Abstract
Severe bronchospasm refractory to β-agonists is a challenging aspect of asthma therapy, and novel therapeutics are needed. β-agonist–induced airway smooth muscle (ASM) relaxation is associated with increases in the phosphorylation of the small heat shock–related protein (HSP) 20. We hypothesized that a transducible phosphopeptide mimetic of HSP20 (P20 peptide) causes relaxation of human ASM (HASM) by interacting with target(s) downstream of the β2-adrenergic receptor (β2AR) pathway. The effect of the P20 peptide on ASM contractility was determined in human and porcine ASM using a muscle bath. The effect of the P20 peptide on filamentous actin dynamics and migration was examined in intact porcine ASM and cultured primary HASM cells. The efficacy of the P20 peptide in vivo on airway hyperresponsiveness (AHR) was determined in an ovalbumin (OVA) sensitization and challenge murine model of allergic airway inflammation. P20 peptide caused dose-dependent relaxation of carbachol-precontracted ASM and blocked carbachol-induced contraction. The β2AR inhibitor, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride (ICI 118,551), abrogated isoproterenol but not P20 peptide–mediated relaxation. The P20 peptide decreased filamentous actin levels in intact ASM, disrupted stress fibers, and inhibited platelet-derived growth factor–induced migration of HASM cells. The P20 peptide treatment reduced methacholine-induced AHR in OVA mice without affecting the inflammatory response. These results suggest that the P20 peptide decreased airway constriction and disrupted stress fibers through regulation of the actin cytoskeleton downstream of β2AR. Thus, the P20 peptide may be a potential therapeutic for asthma refractory to β-agonists.
Keywords: asthma, airway smooth muscle relaxation, ovalbumin mouse model, peptide therapeutic, filamentous actin
Clinical Relevance
Inhaled β-agonists are the mainstay of asthma therapy. However, regular use of these drugs has detrimental effects in some patients with asthma, owing to desensitization and/or genetic polymorphism of the β2-adrenergic receptor. This article demonstrates that a cell-permeant phosphomimetic peptide of heat shock–related protein 20, P20 peptide, reduced airway hypercontractility by regulating the actin cytoskeleton via mechanism(s) downstream of the adrenergic receptor pathway. Furthermore, inhaled delivery of P20 peptide attenuated methacholine-induced airway hyperresponsiveness in a mouse model of allergic airway inflammation that mimics human asthma. Thus, P20 peptide may be a potential therapeutic for asthma refractory to β-agonists.
Asthma is a chronic and complex airway inflammatory disease with bronchoconstriction (1). β-agonists are the most commonly used therapy for acute bronchospasm associated with asthma, but regular use of β-agonists causes detrimental effects on asthma control (2, 3). Furthermore, tolerance to long-acting β-agonists can develop rapidly, occurring even after a single dose (4). Decreased response to β-agonists is partly due to desensitization of the β2-adrenergic receptor (β2AR) (5, 6). High-level activation of the β2AR during regular β-agonist therapy increases the expression of phospholipase C-β in airway smooth muscle (ASM), which might augment the effects of bronchoconstrictors, leading to airway hyperresponsiveness (AHR) (7). Cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PK) A and/or G protein receptor kinase–mediated phosphorylation of β2AR may lead to increased inhibitory G protein, Gi coupling and/or β-arrestin binding, which uncouples the β2AR from its G protein, thereby terminating signaling responses. Arrestin also serves as a scaffolding protein to facilitate internalization of the receptor into endosomes by endocytosis (5, 8, 9). This highlights a need for novel additional therapeutics to manage airway constriction in subjects with asthma refractory to β-agonists.
β-agonists stimulate the β2AR through the heterotrimeric G protein, which, in turn, activates adenylate cyclase and elevates cAMP (5, 10). In ASM, elevated cytosolic cAMP levels activate PKA, which phosphorylates several membrane and/or intracellular proteins, including myosin light chain kinase, myosin light chain phosphatase, high-conductance voltage- and Ca2+-activated big potassium (BKCa) channels, inositol trisphosphate (IP3) receptor, and heat shock–related protein (HSP) 20, promoting ASM relaxation and bronchodilation (10, 11). The proposed mechanisms of PKA-induced relaxation include reduction of intracellular Ca2+ levels, reduction of the sensitivity of the contractile unit to intracellular Ca2+ (5, 10, 12, 13), and changes to the actin cytoskeletal dynamics (14, 15).
It has been established that the small HSP20 (or HSPB6) is a substrate of PKA that mediates relaxation of vascular smooth muscle as well as ASM (14, 16–18). In addition, HSP20 is phosphorylated at serine (Ser) 16 in response to treatment with the β-agonist, isoproterenol (ISO), in bovine and canine ASM (14, 15). HSP20 phosphorylation plays an important role in the cAMP-dependent mechanism of ASM relaxation, and HSP20 may be a target for further development of a new class of bronchodilators (19). The mechanism by which HSP20 mediates relaxation in smooth muscle has not been fully elucidated, but several reports have suggested that it involves actin cytoskeletal regulation (14, 17, 18, 20, 21). Actin plays an important role in the contractility of ASM, because actin polymerization can be triggered by contractile stimuli, and force development can be reduced by treatment with inhibitors of actin polymerization (22, 23). We had demonstrated earlier that mesangial cells from kidneys transfected with wild-type HSP20 had fewer stress fibers that were localized to the periphery of the cell, whereas mesangial cells transfected with a Ser16→alanine 16 mutant HSP20 had abundant stress fibers throughout the cells (21). Furthermore, HSP20 interacts with the adaptor protein 14-3-3, which plays a central role in the HSP20-mediated cytoskeletal rearrangement (24). Taken together, these results suggest a role for phospho-HSP20 in the regulation of actin cytoskeleton.
