Abstract
Although neutrophils play critical roles in innate immunity, in excess these cells cause severe tissue damage. Thus, neutrophil activation must be tightly regulated to prevent indiscriminant damage. Previously, we reported that mice lacking matrix metalloproteinase (MMP) 7 are protected from lung injury owing to markedly impaired neutrophil movement from the interstitium into mucosal lumenal spaces. This phenotype resulted from a lack of MMP7 shedding of syndecan-1, a heparan sulfate proteoglycan that carries the neutrophil chemokine CXCL1 as cargo. Here, we assessed if shedding syndecan-1/CXCL1 complexes affects neutrophil activation. Whereas injured monolayers of wild-type alveolar type II cells potently stimulated neutrophil activation, as gauged by release of myeloperoxidase, cells from Mmp7−/− or syndecan-1-null (Sdc1−/−) mice or human cells with MMP7 knockdown did not. In vivo, we observed reduced myeloperoxidase release relative to neutrophil numbers in bleomycin-injured Mmp7−/− and Sdc1−/− mice. Furthermore, we determined that soluble syndecan-1 directly stimulated neutrophil activation in the absence of cellular damage. These data indicate that MMP7 shedding of syndecan-1/CXCL1 complexes functions as a checkpoint that restricts neutrophil activation at sites of epithelial injury.
Keywords: metalloproteinase, lung injury, inflammation
Clinical Relevance
Although neutrophils are an essential arm of innate immunity, in excess, they can contribute greatly to tissue damage associated with a variety of conditions, including acute lung injury, transfusion-related acute lung injury, severe asthma, and many others. Here, we describe an epithelial mechanism that functions to moderate neutrophil activation at the mucosal surface, which could be manipulated to minimize neutrophil-mediated tissue damage in severe inflammatory conditions.
Neutrophils are an essential arm of innate immunity. However, in excess, they can contribute greatly to tissue damage associated with a variety of conditions, including acute lung injury, transfusion-related acute lung injury, severe asthma, and many others (1–3). Tissue damage likely does not result from just the presence of neutrophils but rather from the indiscriminate damage caused by the oxidative burst of the fully activated cell.
As they advance from the vasculature into injured or infected tissues, neutrophils go through progressive stages of priming and activation, culminating in the release and production of a variety of cytotoxic/bacteriocidal compounds, such as reactive oxygen species and cationic peptides, which counteract microorganisms (4, 5). Neutrophil priming and full activation are stimulated by many factors and events, such as chemokines, cytokines, integrin ligation, selectin ligation, lipid mediators, and bacterial products. Although much is known about specific mechanisms controlling neutrophil activation, particularly at the endothelial interface, less is known about how activation and movement of these granulocytes is controlled within interstitial and epithelial compartments. In addition, it is likely that specific counter-regulatory mechanisms evolved to constrain the activation of neutrophils as they move through different tissue compartments.
Matrilysin/matrix metalloproteinase (MMP) 7 is an epithelial MMP that is induced in response to injury, infection, or allergen challenge (6–8). We reported that, in response to lung or colon injury, transepithelial migration of neutrophils is halted in Mmp7−/− mice (9, 10). Furthermore, this phenotype was accompanied by markedly reduced levels within the alveolar or colon lumenal spaces of CXCL1/KC (9, 10), an acute-phase neutrophil chemokine that is the murine functional ortholog of IL-8/CXCL8. Interestingly, although CXCL1 expression was not affected in Mmp7−/− mice, total levels of CXCL1 protein were greater in tissue homogenates of injured Mmp7−/− mice compared with wild types. We determined that MMP7 cleaves the juxtamembrane domain of syndecan-1, an abundant transmembrane heparan sulfate (HS) proteoglycan on the basal surface of most epithelial cells, liberating an intact ectodomain (9). In addition, we found that CXCL1 is bound to the glycosaminoglycan (GAG) chains of shed syndecan-1, and that neutrophils and CXCL1 levels are reduced in the alveolar space of bleomycin-injured syndecan-1-null (Sdc1−/−) mice (9).
