduction. Osteogenesis is the process of direct bone formation since osteoprogenitor cells and osteoblasts are present. Osteoinduction is defined as a recruitment process for mesenchymal cells and their differentiation into osteoblastic cells, resulting in bone formation. Osteoconduction is the process of forming a scaffold for new bone growth.[4,5,6]
Bone grafts are classified according to their origin such as autologous, allogeneic (of the same species), alloplastic (synthetic), and xenogenous (individuals of different species). Despite the fact that autogenous grafts are considered the gold standard for bone reconstruction, they require an extra surgical approach for their collection, and thus can increase morbidity, in addition to the fact that they offer a limited amount of bone. Their main advantage is the promotion of osteogenesis, osteoinduction, and osteoconduction.[7,8,9]
The three types of allograft biomaterials most commonly referenced are frozen bone; dry and frozen bone; and demineralized dry, frozen bone. Their main disadvantages are their potential for antigenicity and for transmitting diseases, as reported in the literature.[10] Among the alloplastic biomaterials, hydroxyapatite, calcium phosphates, calcium sulfates, and bioactive glass can be included,[11] and their main property is osteoconduction.[12]
Xenogenic materials have the advantage of unlimited supply source and osteoconductive potential, in addition to being biocompatible and not able to promote excessive inflammatory reaction.[13] They are still considered safe for immune response induction and against the transmission of diseases, as long as laboratory processing is adequate.[14] Thus, xenogeneic bovine bone grafting has been researched to investigate its physical and biological properties.
Given the need for bone tissue repair and the numerous biomaterials currently available, this study aimed to evaluate, through radiographic and histological analysis, the tissue reaction induced by a biomaterial based on deproteinized bovine bone matrix (DBBM) in the muscle of sheep.
MATERIALS AND METHODS
This study was submitted to the Research Ethics Committee on Animal Use at the Positivo University (Protocol 21/2011) in accordance with the provisions of the Arouca Law (11794/2008) and ethical principles of the Brazilian Society of Laboratory Animal Science.
Study design
Sixteen sheep approximately 2 years old, with an average weight of 45 kg, were used. Each sheep received three polyethylene tubes, according to the study groups, in the muscle of the lower back: Group 1 - sham group (negative control, tube without biomaterial), Group 2 - particulate autogenous bone (positive control), and Group 3 - DBBM granules (GenOx Inorg, Baumer, Mogi-Mirim, SP, Brazil). The sheep were euthanized 3 and 6 months after surgery (8 sheep per follow-up time). The animals were randomly divided into the follow-up times by means of a randomization form (Randomizer version 4.0, www.randomizer.org/form.htm).
Surgical procedures
Before the surgery, the animals were on solid fasting for 24 h and absolute fasting for 8 h. The animals received the preanesthetic medication acepromazine (0.55 mg/kg) (Acepran 1%, Vetnil, Louveira, SP, Brazil) and ketamine (20 mg/kg) (injectable Dopalen, Vetbrands, Paulinia, SP, Brazil) intramuscularly. For the anesthetic induction and maintenance, sodium thiopental (5 mg/kg) was used intravenously (Thiopentax, Cristália, São Paulo, SP, Brazil), as well as isoflurane vaporization with a flow of 3 L/min of oxygen (Isoflurano, BioChimico, Itatiaia, RJ, Brazil). During the surgical procedure, the animals received ketoprofen 10% (3 mg/kg) (10% Biofen, Biofarm Química e Farmacêutica Ltda., Jaboticabal, SP, Brazil) and enrofloxacin 10% (2.5 mg/kg) (injectable Chemitril 10%, Chemitec Agro Veterinary Ltda., São Paulo, SP, Brazil) intramuscularly.