We previously developed a cell-permeant phosphopeptide mimetic of HSP20 (P20 peptide, YARAAARQARAWLRRAS(PO3)APLPGLK) that contains 13 amino acids surrounding the phosphorylated Ser16 of HSP20 protein attached to an optimized 11–amino acid protein transduction domain (YARAAARQARA) from human immunodeficiency virus TAT protein (21, 25). P20 peptide has been demonstrated to mimic the biological effect of the entire HSP20 molecule, inducing relaxation in bovine carotid artery (21), porcine coronary artery (26), human saphenous vein (27), human umbilical artery (28), rat aorta (29), bovine ASM (14), and canine ASM (15). In National Institutes of Health 3T3 cells and human ASM (HASM) cells, treatment with the P20 peptide led to depolymerization of actin, resulting in disruption of actin stress fibers (14, 15, 24). However, the role of HSP20 on relaxation of HASM is unknown. In this study, we hypothesized that P20 peptide relaxes constricted HASM via mechanism(s) downstream of the β2AR pathway. We determined the effect of the P20 peptide on intact HASM pretreated with a β2AR inhibitor, and tested the in vivo effect of P20 peptide on methacholine-induced AHR in the ovalbumin (OVA) sensitization and challenge mouse model of allergic airway inflammation that mimics human asthma.
Materials and Methods
Detailed methods are provided in the online supplement. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless specified otherwise. P20 peptide (YARAAARQARAWLRRAS(PO3)APLPGLK) and the control scrambled (Scr) peptide (YARAAARQARAPRKS(PO3)LWALGRPLA) were made by either American Peptide Co. (Sunnyvale, CA) or EZBiolab Inc. (Carmel, IN).
Human and Porcine Lung Procurement and Physiological Measurement of Smooth Muscle Functional Viability
Nontransplantable human lungs were collected after obtaining approval of the Institutional Review Boards of the Veterans Affairs Medical Center, Vanderbilt University Medical Center, and the Tennessee Donor Services (all Nashville, TN). Porcine lungs were collected from killed pigs from the surgical suite at Vanderbilt University Medical Center following study protocols approved by the Vanderbilt Institutional Animal Care and Use Committee or from the abattoir (Triune, TN). Bronchial rings (5–7 mm diameter) were isolated and equilibrated in an organ bath, and the smooth muscle contraction, relaxation, and inhibition of contraction were determined as described previously (14) and in the online supplement.
HSP20 Phosphorylation
Phosphorylation of HSP20 in response to bronchodilators was examined by isoelectric focusing, which separates the phospho and nonphospho forms of HSP20, and phospho-HSP20 level was measured by Western blotting, as described previously (30).
Cytoskeletal Dynamics
The ratio of filamentous (F)-actin versus globular (G)-actin in intact porcine ASM (PASM) tissue was measured using the G-actin/F-actin In Vivo Assay kit (Cytoskeleton, Denver, CO), per the manufacturer’s protocol, as described previously (30).
Measurement of Stress Fiber Disruption and Migration in HASM Cells
Primary HASM cells (Lonza Group Ltd., Basel, Germany) were serum starved, either left untreated or treated with carbachol (CCH) (0.15 μM), 200 μM P20 peptides, or 200 μM P20 peptides followed by CCH (0.15 μM), and were stained with Alexa568-conjugated phalloidin and 4′,6-diamidino-2-phenylindole. Stress fibers were visualized by indirect immunofluorescence microscopy. The effect of 200 μM P20 peptide for 24 hours on platelet-derived growth factor (PDGF; 10 ng/ml)-induced migration of HASM cells was assessed by a scratch assay and by using wound assay chambers (ibidi GmbH, Munich, Germany), where cell migration into the defined cell-free gap (500 μm) was determined (details provided in the online supplement).
Toxicity Studies
Mice were treated with vehicle (PBS) or with two doses of P20 peptide in PBS (2 and 5 μg/g body weight) intranasally for 5 days, and full necropsy, including blood counts and serum chemistry analysis, were performed and analyzed by the Translational Pathology Shared Resource at Vanderbilt University Medical Center.
Mouse Model of OVA-Induced Airway Inflammation
Pathogen-free, 8- to 10-week-old female BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA). Mice were categorized into three groups: mock/PBS, OVA/PBS, OVA/P20 peptide (eight mice per group) and challenged with either vehicle or P20 peptide (1.5 mg/g body weight). OVA-induced airway inflammation has been previously described (31, 32). Detailed methods of OVA model and endpoint analysis are in the online supplement.
Statistical Analysis
Values are reported as mean (±SEM). Statistical analysis was performed by Student’s t test or ANOVA, followed by Tukey’s post test (GraphPad Software, Inc., San Diego, CA). The criterion for significance was a P value less than 0.05.
Results
P20 Peptide Relaxes Precontracted ASM and Prevents the Initiation of CCH-Induced Contraction
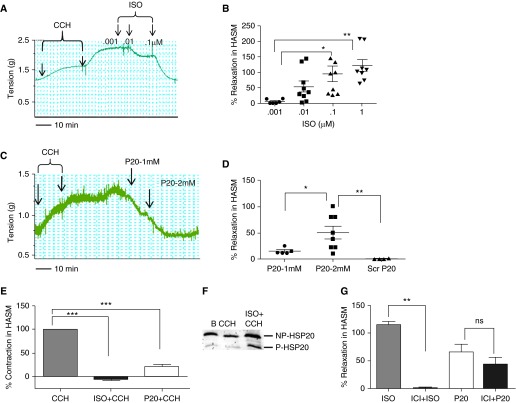
Novel therapeutics are needed to manage airway constriction in patients with asthma who are refractory to β-agonists, and we determined if the HSP20 peptide mimetic (P20 peptide) relaxed precontracted HASM. Similar to previous data reported by others and us (14, 15), ISO induced a dose-dependent relaxation of CCH precontracted HASM rings (Figures 1A and 1B). The P20 peptide also induced relaxation of CCH precontracted HASM in a dose-dependent manner (Figures 1C and 1D). The Scr P20 peptide did not have any effect on relaxation of HASM (Figure 1D), as observed previously in vascular smooth muscle tissues and cells (21, 24, 26). Because the Scr P20 peptide had no effect, it was not used in further experiments. We next determined if P20 pretreatment blocked CCH-induced contraction in HASM, using ISO treatment as a positive control. Both ISO and P20 pretreatment blocked CCH-induced contraction in HASM (Figure 1E). Furthermore, HSP20 phosphorylation was increased significantly in ISO-treated HASM rings when compared with untreated (basal) or CCH-treated HASM (Figure 1F). Phosphorylation was increased from 1.2 (±0.2)- to 6.7 (±2.4)-fold for CCH and ISO plus CCH, respectively, compared with untreated tissue (basal), respectively (P = 0.003, n = 5).
