Abstract
Asymmetric dimethylarginine (ADMA) induces the mitochondrial translocation of endothelial nitric oxide synthase (eNOS) through the nitration-mediated activation of Akt1. However, it is recognized that the activation of Akt1 requires phosphorylation events at threonine (T) 308 and serine (S) 473. Thus, the current study was performed to elucidate the potential effect of ADMA on Akt1 phosphorylation and the mechanisms that are involved. Exposure of pulmonary arterial endothelial cells to ADMA enhanced Akt1 phosphorylation at both threonine 308 and Ser473 without altering Akt1 protein levels, phosphatase and tensin homolog activity, or membrane Akt1 levels. Heat shock protein (Hsp) 90 plays a pivotal role in maintaining Akt1 activity, and our results demonstrate that ADMA decreased Hsp90–Akt1 interactions, but, surprisingly, overexpression of a dominant-negative Hsp90 mutant increased Akt1 phosphorylation. ADMA exposure or overexpression of dominant-negative Hsp90 increased Hsp70 levels, and depletion of Hsp70 abolished ADMA-induced Akt1 phosphorylation. ADMA decreased the interaction of Akt1 with its endogenous inhibitor, carboxyl-terminal modulator protein (CTMP). This was mediated by the proteasomal-dependent degradation of CTMP. The overexpression of CTMP attenuated ADMA-induced Akt1 phosphorylation at Ser473, eNOS phosphorylation at Ser617, and eNOS mitochondrial translocation. Finally, we found that the mitochondrial translocation of eNOS in our lamb model of pulmonary hypertension is associated with increased Akt1 and eNOS phosphorylation and reduced Akt1–CTMP protein interactions. In conclusion, our data suggest that CTMP is directly involved in ADMA-induced Akt1 phosphorylation in vitro and in vivo, and that increasing CTMP levels may be an avenue to treat pulmonary hypertension.
Keywords: asymmetric dimethylarginine, Akt1, mitochondrion, proteasome
Clinical Relevance
Our data identify a new mechanism by which the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine, enhances Akt1 phosphorylation and activation. Akt1 activation is being investigated for its role in the development of pulmonary hypertension. Thus, our studies identifying the loss of the Akt1 inhibitory protein carboxyl-terminal modulator protein (CTMP) in the activation of Akt1 suggest that increasing CTMP levels could be a new avenue for treating pulmonary hypertension.
Akt1 plays an important role in regulating multiple cellular processes, including metabolism, proliferation, cell survival, and transformation (1, 2). Dysregulation of Akt1 is associated with a wide range of pathogenesis disorders, including cardiovascular disease (3). More recently, studies have shown that Akt1 deficiency attenuates the development of pulmonary hypertension (4). Canonical Akt1 activation occurs through a dual regulatory mechanism that requires both translocation to the plasma membrane and phosphorylation at pThreonine (T) 308Akt1 (T308) by PDK1 and pSerine (S) 473Akt1 (S473) by mTORC2 (PDK2) (5, 6). Akt1 activation is also regulated through its interaction with other proteins (1, 6). For example, the interaction of heat shock protein (Hsp) 90 with the Akt1 kinase domain prevents its dephosphorylation and degradation and increases the Akt1 activity (5, 6). Akt1 activity can be attenuated through its binding to carboxy-terminal modulator protein (CTMP) (7). CTMP appears to bind to the C-terminal region of Akt1 at the plasma membrane, where it forms a complex that prevents Akt1 phosphorylation at T308 and especially, S473 (7). Other binding partners of CTMP are unclear, although recently it has been shown that Hsp70 associates with CTMP to mediate cell apoptosis (1, 2), suggesting that Hsp70 may be involved in Akt1 signal transduction.
Recently, we demonstrated that asymmetric dimethylarginine (ADMA) can activate Akt1 through its ability to increase peroxynitrite generation in endothelial cells and induce a single nitration event at tyrosine (Y) 350 (Y350) in Akt1 (8). This nitration-mediated activation of Akt1 is a key component in the mitochondrial translocation of endothelial nitric oxide (NO) synthase (eNOS) required to modulate mitochondrial function (8). However, as noted previously here, the activation of Akt1 is dependent on phosphorylation events at T308 and S473 (3, 5). The potential impact of ADMA on Akt1 phosphorylation and the possible molecular mechanisms required in this process has not been elucidated. Thus, the purposes of this study were to: investigate whether ADMA mediates Akt1 activation through phosphorylation as well as nitration events; to elucidate the molecular mechanisms underlying this process; and to determine whether the same process and mechanisms are recapitulated in vivo in a lamb model of pulmonary hypertension. In cultured pulmonary arterial endothelial cells (PAECs) we found that ADMA decreased Akt1–CTMP interactions via an Hsp70-facilitated proteasomal degradation of CTMP, increasing Akt1 phosphorylation at T308 and S473. Overexpression of CTMP attenuated ADMA-induced eNOS mitochondrial translocation. Overexpression of S473A Akt1 abolished ADMA-mediated both eNOS phosphorylation at S617 and Akt1 nitration at Y350. Finally, we found that the mitochondrial translocation of eNOS in our lamb model of pulmonary hypertension (9) is associated with increased Akt1 and eNOS phosphorylation and reduced Akt1–CTMP protein interactions secondary to reduced CTMP protein levels. Our findings suggest that the loss of CTMP may play an important role in increasing Akt1 activity during the development of pulmonary hypertension. In addition, CTMP may be a promising therapeutic target for alleviating pulmonary hypertension in humans.
Materials and Methods
Surgical Preparations and Care
Surgical preparations and care are described in detail in the online supplement. All protocols and procedures were approved by the Committees on Animal Research at University of California, San Francisco (San Francisco, CA), and the German Heart Center (Munich, Germany).
Antibodies and Chemicals
Antibodies and chemicals are described in detail in the online supplement.
Generation of a Nitration-Specific Akt1 Polyclonal Antibody
A nitro-Tyr350 Akt1–specific antibody was raised against a synthetic peptide antigen CGRLPF(Y-NO2)NQDHEKL, where Y-NO2 represents 3-nitrotyrosine, as previously described (10). This peptide was used to immunize rabbits. Tyrosine nitration–reactive rabbit antiserum was first purified by affinity chromatography. Further purification was performed using immunodepletion and resin chromatography with the column containing nonnitrated peptide (CGRLPFYNQDHEKL), after which the resulting eluate was tested for antibody specificity by immunoblotting.
Cell Culture and Treatment
PAECs were isolated and cultured as described previously (11). Cells were maintained and treated as described in the online supplement.
Immunofluorescence
Immunofluorescence is described in detail in the online supplement.
Imaging and Analysis
Imaging and analysis are described in detail in the online supplement.
Plasmids and Transient Transfection of PAECs
Plasmids and transient transfection of PAECs are described in detail in the online supplement.
Hsp70 RNA Interference Assays
PAECs were transfected with the appropriate small interfering RNA using HiPerFect transfection reagent (Qiagen, Valencia, CA), as described previously (12).
Western Blot Analysis
Western blot analysis is described in detail in the online supplement.
Immunoprecipitation Analysis
The interaction of CTMP with Akt1, Hsp70, C terminus of Hsp70-interacting protein, and ubiquitin were determined by immunoprecipitation analysis, as described previously (13). The online supplement provides details.