The boundary of the surgical area included the promontory of the sacrum bone as an anatomical reference. The surgical area was located 3 cm cranially to the sacral promontory and was expanded 20 cm cranially and 6 cm bilaterally to the median plane (spine). After shaving and disinfecting the surgical site, three incisions on the right and left sides of the spine were made, measuring 2–3 cm, and 4 cm apart from each other [Figure 1]. Incisions were made on the muscular fascia (parallel to the spine) to allow the division of the muscle fibers in the lumbar region (longissimus dorsi) and provide intramuscular space.
Figure 1.

Boundaries and markings before the incisions were made
A single operator performed the insertion of biomaterial in sterile polyethylene tubes, with dimensions of approximately 5 mm in diameter and 10 mm in length [Figure 2]. The autogenous bone inserted in the tubes was removed from the jaw of the same animal and crushed with a pestle bone grinder (Kopp Dental Implants, Curitiba, PR, Brazil). A simple suture of the longissimus dorsi and fascia muscles was performed with 5-0 Vicryl absorbable suture (Ethicon, São Paulo, SP, Brazil) [Figure 3], followed by a continuous skin suture with 5-0 nylon thread (Shalon Surgical Threads Ltda., São Luiz de Montes Belos, GO, Brazil).
Figure 2.

Polyethylene tube filled with GenOx Inorg.
Figure 3.
Suture made on the longissimus dorsi muscle
After surgery, ketoprofen 10% (3 mg/kg) was administered intramuscularly once a day for 3 days (10% Biofen, Biofarm Química e Farmacêutica Ltda., Jaboticabal, SP, Brazil) and enrofloxacin 10% (2.5 mg/kg) once a day for 5 days (injectable Chemitril 10%, Chemitec Agro Veterinária Ltda., São Paulo, SP, Brazil) intramuscularly.
According to the randomization of the animals, they were euthanized after 3 or 6 months with sodium thiopental (8 mg/kg) through a rapid intravenous injection (Thiopentax, Cristália, São Paulo, SP, Brazil). After euthanasia, tissue samples containing the tubes were collected and immediately immersed in 10% formalin fixative solution. After 48 h, the contents of the tubes were removed, kept in the fixative solution and X-rayed.
Processing and histological analysis
Histological processing began after fixing the samples with 10% formalin. Then, washing was conducted with running water for formalin removal. For histological analysis by light microscopy, decalcification of the samples was done in trichloroacetic acid for 40 days followed by blocking in paraffin. Serial sections were obtained with a microtome, and the slides were stained with hematoxylin and eosin. The slides were descriptively analyzed, assessing the tissue reaction and possible ectopic bone formation.
RESULTS
According to the group and follow-up time, the results of radiographic and histological evaluation are described below.
Group 1 (sham group, negative control, tube without biomaterial)
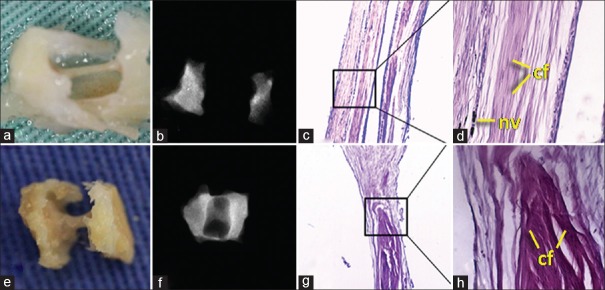
For both 3 and 6 months, macroscopic and radiographic analyses were similar. For the 3-month subgroup, radiographic analysis showed low radiopacity, suggestive of nonmineralized tissue. Macroscopic tissue analysis revealed a very thin line of fibrous tissue. Microscopic analysis indicated the presence of a dense connective tissue permeated by blood vessels, patterned with parallel, uniform fibers. There was no presence of inflammatory process. For the 6-month subgroup, radiographic analysis indicated that low radiopacity was maintained, being similar to the 3-month subgroup. Macroscopic tissue analysis showed a slightly denser tissue. However, in microscopic analysis, a higher concentration of dense, thin collagen fibers was noted, characterizing fibrosis, possibly due to the tissue reaction from placing the polyethylene tube in the ectopic site [Figure 4].