Figure 1.
Isoproterenol (ISO) and P20 peptide dose–response curves and phosphorylation of heat shock–related protein (HSP) 20 in human airway smooth muscle (ASM). (A) Representative trace of the dose–response curve of contraction with carbachol (CCH; 0.15 and 0.2 μM) and relaxation with ISO (0.001, 0.01, 0.1 μM) and (B) cumulative data of percent relaxation induced by ISO (0.001–1 μM; *P < 0.01, n = 6–9, **P < 0.001, n = 8). (C) Representative trace of the dose–response curve of contraction with CCH and relaxation with P20 peptide (1 and 2 mM) and (D) cumulative data of percent relaxation induced by P20 peptide (1 and 2 mM) and scrambled (Scr) peptide (2 mM; *P < 0.05, n = 5–8, **P < 0.001, n = 4–7). (E) Cumulative data of percent inhibition of CCH (0.15 μM) contraction induced by pretreatment with ISO (0.5 μM) and P20 peptide (2 mM) in human ASM (HASM; ***P < 0.0001, n = 4–9). (F) ISO induces phosphorylation of HSP20. Representative Western blot of the phosphorylation of HSP20 in response to ISO (0.1 μM) in untreated tissue, basal (B), CCH (0.15 μM), or 0.1 μM ISO plus 0.15 μM CCH–treated ASM. (G) The β2-adrenergic receptor antagonist (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride (ICI 118,551, ICI) inhibits ISO-induced relaxation but does not block P20 peptide (P20)–induced relaxation of HASM. Cumulative data of percent relaxation induced by ISO (0.1 μM) and P20 peptide (1 mM) with and without the presence of 0.2 μM ICI. **Significant compared with ISO (n = 4, P < 0.001); ns, not significantly different from ICI plus P20 (n = 4, P = 0.283).
β2 AR Antagonist Does Not Block P20 Peptide–Induced Relaxation
To determine whether P20 peptide–induced relaxation requires β2AR activation, P20 peptide–mediated relaxation in the presence of a selective β2AR antagonist, (±)-1-[2,3-(Dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride (ICI 118,551, ICI) was measured. ICI (0.2 μM), a dose chosen from our previous experiments that consistently blocked ISO-induced relaxation in HASM (data not shown), completely blocked ISO-induced relaxation, but did not block P20 peptide–induced relaxation (Figure 1G).
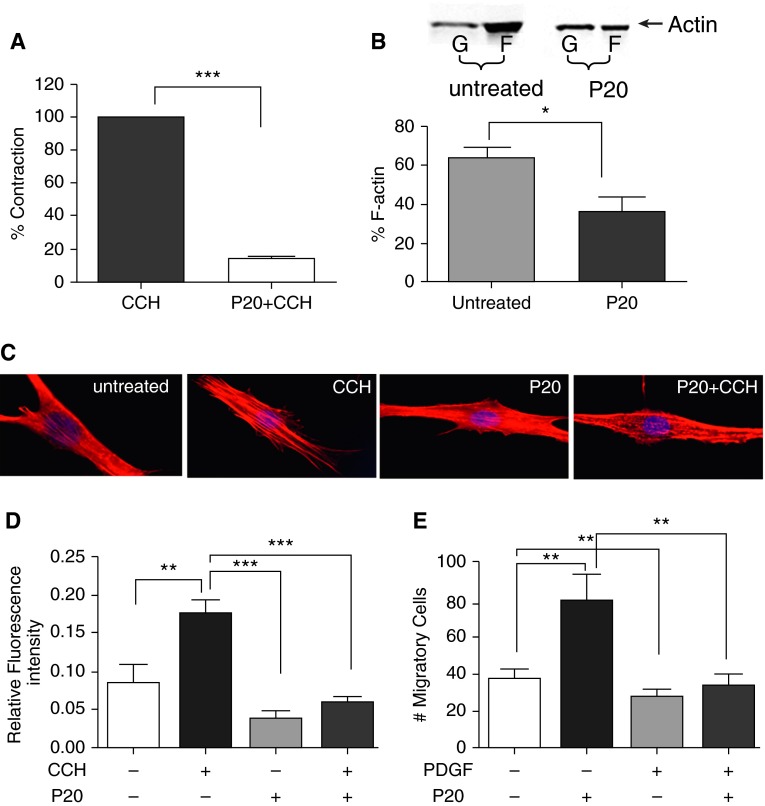
P20 Peptide Treatment Decreases F-Actin Levels in Intact ASM Tissue
Previous investigations by our laboratory demonstrated that P20 peptide caused relaxation of vascular smooth muscle by inducing actin depolymerization (24, 30, 33), and we hypothesized that P20 peptide would also cause actin depolymerization in ASM. Therefore, we measured the actin concentration in intact PASM tissue, as PASM samples have less heterogeneity compared with HASM samples. Pretreatment with the P20 peptide significantly blocked CCH-induced contraction in PASM (Figure 2A) and reduced F-actin levels (63 ± 5 versus 36 ± 7%, P = 0.039, for untreated and P20 peptide–treated PASM, respectively, Figure 2B). P20 peptide treatment also increased G-actin levels (37 ± 5 versus 64 ± 7%, P = 0.04 for untreated and P20 peptide–treated PASM, respectively), indicating that the P20 peptide causes actin depolymerization and inhibition of contraction.
Figure 2.