At first glance, it seemed that MMP7 shedding of syndecan-1/CXCL1 complexes generates a chemokine gradient that neutrophils follow into a lumenal space, but, of course, the cells would be moving against the gradient. Thus, we reasoned that MMP7 shedding of syndecan-1/CXCL1 complexes affects some other neutrophil process. In the present study, we demonstrate that MMP7 and syndecan-1 function together to regulate neutrophil activation as these cells reach a mucosal interface. Our findings show that interaction with cell-bound syndecan-1/CXCL1 complexes restrict neutrophil movement and activation, thereby preventing a damaging oxidative burst at the epithelial cell surface, whereas interaction with soluble syndecan-1/CXCL1 complexes promotes activation, ideally at a safer distance from the mucosal layer (see Figure 6). Thus, we propose that, in addition to essential roles in facilitating re-epithelialization and restoration of barrier function (11), the MMP7–syndecan-1 axis functions also as checkpoint to spatially restrict neutrophil activation near to a compromised epithelium.
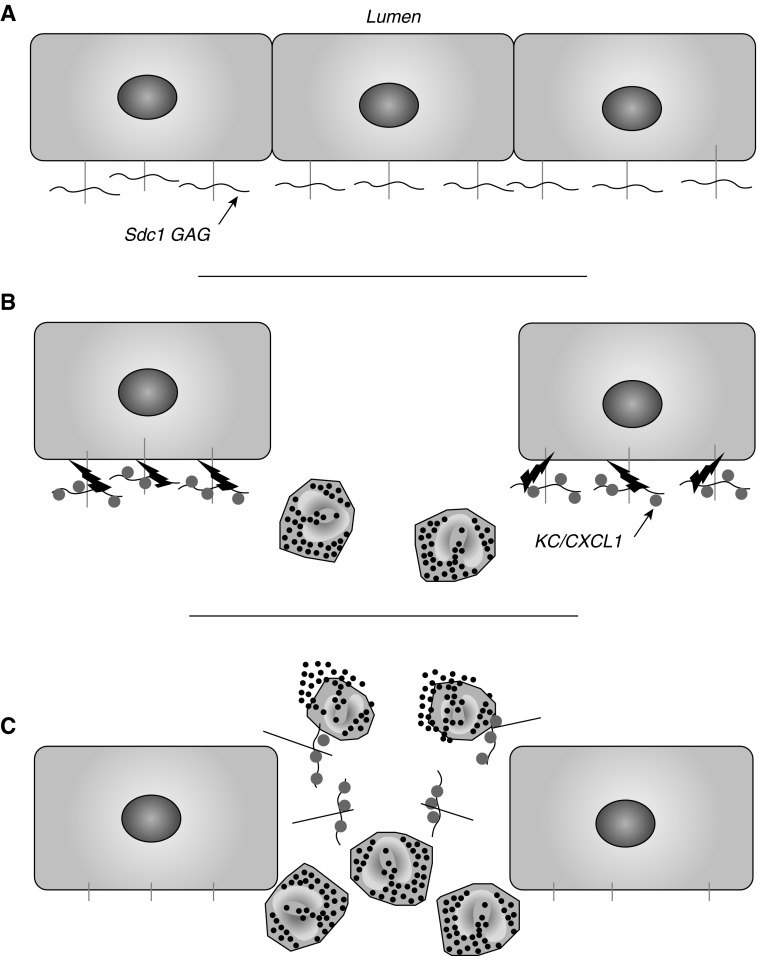
Figure 6.
MMP7 shedding of epithelial Sdc1 functions as a checkpoint of neutrophil activation. (A) Sdc1 is present on the basal–lateral surface of intact lung epithelium. (B) In response to injury, MMP7 (lightning bolt) and KC/CXCL1 are induced, and neutrophils begin to infiltrate to the wound site. Secreted CXCL1 accumulates on the glycosaminoglycan (GAG) chains (horizontal wavy line) of Sdc1, and the complexes are shed by MMP7. (C) Neutrophils interact with shed Sdc1/CXCL1 complexes, which promotes activation and release of cellular contents.
Materials and Methods
Mouse Model
Wild-type, Mmp7−/−, and Sdc1−/− mice (all C57Bl/6; near equal mix of females and males; 8 weeks old) were intubated with 2 × 109 CFU live Pseudomonas aeruginosa (PAO1) or bleomycin (4 U/kg), as previously described (12, 13). Mice were killed at the indicated times, and lungs were either lavaged twice with PBS (1 ml/lavage) or infused with optical cutting temperature compound (VWR, Batavia, IL). Bronchoalveolar lavages (BALs) were processed for cell counts and differentials, as previously described (14). Lavaged lungs were snap frozen in liquid nitrogen for RNA and protein analysis. Mouse protocols were approved by the Office of Animal Welfare at the University of Washington (Seattle, WA), by the Institutional Animal Care and Use Committee at Children’s Hospital (Boston, MA; P. aeruginosa infection), and the Institutional Animal Care and Use Committee at Washington University in St. Louis (glucose uptake studies).