Figure 4.
Group 1 (sham). (a and b) Macroscopic and radiographic aspects (3 months). (c and d) H and E, ×40 and ×200 (3 months). (e and f) Macroscopic and radiographic aspects (6 months). (g and h) H and E, ×40 and ×200 (6 months). cf = Connective fibers; nv = Neovascularization
Group 2 (autogenous bone)
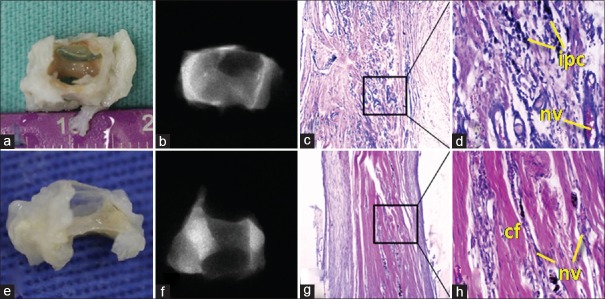
Radiographic and macroscopic results were very similar to Group 1. However, macroscopic tissue evaluation of 3- and 6-month subgroups showed a band of dense fibrous tissue slightly thicker than that of Group 1, but the volume of the biomaterial was not maintained. For the 3-month subgroup, microscopic analysis revealed the presence of dense connective tissue that was fibrous and moderately vascularized, where a chronic inflammatory process was permeated with a prevalence of mononuclear macrophagic and lymphoplasmacytic cells. For the 6-month subgroup, there was a significant decrease in the inflammatory process as well as an exuberant deposition of dense, thin collagen fibers, suggesting a fibrotic response. The presence of osteoid formation or even the deposition of well-formed bone matrix was not observed for both follow-up times. The low radiopacity did not indicate the presence of calcified tissue [Figure 5].
Figure 5.
Group 2 (autogenous bone). (a and b) Macroscopic and radiographic aspects (3 months). (c and d) H and E, ×40 and ×200 (3 months). (e and f) Macroscopic and radiographic aspects (6 months). (g and h) H and E, ×40 and ×200 (6 months). cf = Connective fibers; nv = Neovascularization; ipc = Inflammatory process cells
Group 3 (deproteinized bovinebone matrix)
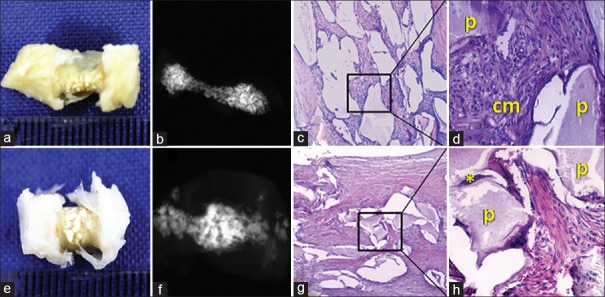
Macroscopic tissue evaluation showed similar characteristics for both follow-up periods. There was a thick tissue in the center of the tube, and maintenance of volume due to the presence of the mineralized particles of xenogenic material. Radiographically, the 6-month subgroup presented a more radiopaque core area compared to the other groups. In the microscopic analysis of the 3-month subgroup, xenogenic material particles were noted, surrounded by dense connective tissue that was highly cellular and with the presence of a granulomatous inflammatory infiltrate consisting of lymphocytes, plasma cells, and macrophages. However, in the 6-month subgroup, a dense connective tissue was found, in which there were fragments of the xenogenic biomaterial particles. In peripheral regions of these particles, the deposition of basophilic material was seen, indicating the formation of mineral particles and tissues with osteoid characteristics [Figure 6].
Figure 6.