P20 peptide inhibits CCH-induced contraction and alters actin cytoskeletal dynamics in porcine ASM (PASM). (A) Cumulative data showing the inhibition of CCH (0.15 μM)-induced contraction by P20 peptide (1 mM, P20; n = 3–4, ***P < 0.0001). (B) P20 peptide decreases filamentous (F)-actin in intact PASM. Top panel, representative Western blot showing actin in the supernatant (globular [G]-actin) and pellet (F-actin) of PASM treated with P20 peptide (1 mM). Bottom panel, cumulative data of percent F-actin levels show significant reduction of F-actin in P20 peptide–treated ASM compared with basal, indicating depolymerization of F-actin, *P < 0.05. (C) P20 peptide prevents CCH-induced stress fiber formation in HASM cells. Representative epifluorescence micrographs at 40× magnification of HASM cells showing stress fiber formation in cells left untreated, or treated with 0.15 μM CCH (CCH), P20 peptide (200 μM for 20 min, P20), or with P20 peptide (200 μM) for 20 minutes before CCH (0.15 μM, P20 + CCH). (D) Relative fluorescence intensity of the stress fiber formation quantitated from the ratio of the fluorescence intensity of the stress fibers over the total stained cell area using Adobe Photoshop CS5 software (**P < 0.001, ***P < 0.0001, n = 6–7). (E) P20 peptide significantly reduced platelet-derived growth factor (PDGF)-induced migration of HASM cells. HASM cells were treated with P20 peptide (200 μM, 20 min), PDGF-BB (10 ng/ml), or pretreated with P20 (200 μM, 20 min) before PDGF-BB (10 ng/ml). Cells were allowed to grow for 24 hours, and images and migratory cell counts were obtained at 0 and 24 hours (**P < 0.001, n = 6–7).
P20 Peptide Reduces CCH-Induced Stress Fibers in HASM Cells
Because P20 peptide decreased F-actin levels in intact ASM tissue, we chose to visualize the effect of P20 peptide on actin stress fiber formation in cultured, primary HASM cells. CCH-induced stress fiber formation throughout the cells (red-stained fibers in the second panel from the left in Figure 2C). Treatment of P20 alone had no significant effect on stress fibers, but pretreatment with P20 for 20 minutes before CCH administration disrupted stress fiber formation (far-right panel in Figure 2C). CCH-induced stress fibers were reduced by 67% with P20 peptide pretreatment (Figure 2D and Figure E1 in the online supplement) (34).
P20 Peptide Treatment Inhibited Migration of HASM Cells
Because P20 peptide treatment led to changes in the actin cytoskeleton, we examined the effect of P20 peptide on migration of HASM cells using a scratch assay, where a “wound gap” in a cell monolayer was created by scratching, and the “healing” of this gap by cell migration and growth toward the center of the gap was monitored and quantitated. P20 peptide reduced PDGF-induced migration of HASM cells by 41 ± 7% when compared with PDGF alone (Figure 2E). These results were confirmed by using the ibidi wound assay chambers, which demonstrated that P20 peptide reduced PDGF-induced migration of HASM cells by 47 ± 9% (Figure E2).
P20 Peptide and Toxicity
To test the toxicity of the P20 peptide, mice were treated with either vehicle or P20 peptide (2 and 5 μg/g body weight) for 5 days, and necropsy was conducted. Intranasal delivery of the P20 peptide was well tolerated, and no apparent toxicity was associated with the 5-day P20 treatment. Complete blood count parameters were within normal limits for all mice, and there were no differences between groups (Table E1). Gross and microscopic examination showed no lesions or evidence of toxicity (Figure E3).
P20 Peptide Reduces Methacholine-Induced AHR in a Mouse Model of OVA-Induced Allergic Airway Inflammation
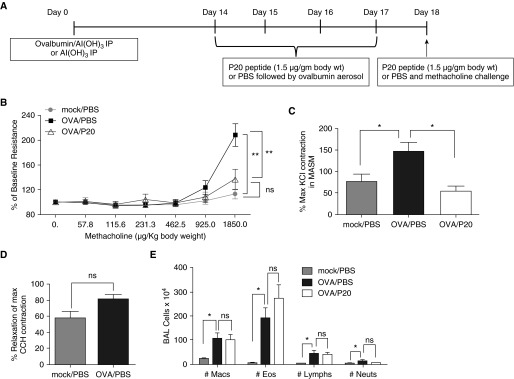
Because P20 peptide caused relaxation of HASM and PASM ex vivo, and there was no evidence of toxicity in vivo, we next determined the effects of the P20 peptide on AHR in vivo using a mouse model of OVA-induced allergic airway inflammation. We hypothesized that the P20 peptide inhibits AHR during allergic airway inflammation. To test our hypothesis, we used an OVA model of allergic airway inflammation. As shown in Figure 3A, mice were sensitized with an intraperitoneal injection of OVA conjugated with Al(OH)3 or an intraperitoneal injection of Al(OH)3 alone on Day 0. At 2 weeks later, mice were administered the P20 peptide (1.5 μg/g body weight) or vehicle (PBS) 30 minutes before OVA aerosol challenge on Days 14–17 and 30 minutes before methacholine challenge on Day 18. Mock/PBS mice received an intraperitoneal injection of Al(OH)3 only on Day 0 and intranasal administration of PBS on Days 14–17. OVA/PBS and OVA/P20 mice received intraperitoneal injection of OVA conjugated with Al(OH)3 on Day 0 and intranasal administration of vehicle (PBS) or the P20 peptide, respectively, 30 minutes before OVA aerosol challenge on Days 14–17. OVA/PBS mice had increased methacholine-induced AHR compared with mock/PBS mice, and AHR was significantly reduced in the OVA/P20 mice compared with OVA/PBS at 1,850 μg/kg body weight (Figure 3B). There was no significant difference in the AHR between mock/PBS and OVA/P20 peptide mice (Figure 3B). There was no significant difference in the elastance or compliance measurements between OVA/PBS and OVA/P20 peptide mice (Figure E4).
Figure 3.