Assays
Immunoprecipitation, immunoblotting, RNA isolation, and quantitative RT-PCR were done as previously described (9, 10). Frozen lungs section (5-μm sections) were immunostained for myeloperoxidase (MPO; RB-373-A1; Thermo Scientific, Fremont, CA), and images were captured using the Nikon Eclipse 90i with NIS-Elements Software (Nikon Instruments Inc., Melville, NY).
Cell Culture Models
Parental SW620 and Mmp7 antisense RNA-expressing cells (SW620AS) were developed and cultured under selective conditions, as previously described (15). Alveolar type II epithelial cells were isolated from lungs of wild-type, Mmp7−/−, and Sdc1−/− mice following the dispase-agarose protocol of Corti and colleagues (16). Briefly, type II cells were isolated from a lung-cell suspension by removing leukocytes via immunoaffinity-magnetic separation. As determined by OsO4/tannic acid staining, we routinely achieved approximately 80–90% type II cells with excellent viability. Type II cells were seeded in 12-well dishes at a near-confluent density (106 cells/well) and were cultured for 4–5 days. Monolayers were either infected with P. aeruginosa (PAO1; 50 multiplicity of infection) for 1 hour and washed thoroughly with antibiotics, as described previously (17), or were given uniform wounds by gently scratching a sterile 20-g needle in a 10 × 10 criss-cross pattern. Injured monolayers (and noninjured controls) were washed three times with sterile PBS and incubated for 2 hours in serum-free Ham’s/F12 medium containing 0.5 mg/ml BSA to allow time to respond to injury.
Blood (5 ml) was obtained from healthy human volunteers (University of Washington Institutional Review Board protocol 03-8666-V/E 04), and neutrophils were isolated using Lympholate-poly (CL5071; Cedarlane Laboratories Ltd., Burlington, NC). Neutrophils were suspended in serum-free medium/BSA at 2 × 106 cells/ml, and 1 ml was added to intact or injured/infected epithelial monolayers or empty wells. Control epithelial cultures received an equal volume of medium/BSA. Neutrophils cultured alone were treated with 1 μM N-formyl-L-methionyl-L-leucyl-L-phenylalanine (nFMLP; Sigma, St. Louis, MO), 1 µM phorbol-12-myristate-13-acetate, or vehicle. After a 3-hour incubation, conditioned media were collected and centrifuged, and the cell layer harvested for RNA isolation. Supernatants were serially diluted 1:3, spotted on PVDF membrane, and MPO was detected using a rabbit anti-human MPO antibody (475915; Calbiochem, San Diego, CA), a horseradish peroxidase–conjugated secondary antibody, and SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Scientific, Waltham, MA). In some experiments, MPO levels were measured by an enzymatic activity assay (Enzchek; Invitrogen, Eugene, OR). Chemiluminescent signal was collected digitally using an EpiChem3 Darkroom (UVP Bioimaging Systems, Upland, CA), and pixel density per spot was normalized to sample volume and expressed in arbitrary units. Isolated neutrophils were also incubated with 100 ng/ml CXCL8, 4 μg/ml low–molecular weight heparin, or a combination of both at different molar ratios. After a 0.5-hour incubation, the conditioned medium was collected, and MPO levels were assayed.
COS7 cells (ATCC, Manassas, VA) were stably transfected using pcDNA 3.3-TOPO TA (Invitrogen, Carlsbad, CA) with empty vector, or syndecan-1 truncated at the juxtamembrane site. Conditioned medium was collected after 12 hours of incubation with stable transfectants and concentrated 10-fold using Amicon 50-kD centrifugal filtration units (Millipore, Billerica, MA). Conditioned medium was incubated with isolated neutrophils (0.5 × 106 cells) for 4 hours at 37°C. After removing the neutrophils by centrifugation at 100 rpm for 5 minutes, MPO activity in the medium was assessed, as described previously here. Syndecan-1 was immunoprecipitated from the conditioned medium using antibody 281-2 (BD Biosciences, San Jose, CA) conjugated to protein G beads (Invitrogen, Carlsbad, CA).