Real-Time Quantitative RT-PCR
Real-time quantitative RT-PCR is described in detail in the online supplement.
Mitochondrial Isolation
Mitochondria from PAECs were isolated using the Pierce Mitochondria isolation kit for cultured cells (Pierce, Rockford, IL), as previously described (8).
Membrane Protein Extraction
Membrane protein extraction is described in detail in the online supplement.
Mitochondrial Localization of eNOS
PAECs were cotransfected with a green fluorescent protein (GFP)-tagged eNOS expression plasmid (eNOS-GFP) (14), the Myc epitope–tagged human CTMP (Origene, Rockville, MD), or the parental vector (pcDNA3). Mitochondrial localization of eNOS was determined by calculating the Pearson product-moment correlation coefficient (15). The online supplement provides details.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software Inc., LaJolla, CA). The mean (±SEM) was calculated for all samples, and significance was determined either by the unpaired t test (for two groups) or ANOVA (for three or more groups) with Newman-Keuls post hoc testing. A value of P less than 0.05 was considered significant.
Results
ADMA Induces Akt1 Phosphorylation in PAECs
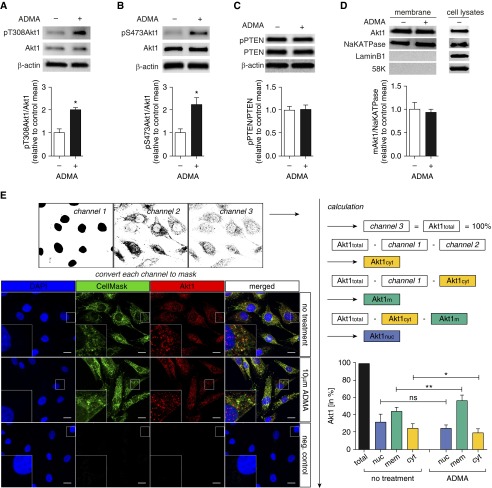
Our previous studies indicate that ADMA induces nitration-mediated Akt1 activation in PAECs (8). However, Akt1 activation is more commonly associated with phosphorylation at T308 and S473. Therefore, we initially examined the effect of ADMA on Akt1 phosphorylation. Western blot analysis demonstrated that, although ADMA (10 μM, 2 h) does not change total Akt1 levels (Figures 1A and 1B). Akt1 phosphorylation at T308 (Figure 1A) and S473 (Figure 1B) were significantly increased. As the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) has been shown to be involved in regulating Akt1 recruitment to plasma membrane (16), next we examined PTEN activity and membrane Akt1 levels. PTEN possesses a carboxy-terminal, noncatalytic regulatory domain with three phosphorylation sites (serine [Ser] 380, threonine [Thr] 382, and Thr383) that regulate PTEN stability and its biological activity (17, 18). Thus, we used the phosphorylation of PTEN to estimate changes in PTEN activity. Our data indicate that PTEN phosphorylation is unchanged (Figure 1C), suggesting that PTEN is not involved in the activation of Akt1 by ADMA. We next evaluated whether ADMA altered Akt1 levels on the plasma membrane. When Akt1 levels were analyzed using a membrane protein extraction method followed by immunoblotting, we found that Akt1 plasma membrane levels were unchanged (Figure 1D). However, when we used the more sensitive technique of confocal imaging, we found that ADMA treatment significantly increased Akt1 translocation from cytosol to the plasma membrane (Figure 1E), suggesting that. upon ADMA stimulation, Akt1 is activated, at least in part, through recruitment to that plasma membrane (19).
Figure 1.
Asymmetric dimethylarginine (ADMA) induces Akt1 phosphorylation in pulmonary arterial endothelial cells (PAECs). PAECs were exposed to ADMA (10 μM, 2 h) and whole-cell extracts (20 μg) subjected to immunoblot (IB) analysis using antibodies raised against pThreonine (T) 308Akt1 (pT308Akt1), pSerine (S) 473Akt1 (pS473Akt1), or phosphatase and tensin homolog deleted on chromosome 10 (pPTEN). Blots were then stripped and reprobed using antibodies raised against β-actin, Akt1, or PTEN. Representative images from duplicate blots are shown. ADMA significantly increased Akt1 phosphorylation at T308 (A) and S473 (B). PTEN activity was unchanged, as estimated by measuring pPTEN levels (C). Plasma membrane protein extracts (20 μg) were also subjected to IB analysis using an antibody raised against Akt1. Reprobing the plasma membrane IBs with an antibody raised against NaKATPase was used to normalize loading. Plasma membrane IBs were reprobed with antibodies raised against laminB1 or 58K to demonstrate no cross-contamination with nuclear or Golgi fractions, respectively. A separate gel was run using a PAEC whole-cell lysate (20 μg) to confirm that each antibody recognizes the ovine protein (D). ADMA does not change Akt1 levels in membrane extracts (D). Changes in plasma membrane and cytosolic Akt1 levels in response to ADMA were also evaluated by confocal microscopy. Representative images, including insets with additional higher magnifications, are shown (E). After converting every channel to a binary mask, signals were subtracted from each other to obtain cytosolic, membrane, and nuclear localized Akt1. There was a significant increase in Akt1 at the membrane and a significant decrease in the cytosol after ADMA treatment (E). The level of Akt1 at the plasma membrane combined with the Akt1 in the cytosol was unchanged, suggesting that total Akt1 levels were unchanged. Akt1total, total Akt1; Akt1cyt, cytosolic Akt1; Akt1mem, membrane Akt1; Akt1nuc, nuclear Akt1; DAPI, 4′,6-diamidino-2-phenylindole; neg. control, negative control. Scale bars: 10 μm. Values are means (±SEM); n = 3–7. *P < 0.05 versus untreated, **P < 0.01 versus untreated. ns, not significant.