P20 peptide inhibits allergen-induced airway hyperresponsiveness to methacholine in ovalbumin (OVA) mice. (A) Timeline of experimental protocol for OVA sensitization/challenge protocol and P20 peptide treatment in mice. (B) Lung resistance with increasing concentrations of intravenous methacholine normalized to baseline. **Mock/PBS versus OVA/PBS, P < 0.004; **OVA/PBS versus OVA/P20, P = 0.001; ns, not significant, mock/PBS versus OVA/P20, P = 0.333; n = 8–9 mice per group, ANOVA. (C) Ex vivo effect of CCH-induced contraction in tracheas of mock/PBS, OVA/PBS, and OVA/P20 mice. CCH (0.15 μM)-induced contraction was significantly increased from mock/PBS compared with OVA/PBS (*P = 0.04) and decreased for OVA/P20 peptide (*P < 0.05, n = 3–4) –treated mice compared with OVA/PBS-treated mice. (D) Smooth muscle relaxation in tracheas from mock/PBS and OVA/PBS mice treated ex vivo with P20 peptide (2 mM; P = 0.09, n = 3–4). (E) P20 peptide (1.5 μg/g body weight) does not significantly change the infiltration of inflammatory cells in bronchoalveolar lavage (BAL) fluid. Eos, eosinophils; Lymphs, lymphocytes; Macs, macrophages; Neuts, neutrophils; ns, not significant, P = 0.25, *P < 0.05, n = 6–8 mice.
Measuring AHR is critical for determining the effect of the P20 peptide on the responses of the fully integrated in vivo system. To directly measure the effect of the P20 peptide treatment on ASM contractility in OVA mice, tracheas were isolated immediately after the plethysmography, and contractile function was measured in the muscle bath. Contraction induced by CCH was significantly higher for tracheas isolated from OVA/PBS mice compared with mock/PBS mice or OVA/P20 peptide mice (Figure 3C). No significant difference in the CCH-induced contraction was observed between mock/PBS and OVA/P20 peptide mice (Figure 3C). ISO induced a dose-dependent relaxation of tracheas isolated from mock/PBS, OVA/PBS, and OVA/P20 peptide mice. (Figure E5). There was no significant difference in the relaxation induced by P20 peptide ex vivo in trachea isolated from mock/PBS or OVA/PBS mice (Figure 3D), suggesting that P20 peptide–mediated relaxation was not affected by OVA-induced inflammation. Tracheas from the OVA/P20 peptide group were not further relaxed with P20 peptide ex vivo, as the mice were treated with the P20 peptide in vivo.
P20 Peptide Does Not Reduce OVA-Induced Infiltration of Inflammatory Cells
We next examined whether the P20 peptide altered OVA-induced airway inflammation in mice. The total number of inflammatory cells, macrophages, eosinophils, lymphocytes, and neutrophils in the bronchoalveolar lavage fluid were significantly increased in the OVA/PBS and OVA/P20 groups compared with mock/PBS (data not shown and Figure 3). However, there was no significant difference between the OVA/PBS and OVA/P20 mice (data not shown and Figure 3E). There was no significant difference between the numbers of macrophages, eosinophils, lymphocytes, or neutrophils in OVA/PBS and OVA/P20 peptide mice (Figure 3E). Furthermore, we determined protein expression of IL-5, IL-13, CCL11 (eotaxin 1), and CCL24 (eotaxin 2) in whole-lung homogenates. OVA/PBS- and OVA/P20-treated mice had a significant increase in IL-5, IL-13, CCL11, and CCL24 compared with mock/PBS (Figure E6), but no significant difference in cytokine or chemokine expression was determined in whole-lung homogenates from OVA/PBS- and OVA/P20 peptide–treated mice.
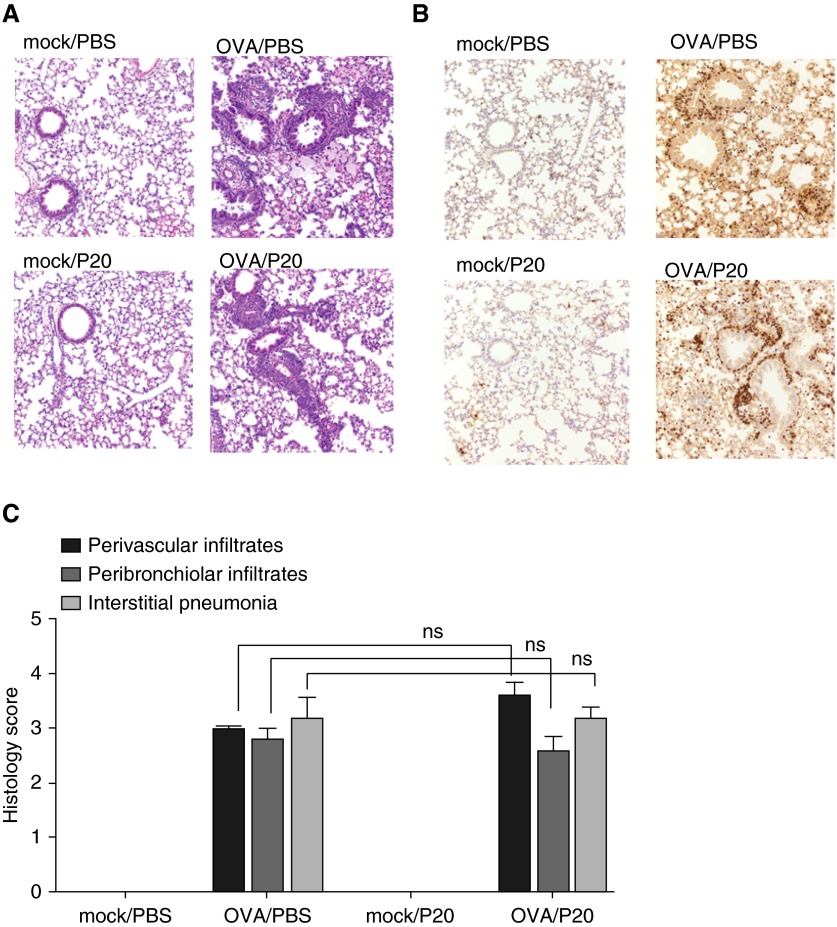
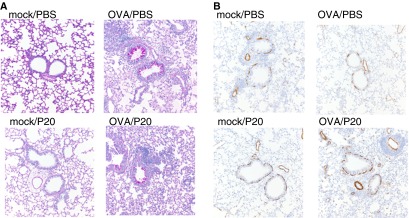
We also determined airway inflammation by hematoxylin and eosin staining of histologic sections. OVA/PBS and OVA/P20 mice had a significant increase in airway inflammation compared with mock/PBS and mock/P20 mice (Figures 4A and 4C). However, there was no difference in the inflammation observed in histological sections stained with hematoxylin and eosin from OVA/PBS and OVA/P20 mice (Figures 4A and 4C). Immunohistochemistry for major basic protein showed increased eosinophil infiltration into the lung of OVA/PBS and OVA/P20 mice compared with mock/PBS and mock/P20 mice, but no difference between OVA/PBS and OVA/P20 mice (Figure 4B).