Group 3 (deproteinized bovine bone matrix). (a and b) Macroscopic and radiographic aspects (3 months). (c and d) H and E, ×40 and ×200 (3 months). (e and f) Macroscopic and radiographic aspects (6 months). (g and h) H and E, ×40 and ×200 (6 months). p = deproteinized bovine bone matrix particle; * = Tissue formation with characteristics similar to osteoids; cm = connective matrix
DISCUSSION
The ideal material for bone grafting has not yet been developed because it has not been possible to aggregate all the important characteristics into a single biomaterial. It is known that in many situations of bone defects, grafting is not done, hoping that the clot itself induces bone formation in noncritical bone sites. In the negative control group (Group 1), after 3 and 6 months, the presence of dense connective tissue with parallel, uniform fibers was observed. This can be attributed to the fact that there was a reaction caused by the presence of the empty polyethylene tube in the tissue, which demonstrates the biocompatibility of the tube since a chronic inflammatory process was not observed.
The autogenous bone, currently considered the gold standard because of its healing properties (osteogenesis, osteoconduction, and osteoinduction), was used in Group 2 as a positive control.[8,9] However, in this study, ectopic bone formation was not observed. Thus, in the present work, autogenous bone cannot be considered as an osteoinducer, as the result of its application was similar to the negative control/sham group (Group 1). Although unlike the control group, in Group 2, after 3 months, a chronic inflammatory process was observed, with the prevalence of mononuclear (macrophage) and lymphoplasmacytic cells. In Group 2, after 6 months, there was a chronic inflammatory process, but to a lesser extent due to the healing time. The fact that a chronic inflammatory process was seen at both follow-up times can be explained by the presence of autogenous bone inside the tube. The presence of osteoids or even the deposition of a well-formed bone matrix was also not observed, as the volume inside the tube was not maintained, which diverges from the data reported in the literature.[15] One possible explanation for this may be the fact that the autogenous bone was out of the bone site, in an ectopic environment. The size of autogenous bone particles inserted in the tube may also have contributed since the autogenous bone went through a grinding process which may have been unfavorable for obtaining the correct particle size for osteoconduction to be achieved.[16] In addition, Miron et al.,[17] demonstrated that the differentiation and mineralization of primary osteoblasts seeded on autogenous grafts are largely influenced by the methods used in harvesting the bone grafts. Other important factors that may have contributed to this result may be the absence or low amount of living cells, which did not collaborate for osteogenesis, or even the absence or loss of morphogenetic bone proteins during maceration.[18,19]
The use of xenogeneic bone, such as GenOx Inorg, has also been studied. This material is characterized by being based on DBBM obtained through deproteinization at high temperatures (between 950°C and 1000°C). After repeated stringent washes to eliminate blood and fat cells as well as other impurities, it is decalcified and dehydrated through lyophilization process, preventing denaturation of proteins while retaining the active ingredient in the particles. The high processing temperature increases the crystallinity, as well as implicating hydroxyapatite decomposition in other phases, such as with tricalcium phosphate (TCP). A biomaterial that is more crystalline will be less degradable, but the presence of other phases (generally more soluble than hydroxyapatite) can offset or reduce this effect.[20]
The literature reports that these are highly osteoconductive grafts, providing a favorable surface due to their structure and chemical composition, on which new bone can be deposited, thus acting as a framework that facilitates new bone formation.[21] Among the disadvantages of using bovine materials, the risk of disease transmission can be cited. Wenz et al.[14] report that the processing of these materials does not imply a risk of bovine spongiform encephalopathy transmission to graft recipients. Many studies are being conducted with deproteinized bovine materials,[22,23,24,25,26] but little is known about the use of this material in ectopic sites, as in the experimental model used in the present study. The use of biomaterials in ectopic sites is recent. Spalthoff et al.,[27] in an ectopic site model, noted that the amount of bone formation and degeneration of the ceramic matrix is totally dependent on the vascularization of the area, supporting the hypothesis that resorption is mediated by cells.