ADMA Decreases Akt1–Hsp90 Interactions in PAECs
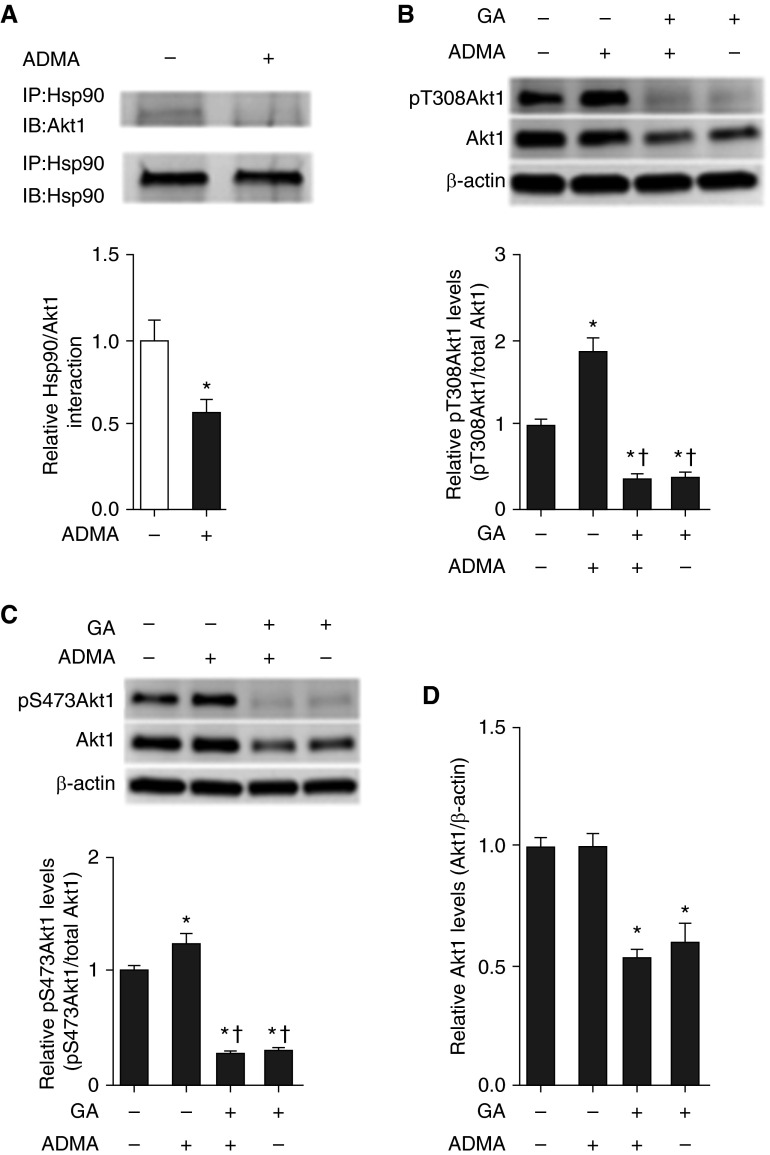
Studies indicate that Hsp90 binding to Akt1 is required to maintain Akt1 phosphorylation (20). To further elucidate the mechanisms of ADMA-induced Akt1 phosphorylation, we examined the effect of ADMA on Akt1–Hsp90 interactions. Our data demonstrate that ADMA decreased Akt1–Hsp90 interactions in PAECs (Figure 2A), which is consistent with our previous findings that ADMA decreases Hsp90 chaperone activity due to a reduction in cellular ATP levels (21). Geldanamycin (GA), which binds to Hsp90 N-terminal ATP binding site, is known as a potent, small molecule inhibitor of Hsp90 (22). To test the effect of the inhibition of Hsp90 in ADMA-mediated Akt1 phosphorylation in PAECs, cells were pretreated with GA (2 μM, 24 h) or vehicle (0.1% DMSO) followed by ADMA (10 μM, 2 h) treatment. Western blot analysis revealed that, although ADMA significantly increased Akt1 phosphorylation at T308 (Figure 2B) and S473 (Figure 2C), GA dramatically reduced Akt1 phosphorylation at T308 (Figure 2B) and S473 (Figure 2C), as well as total Akt1 levels (Figure 2D).
Figure 2.
Heat shock protein (Hsp) 90 is involved in the ADMA-mediated increase in Akt1 phosphorylation in PAECs. PAECs were exposed to ADMA (10 μM, 2 h) and cell lysates (500 μg) were subjected to immunoprecipitation (IP), using an antibody specific to Hsp90, then subjected to IB analysis using an Akt1-specific antibody. Blots were stripped and reprobed for Hsp90 to normalize for the IP efficiency. A representative image is shown (A). ADMA significantly decreases the interactions of Akt1 with Hsp90 (A). PAECs were pretreated with the Hsp90 inhibitor geldanamycin (GA; 2 μM, 24 h) or vehicle (0.1% dimethyl sulfoxide [DMSO], 24 h), followed by exposure to ADMA (10 μM, 2 h). Whole-cell extracts (20 μg), subjected to IB analysis on duplicate gels, revealed that ADMA significantly increased Akt1 phosphorylation at T308 (B) and S473 (C). GA pretreatment completely abolished ADMA-induced Akt1 phosphorylation at T308 (B) and S473 (C) and significantly decreased total Akt1 levels (D). Representative images are shown. Values are means (±SEM); n = 4–9. *P < 0.05 versus untreated; †P < 0.05 versus ADMA alone.
The Inhibition of Hsp90 Stimulates Akt1 Phosphorylation in PAECs
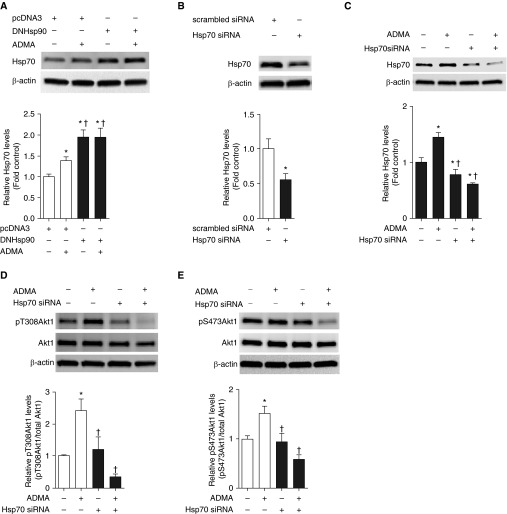
To assess whether more subtle inhibition of Hsp90 modulated ADMA-induced Akt1 phosphorylation, we overexpressed a dominant-negative (DN) mutant of Hsp90 (DN Hsp90; Figure 3A). At 48 hours after transfection, cells were then exposed, or not, to ADMA and Akt1 phosphorylation levels determined. Surprisingly, the overexpression of DN Hsp90 alone increased Akt1 phosphorylation levels at both T308 (Figure 3B) and S473 (Figure 3C).
Figure 3.
The overexpression of dominant-negative (DN) Hsp90 increases Akt1 phosphorylation in PAECs. PAECs were transiently transfected with a DN Hsp90 or pcDNA3 (as a control). At 48 hours after transfection, whole-cell lysates (20 μg) were prepared and subjected to IB analysis to measure Hsp90 levels. A representative image is shown (A). Transfection of DN Hsp90 increases Hsp90 protein levels by approximately twofold (A). PAECs were then transfected with DN Hsp90 or pcDNA3 for 48 hours followed by ADMA (10 μM, 2 h) treatment. Whole-cell lysates (20 μg) were prepared and subjected to IB analysis, on duplicate gels, to measure changes in Akt1 phosphorylation at T308 and S473. Representative images are shown (B and C). The phosphorylation of Akt1 at T308 (B) and S473 (C) are increased by DN Hsp90 overexpression. However, there is no additive effect by the addition of ADMA (B and C). Values are means (±SEM); n = 5–6. *P < 0.05 versus untreated or empty vector transfection without ADMA treatment.
Hsp70 Participate ADMA-Mediated Akt1 Phosphorylation in PAECs
Accumulating evidence indicates that Hsp70 is involved in both folding and degradation of Hsp90 client proteins (23). In addition, inhibition of Hsp90 function is known to induce expression of protective chaperone Hsp70 expression (24). Prenatal increases in ADMA are also associated with increased placental Hsp70 (25). Therefore, we examined the effect of ADMA (10 μM, 2 h) or overexpressed DN Hsp90 on Hsp70 protein levels in PAECs. Consistent with our previous studies (12), ADMA as well as DN Hsp90 overexpression increased Hsp70 protein levels (Figure 4A). Interestingly, DN Hsp90 overexpression increased Hsp70 protein levels to a greater extent than ADMA (Figure 4A), suggesting that Hsp90 inhibition may produce a more severe affect compared with ADMA. We next determined whether increases in Hsp70 were causally involved in the ADMA-mediated phosphorylation of Akt1. Our data indicate that small interfering RNA–mediated down-regulation of Hsp70 (Figure 4B) completely abolished ADMA-induced Akt1 phosphorylation in PAECs (Figures 4C and 4D).