Figure 4.
P20 peptide does not reduce allergic airway inflammation in mice. Histological sections of mice after OVA-induced airway inflammation in the presence of PBS or P20 peptide. (A) Representative sections of lung hematoxylin and eosin stain (magnification, ×200). (B) Representative sections of lung immunohistochemistry for major basic protein (magnification, ×200; n = 5 mice per group). (C) Histology score of the hematoxylin and eosin slides showing the perivascular and peribronchiolar infiltrates and interstitial pneumonia. Scoring method: 0 = no inflammation; 1 = minimal; 2 = mild; 3 = moderate; and 4 = severe inflammatory infiltration. ns, not significant.
To determine if P20 peptide altered OVA-induced airway mucus production, we stained histologic sections with periodic acid Schiff stain. OVA/PBS and OVA/P20 mice had a significant increase in mucus production compared with mock/PBS and mock/P20 mice, but there was no difference in the amount of mucus between the OVA/PBS and OVA/P20 (Figure 5A). Furthermore, immunohistochemistry analysis for smooth muscle actin revealed no difference in the expression in the mock/PBS-, mock/P20-, OVA/PBS-, or OVA/P20-treated mice (Figure 5B), suggesting no effect on smooth muscle mass. These results reveal that the P20 peptide inhibited OVA-induced methacholine reactivity, while not reducing allergic inflammation or mucus production.
Figure 5.
P20 peptide does not reduce mucus production or affect smooth muscle mass in the OVA mice. (A) Representative sections of lung periodic acid–Schiff stain (magnification, ×200) on Day 5 of OVA protocol. (B) Representative sections of lung immunohistochemistry for smooth muscle actin (magnification, ×200; n = 5).
Off-Target Effects of P20 Peptide
To determine if there were any off-target effects on the vasculature, aortae were isolated from the P20-treated naive mice and contraction to phenylephrine and relaxation to sodium nitroprusside were determined. The phenylephrine-induced contraction of aortae isolated from PBS mice or P20-treated naive mice was not significantly different (PBS versus P20 peptide, 2 μg and 5 μg/g body weight treated mice, P = 0.518, n = 4; Figure E7). Similarly, ex vivo sodium nitroprusside–induced relaxation of aortae isolated from mock and P20 peptide–treated mice were not significantly different (PBS versus P20 peptide, 2 and 5 μg/g body weight treated mice, P = 0.522, n = 4; Figure E7). These results suggest that P20 peptide did not exhibit significant off-target effects in naive mice.
Discussion
In the present study, we demonstrated that a cell-permeant phosphomimetic peptide of HSP20, the P20 peptide, reduces ASM hypercontractility by disruption of the actin cytoskeleton. Increased phosphorylation of HSP20 correlated with relaxation of HASM (Figures 1A and 1F), confirming the integral role of HSP20 in the relaxation of HASM (14, 15). Importantly, P20 peptide relaxed HASM in the presence of a selective inhibitor of β2AR, suggesting that P20 peptide–mediated relaxation of ASM does not require activation of the β2AR (Figure 1G).
Several studies have shown that P20 peptide relaxed vascular smooth muscle preparations from various tissues and species by regulating the actin cytoskeleton (reviewed in Refs. 18, 29). P20 peptide decreased F-actin and increased G-actin in intact ASM tissue, and caused disruption of stress fibers in HASM cells, suggesting that the P20 peptide induced relaxation through actin disruption (Figure 2). This ability to disrupt F-actin may also explain the inhibitory effect of P20 peptide on the migration of HASM cells (Figure 2E). P20 peptide has been demonstrated to inhibit migration of vascular smooth muscle cells and inhibit intimal hyperplasia formation in human saphenous vein in an organ culture model (27), suggesting that the P20 peptide may also have a role in smooth muscle remodeling. However, the P20 peptide did not have an effect on the proliferation of HASM cells (data not shown). Previous investigations have determined that vaso- or bronchodilator-induced phosphorylation of HSP20 or the P20 peptide reduced F-actin filaments in cultured Swiss 3T3 cells (24), HASM cells (14, 15), and keloid fibroblast cells (33). Moreover, we reported earlier that phosphorylated HSP20 or the P20 peptide binds to the adaptor protein 14-3-3 that is bound to cofilin, leading to the displacement, dephosphorylation, and activation of cofilin as an actin depolymerizing factor mediating relaxation (24). Thus, results presented here also suggest that the P20 peptide–mediated actin depolymerization in the intact HASM may cause relaxation of HASM through activation of cofilin as an actin depolymerizing factor.
Using an OVA model of allergic airway inflammation that is a mouse model with features of asthma in humans, we demonstrated that intranasal delivery of P20 peptide attenuated methacholine-induced AHR in mice (Figure 3B). In addition, P20 decreased CCH-induced contraction in ASM from OVA/P20 mice compared with OVA/PBS mice (Figure 3C). Because P20 reduced F-actin content in intact HASM tissues (Figure 2B), the reduction in AHR may be due to the ability of P20 peptide to modulate actin cytoskeleton during the peptide treatment.