The Group 3 group, in which DBBM was used, was the only one that showed tissue volume maintenance within the tube. This is justified by the presence of mineral particles, as the inorganic bovine bone is free of proteins and cells and is characterized by high hydroxyapatite content.[20] It was observed in Group 3, after 3 months, the presence of a highly cellular, dense connective tissue around the particles, a fact that suggests the possible formation of a cementitious line around the particles. In Group 3, after 6 months, on the peripheral regions of the particles, deposition of a basophilic material was noted, indicating the formation of a small portion of minerals and tissue formation with characteristics similar to an osteoid, a fact that corroborates the literature.[15] However, with the data obtained so far, it was not possible to conclude that GenOx Inorg presented characteristics of an osteoinductive material in an ectopic site. It is noteworthy that in recent studies associating β-TCP with iliac autogenous bone; ectopic bone formation was satisfactory.[27]
Finally, it should be pointed out that this study has limitations, among which are the limited number of samples, the use of a single xenogeneic biomaterial in an ectopic site and only two follow-up times. Therefore, more studies are needed to evaluate the biological properties, tissue response, and possible induction of ectopic bone formation associated with the use of the DBBM evaluated.
CONCLUSION
It is possible to infer that the biomaterial based on DBBM led, in this study, to the formation of tissue with characteristics that are similar to an osteoid when placed in contact with the muscle tissue in sheep in a postoperative period of up to 6 months.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Horowitz RA, Leventis MD, Rohrer MD, Prasad HS. Bone grafting: History, rationale, and selection of materials and techniques. Compend Contin Educ Dent. 2014;35(4 Suppl):1–6. [PubMed] [Google Scholar]
- 2.Martinez A, Balboa O, Gasamans I, Otero-Cepeda XL, Guitian F. Deproteinated bovine bone vs. Beta-tricalcium phosphate as bone graft substitutes: Histomorphometric longitudinal study in the rabbit cranial vault. Clin Oral Implants Res. 2015;26:623–32. doi: 10.1111/clr.12349. [DOI] [PubMed] [Google Scholar]
- 3.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol 2000. 2015;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 4.Baldini N, De Sanctis M, Ferrari M. Deproteinized bovine bone in periodontal and implant surgery. Dent Mater. 2011;27:61–70. doi: 10.1016/j.dental.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Ilan DI, Ladd AL. Bone graft substitutes. Oper Tech Plast Reconstr Surg. 2002;9:151–60. [Google Scholar]
- 6.Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011;42(Suppl 2):S16–21. doi: 10.1016/j.injury.2011.06.199. [DOI] [PubMed] [Google Scholar]
- 7.Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466:2973–80. doi: 10.1007/s11999-008-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rokn AR, Khodadoostan MA, Reza Rasouli Ghahroudi AA, Motahhary P, Kharrazi Fard MJ, Bruyn HD, et al. Bone formation with two types of grafting materials: A histologic and histomorphometric study. Open Dent J. 2011;5:96–104. doi: 10.2174/1874210601105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang HL, Cooke J. Periodontal regeneration techniques for treatment of periodontal diseases. Dent Clin North Am. 2005;49:637–59, vii. doi: 10.1016/j.cden.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Vaishnav S, Thomas Vangsness C, Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28:127–41. doi: 10.1016/j.csm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: The reconstructive surgeon's point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: Bone replacement grafts. Dent Clin North Am. 2010;54:55–71. doi: 10.1016/j.cden.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Wanschitz F, Nell A, Patruta S, Wagner A, Ewers R. Influence of three currently used bone replacing materials on the in vitro proliferation of human peripheral blood mononuclear cells. Clin Oral Implants Res. 2005;16:570–4. doi: 10.1111/j.1600-0501.2005.01150.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenz B, Oesch B, Horst M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials. 2001;22:1599–606. doi: 10.1016/s0142-9612(00)00312-4. [DOI] [PubMed] [Google Scholar]