Figure 4.
Depletion of Hsp70 abolishes ADMA-mediated Akt1 phosphorylation in PAECs. PAECs were transfected with DN Hsp90 or pcDNA3 for 48 hours, followed by ADMA (10 μM, 2 h) treatment. Whole-cell lysates (20 μg) were prepared and subjected to Western blot analysis to determine Hsp70 levels. A representative image is shown (A). Both ADMA and DN Hsp90 overexpression increase Hsp70 levels (A). PAECs were transiently transfected with a small interfering RNA (siRNA) for Hsp70 or a control siRNA. At 48 hours after transfection, whole-cell lysates (20 μg) were prepared and subjected to IB analysis to determine Hsp70 levels. A representative image is shown. Transfection with the Hsp70 siRNA reduces Hsp70 protein levels by approximately 50% (B). The effect of ADMA (10 μM, 2 h) on Akt1 phosphorylation at T308 and S473 after Hsp70 knockdown was then determined using duplicate blots. Representative images are shown for Hsp70 (C), T308 (D), and S473 (E). ADMA increased Hsp70 levels (C). Depleting Hsp70 protein levels (C) completely abolished ADMA-induced Akt1 phosphorylation at T308 (D) and S473 (E). Values are means (±SEM); n = 4–6. *P < 0.05 versus control siRNA; †P < 0.05 versus control siRNA with ADMA treatment.
ADMA Mediates CTMP Degradation via an Ubiquitination–Proteasome Pathway in PAECs
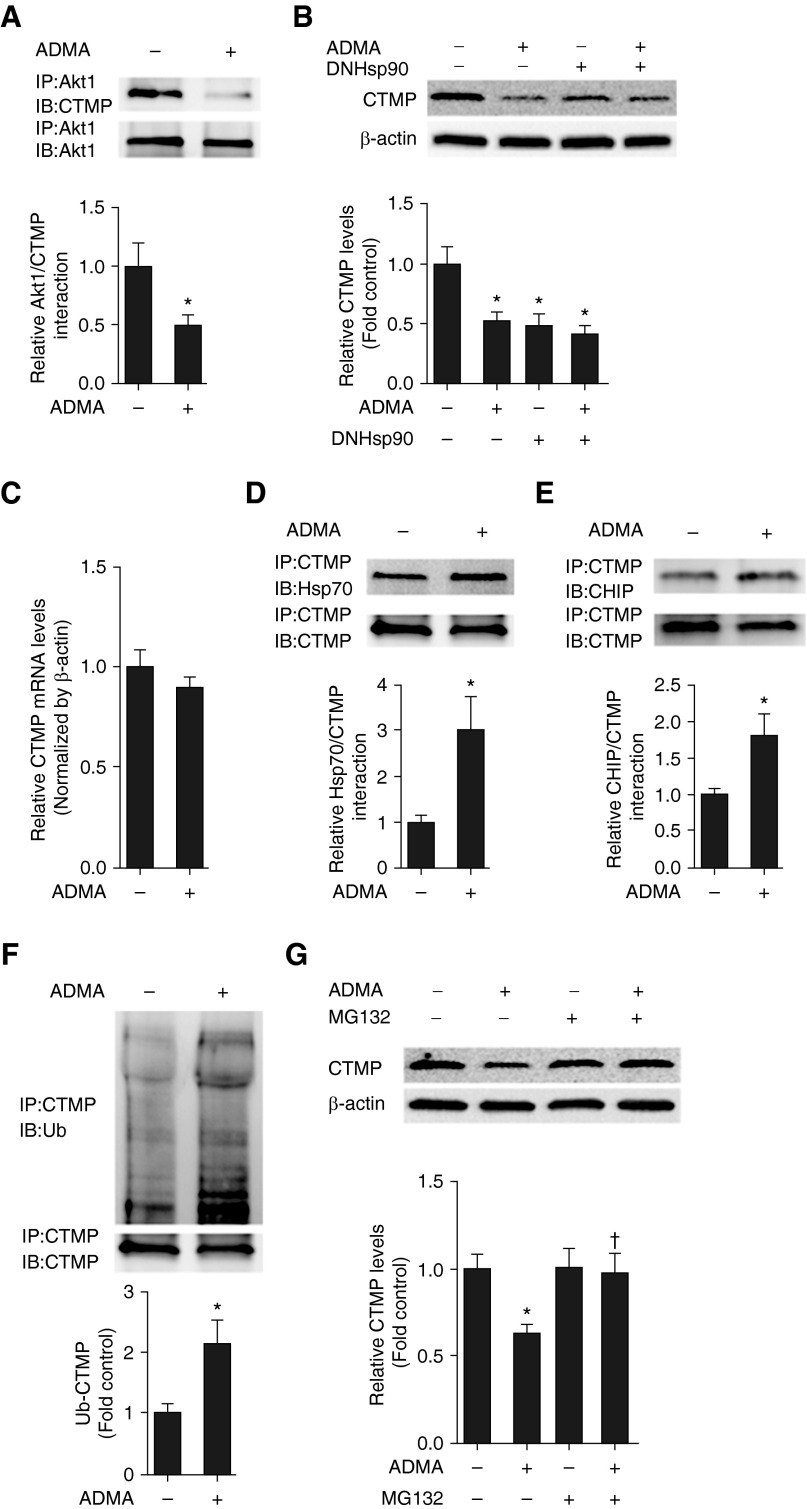
CTMP is regarded as an Akt1 inhibitor through its ability to block Akt1 phosphorylation (7). Thus, we determined if ADMA was altering the regulation of CTMP with Akt1. Our data indicate that ADMA exposure significantly decreased the interactions of CTMP with Akt1 (Figure 5A). Our previous studies indicate that ADMA increases Hsp70-dependent proteasomal degradation in PAECs (13), and recent studies suggest that the CTMP may be an Hsp70 binding protein (2). To determine if ADMA was reducing the interaction of CTMP with Akt1 by stimulating CTMP degradation, we evaluated the effect of ADMA and DN Hsp90 on CTMP protein and mRNA levels. Both ADMA and DN Hsp90 decreased CTMP protein (Figure 5B), but not mRNA (Figure 5C) levels, suggesting that ADMA induces CTMP degradation. Furthermore, the exposure of PAECs to ADMA led to a significant increase in the associations of CTMP with both Hsp70 (Figure 5D) and the C terminus of Hsp70-interacting protein (E3 ubiquitin ligase) (Figure 5E). This led to an increase in CTMP ubiquitination (Figure 5F). Conversely, the proteasome inhibitor, N-(benzyloxycarbonyl)leucinylleucinylleucinal Z-Leu-Leu-Leu-al (MG132), attenuated the CTMP degradation associated with ADMA exposure (Figure 5G).
Figure 5.