Because the 5-day treatment of P20 peptide protocol used in this study did not have an effect on the infiltration of inflammatory cells (Figures 3E, 4, and 5), the marked reduction in airway resistance observed after P20 treatment was not a result of an effect on inflammation, but was instead likely due to the bronchodilatory properties of the peptide. There was no significant difference in the OVA-induced increase in IL-5, IL-13, CCL11, and CCL24 protein expression (Figure E6) upon peptide treatment, suggesting that the peptide had no effect on production of these cytokines and chemokines. These data suggest that the peptide may be effective in relieving acute bronchoconstriction. In addition, P20 peptide may be used in combination therapy to enhance the effect of antiinflammatory drugs in long-term asthma management, because no apparent toxicity was associated with the nasal delivery of the P20 peptide in the mouse model (Figure E3), and P20 peptide had no effect on infiltration of inflammatory cells.
Although inhaled β-agonists are the mainstay of asthma therapy, regular use of these drugs has detrimental effects in some patients with asthma, due to desensitization and/or genetic polymorphism of the β2AR (2, 3, 5, 6, 35, 36). Thus, a drug that can cause bronchodilation without the activation of the β2AR may be very valuable in asthma treatment. We demonstrated in the current study that P20 peptide relaxed HASM pretreated with a β2AR antagonist, ICI 118,551 (Figure 1G), suggesting that P20 peptide–induced relaxation is not mediated through the activation of β2AR. Along with its ability to reduce methacholine-induced hyperresponsiveness in the OVA mouse model (Figure 3), the development of the P20 peptide into an effective bronchodilator in clinical settings has promising potential. Thus, P20 peptide emerges as a potential asthma therapeutic that causes ASM relaxation, joining other bronchodilators that can cause relaxation of ASM, such as bitter taste receptor activators (37), chloride channel blockers (38), and calcium-sensing receptor antagonists (calcilytics) (39). One of the limitations of this study is that the HASM used did not constitute samples from patients with asthma. Future studies are needed to evaluate the effectiveness of the peptide in relaxing ASM isolated from patients with asthma and β-agonist–resistant ASM tissues.
ASM constriction plays a major role in asthma pathogenesis, and several therapeutics have been developed that target ASM (19, 40); however, no new class of drugs have been added recently. A potential solution to this problem is to develop a peptide therapeutic that targets protein effectors downstream of the receptor. Results presented in this study demonstrate that P20 peptide mediated relaxation of HASM through modulation of actin cytoskeletal dynamics via mechanism(s) that are downstream of the β2AR, and reduced methacholine-induced AHR in the OVA mouse model, thus having the potential to be developed as a therapeutic for β2AR-resistant asthma.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank the staff at the Tennessee Donor Services (Nashville, TN), the donor families for the human specimens used in this study, and Dr. Kyle Hocking and Sydney Pedigo for quantitating the stress fiber images.
Footnotes
This work was supported by National Institutes of Health grants R56-HL122735–01 (P.K.), R01 AI 111,820 (R.S.P.), 2I01BX000624 (R.S.P.), U19 AI 095227–02 (R.S.P.), R01 HL 090664–04 (R.S.P.), RO1 HL122554 (D.C.N.), and R21 AI121420 (D.C.N.), and a Clinical and Translational Service Award award UL1TR000445, from the National Center for Advancing Translational Sciences (P.K.), and in part by resources and materials from the Veterans Affairs Tennessee Valley Healthcare System.
The contents of this article are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author Contributions: Conception and design—J.C.-F., K.L.B., D.C.N., R.S.P., and P.K.; acquisition, analysis, and interpretation of data—A.B., J.C.-F., K.G., K.L.B., D.C.N., R.S.P., and P.K.; drafting of the manuscript—A.B. and P.K.; review of the manuscript—A.B., J.C.-F., K.G., K.L.B., D.C.N., R.S.P., and P.K.; final approval of the manuscript—P.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0139OC on February 24, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 2.Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, Yates DM, Lucas MK, Herbison GP. Regular inhaled β-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DR, Sears MR, Herbison GP, Flannery EM, Print CG, Lake DC, Yates DM, Lucas MK, Li Q. Regular inhaled β agonist in asthma: effects on exacerbations and lung function. Thorax. 1993;48:134–138. doi: 10.1136/thx.48.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 5.Shore SA, Moore PE. Regulation of β-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol. 2003;137:179–195. doi: 10.1016/s1569-9048(03)00146-0. [DOI] [PubMed] [Google Scholar]
- 6.Penn RB. Embracing emerging paradigms of G protein coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:149–169. doi: 10.1007/s00210-008-0263-1. [DOI] [PubMed] [Google Scholar]
- 7.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway β-agonist paradox. J Clin Invest. 2003;112:619–626. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shore SA, Drazen JM. β-Agonists and asthma: too much of a good thing? J Clin Invest. 2003;112:495–497. doi: 10.1172/JCI19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande DA, Theriot BS, Penn RB, Walker JK. β-Arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penn RB, Benovic JL. Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am Thorac Soc. 2008;5:47–57. doi: 10.1513/pats.200705-054VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thirstrup S. Control of airway smooth muscle tone: II pharmacology of relaxation. Respir Med. 2000;94:519–528. doi: 10.1053/rmed.1999.0738. [DOI] [PubMed] [Google Scholar]
- 12.Kotlikoff MI, Kamm KE. Molecular mechanisms of β-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol. 1996;58:115–141. doi: 10.1146/annurev.ph.58.030196.000555. [DOI] [PubMed] [Google Scholar]
- 13.Penn RB, Panettieri RA, Jr, Benovic JL. Mechanisms of acute desensitization of the β2AR-adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol. 1998;19:338–348. doi: 10.1165/ajrcmb.19.2.3025. [DOI] [PubMed] [Google Scholar]