- 15.Pripatnanont P, Nuntanaranont T, Vongvatcharanon S. Proportion of deproteinized bovine bone and autogenous bone affects bone formation in the treatment of calvarial defects in rabbits. Int J Oral Maxillofac Surg. 2009;38:356–62. doi: 10.1016/j.ijom.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Ullmark G. Bigger size and defatting of bone chips will increase cup stability. Arch Orthop Trauma Surg. 2000;120:445–7. doi: 10.1007/s004029900122. [DOI] [PubMed] [Google Scholar]
- 17.Miron RJ, Hedbom E, Saulacic N, Zhang Y, Sculean A, Bosshardt DD, et al. Osteogenic potential of autogenous bone grafts harvested with four different surgical techniques. J Dent Res. 2011;90:1428–33. doi: 10.1177/0022034511422718. [DOI] [PubMed] [Google Scholar]
- 18.Jakse N, Seibert FJ, Lorenzoni M, Eskici A, Pertl C. A modified technique of harvesting tibial cancellous bone and its use for sinus grafting. Clin Oral Implants Res. 2001;12:488–94. doi: 10.1034/j.1600-0501.2001.120509.x. [DOI] [PubMed] [Google Scholar]
- 19.Mazock JB, Schow SR, Triplett RG. Proximal tibia bone harvest: Review of technique, complications, and use in maxillofacial surgery. Int J Oral Maxillofac Implants. 2004;19:586–93. [PubMed] [Google Scholar]
- 20.Accorsi-Mendonça T, Conz MB, Barros TC, de Sena LA, Soares Gde A, Granjeiro JM. Physicochemical characterization of two deproteinized bovine xenografts. Braz Oral Res. 2008;22:5–10. doi: 10.1590/s1806-83242008000100002. [DOI] [PubMed] [Google Scholar]
- 21.Busenlechner D, Tangl S, Mair B, Fugger G, Gruber R, Redl H, et al. Simultaneous in vivo comparison of bone substitutes in a guided bone regeneration model. Biomaterials. 2008;29:3195–200. doi: 10.1016/j.biomaterials.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Donos N, Lang NP, Karoussis IK, Bosshardt D, Tonetti M, Kostopoulos L. Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects. Clin Oral Implants Res. 2004;15:101–11. doi: 10.1111/j.1600-0501.2004.00986.x. [DOI] [PubMed] [Google Scholar]
- 23.Keller J, Brink S, Busse B, Schilling AF, Schinke T, Amling M, et al. Divergent resorbability and effects on osteoclast formation of commonly used bone substitutes in a human in vitro assay. PLoS One. 2012;7:e46757. doi: 10.1371/journal.pone.0046757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munhoz EA, Bodanezi A, Cestari TM, Taga R, de Carvalho PS, Ferreira O., Jr Long-term rabbits bone response to titanium implants in the presence of inorganic bovine-derived graft. J Biomater Appl. 2012;27:91–8. doi: 10.1177/0885328210396946. [DOI] [PubMed] [Google Scholar]
- 25.Rocha FS, Ramos LM, Batista JD, Zanetta-Barbosa D, Ferro EA, Dechichi P. Bovine anorganic bone graft associated with platelet-rich plasma: Histologic analysis in rabbit calvaria. J Oral Implantol. 2011;37:511–8. doi: 10.1563/AAID-JOI-D-09-00091.1. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000;11:217–29. doi: 10.1034/j.1600-0501.2000.011003217.x. [DOI] [PubMed] [Google Scholar]
- 27.Spalthoff S, Jehn P, Zimmerer R, Möllmann U, Gellrich NC, Kokemueller H. Heterotopic bone formation in the musculus latissimus dorsi of sheep using ß-tricalcium phosphate scaffolds: Evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int J Oral Maxillofac Surg. 2015;44:791–7. doi: 10.1016/j.ijom.2014.11.012. [DOI] [PubMed] [Google Scholar]