ADMA induces carboxyl-terminal modulator protein (CTMP) ubiquitination and proteasomal degradation in PAECs. PAECs were exposed, or not, to ADMA (10 μM, 2 h), then whole-cell lysates (1 mg) were subjected to IP using an antibody specific to Akt1 then analyzed by IB using an antibody specific to CTMP. Blots were stripped and reprobed with the Akt1 antibody to normalize for IP efficiency. A representative image is shown (A). ADMA treatment decreases Akt1–CTMP interactions (A). PAECs were transfected with DN Hsp90 or pcDNA3 for 48 hours, followed by ADMA (10 μM, 2 h) treatment. Whole-cell lysates (20 μg) were prepared and subjected to Western blot analysis to determine CTMP levels. A representative image is shown (B). Both ADMA and DN Hsp90 overexpression decreased CTMP levels (B). CTMP mRNA levels were also determined in ADMA-exposed (10 μM, 2 h) cells. ADMA has no effect on CTMP mRNA levels (C). Cell lysates (1 mg) were also subjected to IP using an antibody specific to CTMP, then subjected to IB analysis using antibodies specific to Hsp70 (D) or C terminus of Hsp70-interacting protein (CHIP) (E). Blots were stripped and reprobed with the CTMP antibody to normalize for IP efficiency. The Hsp70 blot was also stripped and reprobed with an antibody for ubiquitin (F). Representative images are shown for each (D–F). ADMA treatment increases CTMP–Hsp70 (D) and CTMP–CHIP (E) interactions, as well as ubiquitinated CTMP levels (F). PAECs were pretreated with N-(benzyloxycarbonyl)leucinylleucinylleucinal Z-Leu-Leu-Leu-al (MG132) (10 μM) or vehicle (0.1% DMSO) for 30 minutes, followed by exposure, or not, to ADMA (10 μM, 2 h). Whole-cell lysates (20 μg) were prepared and subjected to IB analysis to determine CTMP levels. A representative image is shown (G). The presence of MG132 attenuated the ADMA-mediated decrease in CTMP protein levels (G). Values are means (±SEM); n = 4–12. *P < 0.05 versus untreated; †P < 0.05 versus ADMA alone. Ub, ubiquitin.
CTMP Overexpression Restores ADMA-Mediated Akt1 Phosphorylation and eNOS Mitochondrial Translocation in PAECs
To further confirm the important role of loss of CTMP in the ADMA-mediated increase in Akt1 phosphorylation, we evaluated the effect of CTMP overexpression. Our data indicate that CTMP overexpression (Figure 6A) significantly attenuated the ADMA-mediated increase in Akt1 phosphorylation at S473 (Figure 6B), although the increase in phosphorylation at T308 was unaffected (Figure 6A). We have recently shown the importance of Akt1-mediated phosphorylation of eNOS at S617 in the mitochondrial translocation of eNOS induced by ADMA (8). Thus, we next evaluated whether CTMP overexpression could attenuate these downstream events. Using an antibody specific to nitrated Akt1 (see Figure E1 in the online supplement for validation), we found that CTMP overexpression attenuated the ADMA-mediated increase in Akt1 nitration at Y350 (Figure 6C). In addition, immunoblot analysis indicated that CTMP overexpression attenuated the eNOS phosphorylation at S617 in response to ADMA (Figure 6D), and completely abolished the ADMA-mediated increase in eNOS mitochondrial translocation (Figures 6E and 6F). Furthermore, overexpression of Akt1 mutant, where S473 was mutated to an alanine (Figure 6G), attenuated ADMA-mediated eNOS phosphorylation at S617 (Figure 6H) and Akt1 nitration at Y350 (Figure 6I).
Figure 6.
The overexpression of CTMP attenuates ADMA-induced Akt1 phosphorylation and endothelial nitric oxide synthase (eNOS) mitochondrial translocation in PAECs. PAECs were transfected with a myc-tagged CTMP expression plasmid or pcDNA3 (as a control) for 48 hours, followed by exposure to ADMA (10 μM, 2 h). Whole-cell lysates (20 μg) were subjected to IB analysis on duplicate blots. CTMP overexpression was confirmed by probing each blot with an antibody specific for myc (A–D). Changes in Akt1 phosphorylation at T308 (A) and S473 (B), Akt1 nitration at tyrosine (Y) 350 (Y350) (C), and eNOS phosphorylation at serine (Ser) 617 (D) were also determined. A representative image is shown for each (A–D). CTMP overexpression does not change ADMA-induced Akt1 phosphorylation at T308 (A) but abolishes ADMA-mediated Akt1 phosphorylation at S473 (B), Akt1 nitration at Y350 (C), and eNOS phosphorylation at S617 (D). Cells were cotransfected with eNOS-green fluorescent protein (GFP) and CTMP or the parental vector pcDNA3. After 24 hours, cells were seeded in Tek-chambered coverglass slides and allowed to attach overnight. Cells were labeled with MitoTracker (100 nM, 30 min, red; Molecular Probes, Eugene, OR), then exposed to ADMA (10 μM, 2 h). The extent of mitochondrial localization of eNOS-GFP (green) was then determined by measuring the intensity of yellow fluorescence (overlap of red fluorescence of MitoTracker and green fluorescence of eNOS-GFP) (E). CTMP overexpression abolished ADMA-mediated eNOS mitochondrial translocation (E). Mitochondrial fractions were also isolated. Mitochondrial protein extracts (5 μg) were subjected to IB analysis using an antibody raised against eNOS. ADMA increased eNOS accumulation in the mitochondria (F). CTMP overexpression attenuated ADMA-induced eNOS mitochondrial translocation (F). Loading was normalized by reprobing with the mitochondrial protein Hsp60. PAECs were transiently transfected with a mutant in which Ser473 was replaced with alanine (S473A Akt). Initial Western blotting confirmed overexpression of the construct (G). PAECs were transfected with an S473A Akt1 or pcDNA3 (as a control) for 48 hours, followed by exposure to ADMA (10 μM, 2 h). The effects on eNOS phosphorylation (H) and Akt1 nitration (I) were determined. Representative images are shown (H and I). S473A Akt1 overexpression attenuated ADMA-mediated eNOS phosphorylation at S617 (H) and Akt1 nitration at Y350 (I). Values are means (±SEM); n = 6. *P < 0.05 versus empty vector transfection without ADMA treatment; †P < 0.05 versus pCDNA3 transfection with ADMA treatment. WT, wild type.