- 14.Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, Guo M, Pallero MA, Murphy-Ullrich JE, Brophy CM. The small heat shock–related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L69–L78. doi: 10.1152/ajplung.00235.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ba M, Singer CA, Tyagi M, Brophy C, Baker JE, Cremo C, Halayko A, Gerthoffer WT. HSP20 phosphorylation and airway smooth muscle relaxation. Cell Health Cytoskelet. 2009;2009:27–42. doi: 10.2147/chc.s5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beall A, Bagwell D, Woodrum D, Stoming TA, Kato K, Suzuki A, Rasmussen H, Brophy CM. The small heat shock–related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem. 1999;274:11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- 17.Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524:865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119:44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerthoffer WT, Solway J, Camoretti-Mercado B. Emerging targets for novel therapy of asthma. Curr Opin Pharmacol. 2013;13:324–330. doi: 10.1016/j.coph.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tessier DJ, Komalavilas P, Panitch A, Joshi L, Brophy CM. The small heat shock protein (HSP) 20 is dynamically associated with the actin cross-linking protein actinin. J Surg Res. 2003;111:152–157. doi: 10.1016/s0022-4804(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 21.Woodrum D, Pipkin W, Tessier D, Komalavilas P, Brophy CM. Phosphorylation of the heat shock–related protein, HSP20, mediates cyclic nucleotide–dependent relaxation. J Vasc Surg. 2003;37:874–881. doi: 10.1067/mva.2003.153. [DOI] [PubMed] [Google Scholar]
- 22.Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol. 2005;83:851–856. doi: 10.1139/y05-088. [DOI] [PubMed] [Google Scholar]
- 23.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreiza CM, Brophy CM, Komalavilas P, Furnish EJ, Joshi L, Pallero MA, Murphy-Ullrich JE, von Rechenberg M, Ho Y-SJ, Richardson B, et al. Transducible heat shock protein 20 (hsp20) phosphopeptide alters cytoskeletal dynamics. FASEB J. 2004:04–2911fje. doi: 10.1096/fj.04-2911fje. [DOI] [PubMed] [Google Scholar]
- 25.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–477. [PubMed] [Google Scholar]
- 26.Flynn CR, Komalavilas P, Tessier D, Thresher J, Niederkofler EE, Dreiza CM, Nelson RW, Panitch A, Joshi L, Brophy CM. Transduction of biologically active motifs of the small heat shock–related protein HSP20 leads to relaxation of vascular smooth muscle. FASEB J. 2003;17:1358–1360. doi: 10.1096/fj.02-1028fje. [DOI] [PubMed] [Google Scholar]
- 27.Tessier DJ, Komalavilas P, Liu B, Kent CK, Thresher JS, Dreiza CM, Panitch A, Joshi L, Furnish E, Stone W, et al. Transduction of peptide analogs of the small heat shock–related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40:106–114. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Flynn CR, Brophy CM, Furnish EJ, Komalavilas P, Tessier D, Thresher J, Joshi L. Transduction of phosphorylated heat shock–related protein 20, HSP20, prevents vasospasm of human umbilical artery smooth muscle. J Appl Physiol (1985) 2005;98:1836–1845. doi: 10.1152/japplphysiol.01043.2004. [DOI] [PubMed] [Google Scholar]
- 29.Dreiza CM, Komalavilas P, Furnish EJ, Flynn CR, Sheller MR, Smoke CC, Lopes LB, Brophy CM. The small heat shock protein, HSPB6, in muscle function and disease. Cell Stress Chaperones. 2010;15:1–11. doi: 10.1007/s12192-009-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hocking KM, Baudenbacher FJ, Putumbaka G, Venkatraman S, Cheung-Flynn J, Brophy CM, Komalavilas P. Role of cyclic nucleotide-dependent actin cytoskeletal dynamics: [Ca2+]i and force suppression in forskolin-pretreated porcine coronary arteries. PLoS One. 2013;8:e60986. doi: 10.1371/journal.pone.0060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peebles RS, Jr, Sheller JR, Johnson JE, Mitchell DB, Graham BS. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbumin-sensitized mice. J Med Virol. 1999;57:186–192. doi: 10.1002/(sici)1096-9071(199902)57:2<186::aid-jmv17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Peebles RS, Jr, Sheller JR, Collins RD, Jarzecka AK, Mitchell DB, Parker RA, Graham BS. Respiratory syncytial virus infection does not increase allergen-induced type 2 cytokine production, yet increases airway hyperresponsiveness in mice. J Med Virol. 2001;63:178–188. [PubMed] [Google Scholar]
- 33.Lopes LB, Furnish EJ, Komalavilas P, Flynn CR, Ashby P, Hansen A, Ly DP, Yang GP, Longaker MT, Panitch A, et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-β1–induced CTGF expression in keloid fibroblasts. J Invest Dermatol. 2009;129:590–598. doi: 10.1038/jid.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans BC, Hocking KM, Kilchrist KV, Wise ES, Brophy CM, Duvall CL. Endosomolytic nano-polyplex platform technology for cytosolic peptide delivery to inhibit pathological vasoconstriction. ACS Nano. 2015;9:5893–5907. doi: 10.1021/acsnano.5b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Effect of polymorphism of the β2-adrenergic receptor on response to regular use of albuterol in asthma. Int Arch Allergy Immunol. 2001;124:183–186. doi: 10.1159/000053705. [DOI] [PubMed] [Google Scholar]
- 36.Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between β2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997;350:995–999. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- 37.Liggett SB. Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert Opin Ther Targets. 2013;17:721–731. doi: 10.1517/14728222.2013.782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danielsson J, Yim P, Rinderspacher A, Fu XW, Zhang Y, Landry DW, Emala CW. Chloride channel blockade relaxes airway smooth muscle and potentiates relaxation by β-agonists. Am J Physiol Lung Cell Mol Physiol. 2014;307:L273–L282. doi: 10.1152/ajplung.00351.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarova PL, Stewart AL, Sathish V, Britt RD, Jr, Thompson MA, P Lowe AP, Freeman M, Aravamudan B, Kita H, Brennan SC, et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med. 2015;7:284ra60. doi: 10.1126/scitranslmed.aaa0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui S, Redhu NS, Ojo OO, Liu B, Irechukwu N, Billington C, Janssen L, Moir LM. Emerging airway smooth muscle targets to treat asthma. Pulm Pharmacol Ther. 2013;26:132–144. doi: 10.1016/j.pupt.2012.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.