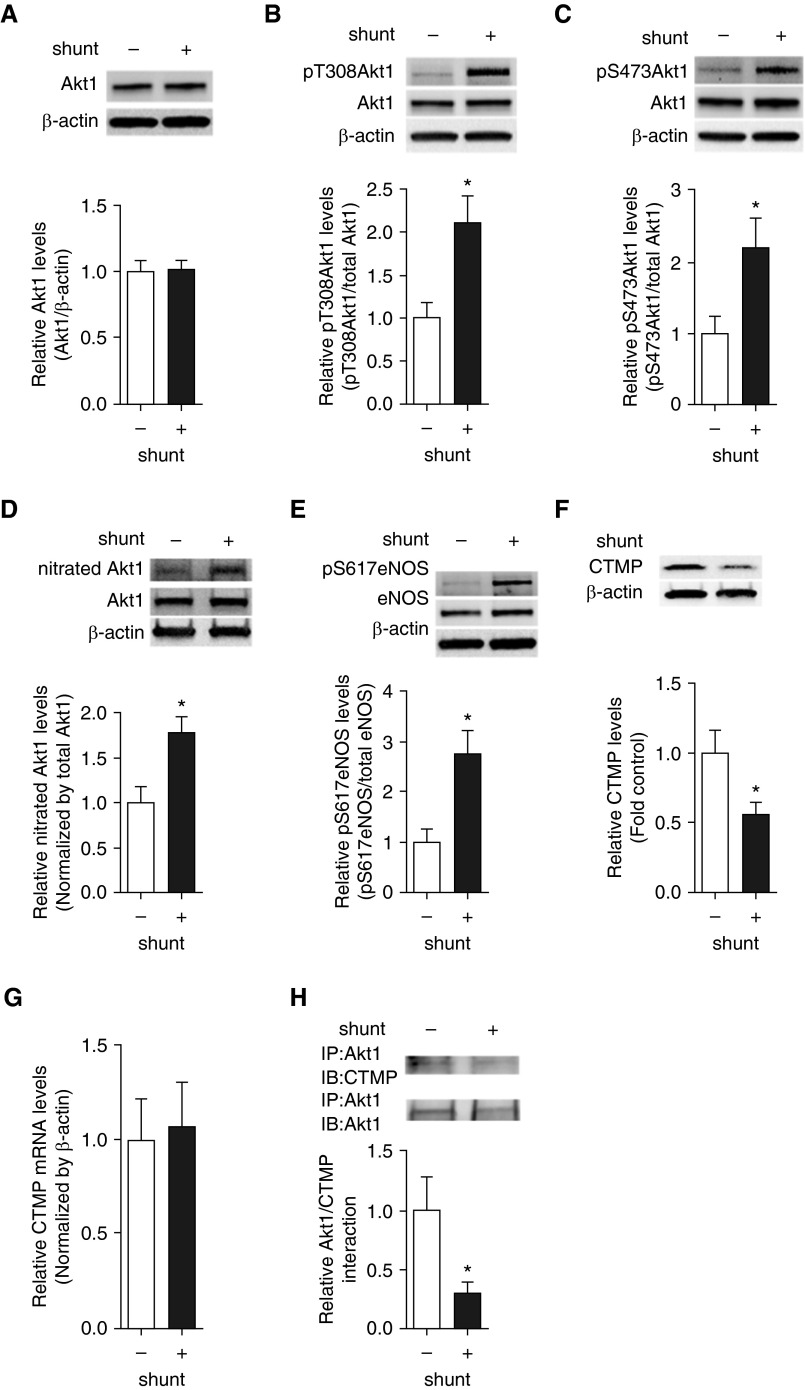
Increased Akt1 Phosphorylation Secondary to Decreased Akt1–CTMP Interactions in a Lamb Model with Increased Pulmonary Blood Flow
Our previous studies have demonstrated that ADMA levels are elevated in our shunt model of increased pulmonary blood flow (13). Therefore, we examined our lamb model to determine if the same signaling pathway exists in vivo. Our data indicate that, although total Akt1 levels are unchanged (Figure 7A), there is a significant increase in pT308 Akt1 (Figure 7B), pS473Akt1 (Figure 7C), nitrated Akt1 (Figure 7D), and pS617eNOS (Figure 7E) in shunt lambs. Furthermore, these changes correlated with a reduction in CTMP protein levels (Figure 7F), although CTMP mRNA levels were unchanged (Figure 7G), and a significant decrease in CTMP–Akt1 interactions shunt lambs (Figure 7H).
Figure 7.
Increased Akt1 phosphorylation and the CTMP degradation are recapitulated in lambs with increased pulmonary blood flow. Protein extracts (20 μg), prepared from peripheral lung of shunt and control lambs, were subjected to IB analysis on duplicate gels using antibodies specific to pT308Akt1, pS473Akt1, pS617eNOS, and CTMP. Blots were then stripped and reprobed with antibodies raised against β-actin, Akt1, or eNOS. A representative image is shown for each (A–E). Akt1 protein levels did not change in shunt lambs (A). The levels of pT308Akt1 (B), pS473Akt1 (C), and pS617eNOS (D) were significantly increased, whereas CTMP levels (E) were decreased in shunt lambs. However, CTMP mRNA levels were unchanged (F). Protein extracts (1 mg) were also subjected to IP using an antibody specific to Akt1, then analyzed by IB analysis using an antibody raised against CTMP. A representative image is shown (G). The association of Akt1 with CTMP was significantly reduced in shunt lambs (H). Values are means (±SEM); n = 6–10. *P < 0.05 versus control.
Discussion
As an endogenous NOS inhibitor, increased levels of ADMA are thought to be a biomarker of endothelial dysfunction in various cardiovascular diseases (26). This concept is supported by studies showing that cellular ADMA concentrations tightly mediate the local NO–reactive oxygen species (ROS) balance (27, 28). An imbalance in NO–ROS is associated with a hypertensive phenotype (29). The study we present here provides further evidence for the importance of increased ADMA in modulating cellular signaling.
Akt1 belongs to the Ser/Thr protein kinase family, and is known to phosphorylate eNOS at S617 (30) and S1177 (31). Phosphorylation of these sites increases eNOS activity by enhancing calcium responsiveness (32). However, it should be noted that these studies were performed using purified enzymes, and the in vivo situation will be more complex. Indeed, we have identified a novel role for these phosphorylation sites, mediated by nitrated Akt1, in regulating the mitochondrial translocation of eNOS, independent of their role in regulating eNOS activity (8). Although pS1177 is involved, pS617 drives the process by exposing the normally buried mitochondrial translocation sequence (8). S617 is not easily accessible to Akt1, and its phosphorylation in vivo appears to be dependent on the formation of a salt bridge between nitrated Y350 on Akt1 and K612 of eNOS (8). Preventing Akt1 nitration at Y350 prevents pS617 phosphorylation and eNOS mitochondrial translocation (8). The data we present here add to our understanding of this process by demonstrating that ADMA also stimulates Akt1 activity by increasing the phosphorylation at T308 and S473 through an Hsp70-dependent increase in CTMP proteasomal degradation. Consistent with our findings, prior work has shown that vascular endothelial growth factor stimulates endothelial cell proliferation through an ROS-dependent increase in Akt1 phosphorylation (33).
The mechanism of Akt1 activation is complex (19). It is primarily controlled in a positive manner by phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and a negative manner by the action of PTEN via the modulation of phosphatidylinositol (3-5)-trisphosphate levels, which modulate Akt1 translocation to the plasma membrane (16). Our data indicate that ADMA does not appear to modulate PTEN activity. However, we obtained conflicting data that ADMA was able to enhance Akt1 plasma membrane translocation with immunoblot analysis of plasma membrane fractions, suggesting that Akt1 levels were unchanged, whereas the more sensitive technique, confocal microscopy, indicated that plasma membrane Akt1 was increased. Thus, although PTEN activity was unchanged, ADMA may be able to stimulate PI3K activity and Akt1 plasma membrane translocation. Although our studies did not test this possibility, there is support for a mechanism by which ROS can increase PI3K activity by stimulating its interaction with p21 rat sarcoma (34), and we have shown that ADMA increases ROS generation in PAECs (21). In addition to its translocation to the plasma membrane, Akt1 activity is also regulated by phosphorylation. T308 phosphorylation increases Akt1 activity by approximately 100-fold (3). Phosphorylation at S473 can further increase Akt1 activity 7- to 10-fold (2). Mutations of either T308 or S473 to Ala have been shown to have no effect on the other residue being phosphorylated in response to insulin, suggesting independence (19). However, other studies using an RNA interference approach to attenuate Rictor expression or a small molecule inhibitor of mammalian target of rapamycin reduced both S473 and T308 phosphorylation, suggesting that the phosphorylation events are interdependent (3). In our studies, CTMP overexpression did not prevent the ADMA-mediated increase in T308 phosphorylation, but did attenuate the phosphorylation of S473. It is unclear why this occurred, but a possible explanation is that the binding site of CTMP on Akt1 (411–480) (7) likely blocks S473 from phosphorylation. A similar effect has also been seen in human hepatocellular carcinoma cells, where fenofibrate (a peroxisome proliferator–activated receptor α agonist) increased CTMP protein levels, and this correlated with a decrease in Akt1 phosphorylation at S473, but not T308 (35). These data also suggest that, as blocking S473 phosphorylation is sufficient to abolish Akt1 downstream signaling, it may play a more important role than T308 phosphorylation to determine Akt1 activity, at least in mediating the mitochondrial translocation of eNOS. However, although the crystal structure of Akt1 suggests that S473 phosphorylation is pivotal for kinase activation and stabilization (36), patients with non–small cell lung cancer have increased Akt1 signaling, which correlates with higher levels of T308, but not S473, phosphorylation (3). Thus, the regulation of Akt1 phosphorylation and the involvement of the different phosphorylation sites in transducing an upstream signal is complex and will need further study to unravel.
Hsp90 is a highly conserved and abundant cytosolic protein, which serves as an ATPase-dependent chaperone participating in protein folding and signal transduction (20). Hsp90 interacts with a number of proteins that are required for efficient NO biosynthesis, including eNOS (37), Akt1 (20), and, as we have recently shown, GTP cyclohydrolase I, the rate-limiting enzyme in tetrahydrobiopterin biosynthesis (13). Amino acids 229–309 in Akt1 are required to bind Hsp90 (20). The binding of Hsp90 to Akt1 enhances its activity by preventing phosphatase 2A (PP2A)–mediated dephosphorylation of Akt1 at T308 (20). In our current study, we evaluated Akt1 phosphorylation using both pharmacologic and molecular interventions determined to inhibit Hsp90. Interestingly, the results obtained are contradictory with pharmacologic inhibition decreasing and overexpressing a DN Hsp90 increasing Akt1 phosphorylation. This discrepancy is likely related to the fact that the Hsp90 inhibitor, GA decreased Akt1 protein levels, most likely via increased Akt1 proteasomal degradation. Furthermore, our data indicate that ADMA mimics the effect of DN Hsp90 overexpression. However, we did not find a synergistic increase in Akt1 phosphorylation when used in combination, suggesting that either intervention is sufficient to drive maximal Akt1 phosphorylation. Moreover, our data show that, even when ADMA decreased Hsp90–Akt1 interactions, Akt1 phosphorylation was still increased, due to the concomitant increase in CTMP degradation. This suggests that CTMP-mediated increases in Akt1 phosphorylation are sufficient to overcome any increases in PP2A-stimulated Akt1 dephosphorylation, although it is possible that ADMA may also impair PP2A activity. Further examinations will be needed to investigate this possibility.
CTMP was initially identified as a negative regulator of Akt1 through its binding to Akt1 at the plasma membrane and the subsequent inhibition of Akt1 phosphorylation and activity (7). Thus, modulating CTMP expression may provide a potential therapeutic strategy to target Akt1 signaling pathways and Akt1-associated diseases. Indeed, increasing CTMP expression reduces non–small cell lung tumor progression in mice (38), exerts robust protection against ischemia-induced neuronal death (39), and restores immune function (40). It is, however, worth noting that, although the preponderance of evidence supports CTMP as a negative regulator of Akt1 phosphorylation and activation (7, 39, 41), a few studies suggest that CTMP can act as a positive regulator of Akt1 (6, 42, 43). In our work, we have elucidated a new regulatory mechanism by which the proteasomal degradation of CTMP leads to an increase in Akt1 phosphorylation. CTMP, similar to GTP cyclohydrolase I (13), can be post-translationally regulated through an Hsp70-facilitated, ubiqutin-dependent degradation. Our findings are supported by previous studies, in which vascular endothelial growth factor–induced Akt1 phosphorylation is attenuated when heat shock cognate protein 70 is depleted (44). Interestingly, emerging studies also indicate that CTMP can localize to the mitochondria (45). This mitochondrial localized CTMP appears to have a major function in the regulation of mitochondrial dynamics, and may also be involved in the regulation of mitochondrial function (46). This is important, as mitochondrial dysfunction is now appreciated to be a critical event in many diseases, including pulmonary hypertension (47). Our previous studies have shown that mitochondrial function is decreased in lambs with pulmonary hypertension (48). The decrease in mitochondrial function in these lambs is induced by the mitochondrial translocation of eNOS, and the data we present here shows that this is likely due to increased Akt1 activation and the subsequent phosphorylation of eNOS at S617, and that this is driven by decreases in CTMP protein and a subsequent reduction in Akt1–CTMP interactions. However, it should be noted that the animal studies were performed using random lobe biopsies from peripheral lung, and thus contain distal intrapulmonary segments, and we did not localize the events in the lambs to the endothelium. It is therefore possible that other cell types in the lung may also undergo similar regulation of the Hsp90–Akt1–CTMP pathway. However, further studies will be required to investigate this possibility. However, overall, our data allow us to conclude that CTMP plays a pivotal role in ADMA-mediated Akt1 phosphorylation in vitro and in vivo, and we speculate that CTMP may be a potential therapeutic target to treat pulmonary hypertension.
Supplementary Material
Footnotes
This work was supported in part by National Institutes of Health grants HL60190 (S.M.B.), HL67841 (S.M.B.), HL101902 (S.M.B.), HD039110 (S.M.B.), and HL61284 (J.R.F.), and American Heart Association National Office Scientist Development Grant 14SDG20480354 (R.R.).
Author Contributions: Conception and design—X.S., M.K., S.F., R.R., J.R.F., and S.M.B.; analysis and interpretation—X.S., Q.L., A.K., N.Q., C.K., S.F., R.R., J.R.F., and S.M.B.; drafting the manuscript for important intellectual content—X.S., M.K., A.A.D., T.W., S.F., J.X.-J.Y., J.R.J., J.G.N.G., J.R.F., and S.M.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0185OC on March 9, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brazil DP, Park J, Hemmings BA. PKB binding proteins: getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 2.Piao L, Li Y, Yang KJ, Park KA, Byun HS, Won M, Hong J, Kim JL, Kweon GR, Hur GM, et al. Heat shock protein 70–mediated sensitization of cells to apoptosis by carboxyl-terminal modulator protein. BMC Cell Biol. 2009;10:53. doi: 10.1186/1471-2121-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, et al. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;308:L208–L220. doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Liu YP, Liao WC, Ger LP, Chen JC, Hsu TI, Lee YC, Chang HT, Chen YC, Jan YH, Lee KH, et al. Carboxyl-terminal modulator protein positively regulates Akt phosphorylation and acts as an oncogenic driver in breast cancer. Cancer Res. 2013;73:6194–6205. doi: 10.1158/0008-5472.CAN-13-0518. [DOI] [PubMed] [Google Scholar]
- 7.Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294:374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 8.Rafikov R, Rafikova O, Aggarwal S, Gross C, Sun X, Desai J, Fulton D, Black SM. Asymmetric dimethylarginine induces endothelial nitric-oxide synthase mitochondrial redistribution through the nitration-mediated activation of Akt1. J Biol Chem. 2013;288:6212–6226. doi: 10.1074/jbc.M112.423269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Sharma S, Fratz S, Kumar S, Rafikov R, Aggarwal S, Rafikova O, Lu Q, Burns T, Dasarathy S, et al. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal. 2013;18:1739–1752. doi: 10.1089/ars.2012.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S, Gross CM, Rafikov R, Kumar S, Fineman JR, Ludewig B, Jonigk D, Black SM. Nitration of tyrosine 247 inhibits protein kinase G-1α activity by attenuating cyclic guanosine monophosphate binding. J Biol Chem. 2014;289:7948–7961. doi: 10.1074/jbc.M113.534313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L984–L991. doi: 10.1152/ajplung.00224.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Sun X, Kumar S, Rafikov R, Aramburo A, Kalkan G, Tian J, Rehmani I, Kallarackal S, Fineman JR, et al. Preserving mitochondrial function prevents the proteasomal degradation of GTP cyclohydrolase I. Free Radic Biol Med. 2012;53:216–229. doi: 10.1016/j.freeradbiomed.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Fratz S, Sharma S, Hou Y, Rafikov R, Kumar S, Rehmani I, Tian J, Smith A, Schreiber C, et al. C-terminus of heat shock protein 70–interacting protein-dependent GTP cyclohydrolase I degradation in lambs with increased pulmonary blood flow. Am J Respir Cell Mol Biol. 2011;45:163–171. doi: 10.1165/rcmb.2009-0467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian J, Hou Y, Lu Q, Wiseman DA, Vasconcelos Fonsesca F, Elms S, Fulton DJ, Black SM. A novel role for caveolin-1 in regulating endothelial nitric oxide synthase activation in response to H2O2 and shear stress. Free Radic Biol Med. 2010;49:159–170. doi: 10.1016/j.freeradbiomed.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 16.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 19.Vanhaesebroeck B, Alessi DR. The PI3K–PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 20.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorska M, Popowska U, Sielicka-Dudzin A, Kuban-Jankowska A, Sawczuk W, Knap N, Cicero G, Wozniak F. Geldanamycin and its derivatives as Hsp90 inhibitors. Front Biosci (Landmark Ed) 2012;17:2269–2277. doi: 10.2741/4050. [DOI] [PubMed] [Google Scholar]
- 23.Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- 24.Bhat R, Tummalapalli SR, Rotella DP. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J Med Chem. 2014;57:8718–8728. doi: 10.1021/jm500823a. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Jeon YJ, Ahn YM, Lee HY, Woo SY, Park HS, Ha EH, Pang MG. Prenatal increased asymmetric dimethylarginine is associated with placental heat-shock protein 70 and lectin-like oxidized low-density lipoprotein receptor-1 expression. Arch Med Res. 2007;38:839–845. doi: 10.1016/j.arcmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Zakrzewicz D, Eickelberg O. From arginine methylation to ADMA: a novel mechanism with therapeutic potential in chronic lung diseases. BMC Pulm Med. 2009;9:5. doi: 10.1186/1471-2466-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–460. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwedhelm E, Böger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7:275–285. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- 29.Tain YL, Huang LT. Restoration of asymmetric dimethylarginine-nitric oxide balance to prevent the development of hypertension. Int J Mol Sci. 2014;15:11773–11782. doi: 10.3390/ijms150711773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 31.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran QK, Leonard J, Black DJ, Persechini A. Phosphorylation within an autoinhibitory domain in endothelial nitric oxide synthase reduces the Ca(2+) concentrations required for calmodulin to bind and activate the enzyme. Biochemistry. 2008;47:7557–7566. doi: 10.1021/bi8003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species-dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 34.Deora AA, Win T, Vanhaesebroeck B, Lander HM. A redox-triggered ras-effector interaction: recruitment of phosphatidylinositol 3′-kinase to Ras by redox stress. J Biol Chem. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki D, Kawabe N, Nakamura H, Tachibana K, Ishimoto K, Tanaka T, Aburatani H, Sakai J, Hamakubo T, Kodama T, et al. Fenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARα-independent mechanisms. Eur J Cell Biol. 2011;90:657–664. doi: 10.1016/j.ejcb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J, Cyr D, Babbitt RW, Sessa WC, Patterson C. Chaperone-dependent regulation of endothelial nitric-oxide synthase intracellular trafficking by the co-chaperone/ubiquitin ligase CHIP. J Biol Chem. 2003;278:49332–49341. doi: 10.1074/jbc.M304738200. [DOI] [PubMed] [Google Scholar]
- 38.Hwang SK, Lim HT, Minai-Tehrani A, Lee ES, Park J, Park SB, Beck GR, Jr, Cho MH. Repeated aerosol delivery of carboxyl-terminal modulator protein suppresses tumor in the lungs of K-rasLA1 mice. Am J Respir Crit Care Med. 2009;179:1131–1140. doi: 10.1164/rccm.200810-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Leeman SE, Amar S. Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proc Natl Acad Sci USA. 2011;108:2867–2872. doi: 10.1073/pnas.1019270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knobbe CB, Reifenberger J, Blaschke B, Reifenberger G. Hypermethylation and transcriptional downregulation of the carboxyl-terminal modulator protein gene in glioblastomas. J Natl Cancer Inst. 2004;96:483–486. doi: 10.1093/jnci/djh064. [DOI] [PubMed] [Google Scholar]
- 42.Ono H, Sakoda H, Fujishiro M, Anai M, Kushiyama A, Fukushima Y, Katagiri H, Ogihara T, Oka Y, Kamata H, et al. Carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways. Am J Physiol Cell Physiol. 2007;293:C1576–C1585. doi: 10.1152/ajpcell.00570.2006. [DOI] [PubMed] [Google Scholar]
- 43.Franke TF. Akt-interacting proteins: attractive opposites: focus on “carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways”. Am J Physiol Cell Physiol. 2007;293:C1768–C1770. doi: 10.1152/ajpcell.00451.2007. [DOI] [PubMed] [Google Scholar]
- 44.Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K, Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol. 2010;30:491–497. doi: 10.1161/ATVBAHA.109.193631. [DOI] [PubMed] [Google Scholar]
- 45.Parcellier A, Tintignac LA, Zhuravleva E, Cron P, Schenk S, Bozulic L, Hemmings BA. Carboxy-terminal modulator protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis. Cell Signal. 2009;21:639–650. doi: 10.1016/j.cellsig.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Parcellier A, Tintignac LA, Zhuravleva E, Dummler B, Brazil DP, Hynx D, Cron P, Schenk S, Olivieri V, Hemmings BA. The carboxy-terminal modulator protein (CTMP) regulates mitochondrial dynamics. PLoS One. 2009;4:e5471. doi: 10.1371/journal.pone.0005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien D, Chunduri P, Iyer A, Brown L. L-carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin Pharmacol Toxicol. 2010;106:296–301. doi: 10.1111/j.1742-7843.2009.00480.x. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L46–L56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.