Glucose Uptake
We quantitated the uptake of the glucose analog [18F]fluorodeoxyglucose ([18F]FDG) as a marker of early neutrophil priming within the circulation. As reported, the uptake of [18F]FDG into circulating neutrophil correlates closely with their influx into lung (18, 19). Mice were injected intravenously with 1 μCi/g body weight [18F]FDG at 1 or 3 days after instillation with saline or bleomycin (0.8 U), and plasma was collected 1 hour later.
Statistical Analysis
Appropriate methods, including unpaired t tests and one-way ANOVAs with a Tukey post hoc test, were used for each experiment (indicated in the figure legends) and P less than 0.05 was considered significant.
Results
Reduced Neutrophil Influx into the Alveolar Space of Infected Mmp7−/− and Sdc1−/− Mice
Using a model of sterile acute lung injury (bleomycin toxicity), we reported that MMP7 shedding of syndecan-1/CXCL1 complexes permits transepithelial efflux of neutrophils into the alveolar space of injured lungs (9). We also reported that P. aeruginosa infection potently induces MMP7 expression and activation (17, 20). Therefore, to determine if this mechanism functions in response to disparate challenges, we assessed if MMP7 and syndecan-1 function in controlling neutrophil influx across the epithelium in response to this relevant lung pathogen.
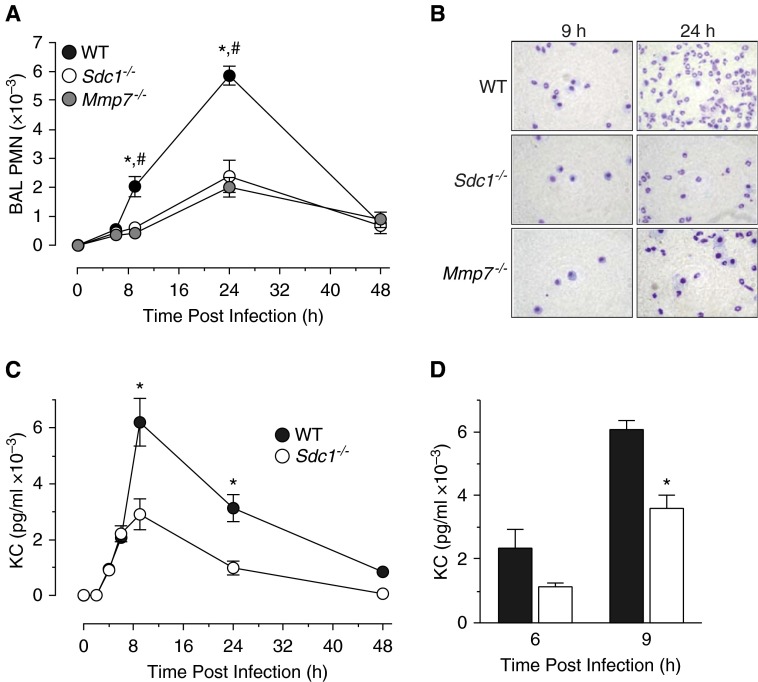
As reported (9), the numbers of circulating neutrophils does not differ among unchallenged wild-type, Mmp7−/−, or Sdc1−/− mice. However, after P. aeruginosa infection, we found that the influx of neutrophils into the airspace was significantly reduced in Mmp7−/− and Sdc1−/− mice compared with infected wild types (Figures 1A and 1B). The numbers of macrophages and lymphocytes did not differ among genotypes (data now shown), and were comparable with the numbers reported in other studies (14, 21). Levels of CXCL1 in BAL were reduced in infected Sdc1−/− mice compared with wild-type mice (Figure 1C). In addition, CXCL1 in the BAL of infected wild-type mice was partially cleared with anti–syndecan-1 antibody (Figure 1D), indicating that the chemokine was complexed with shed syndecan-1. These findings mirror our data with the bleomycin model (9), and indicate that MMP7 shedding of syndecan-1/CXCL1 from lung epithelia controls the transmucosal advancement of neutrophils in response to both noninfectious and infectious insults. Furthermore, we detected no significance difference among endpoints between female and male mice of a given genotype.
Figure 1.
Matrix metalloproteinase (MMP) 7 and syndecan-1 (Sdc1) promote neutrophil influx in response to acute Pseudomonas aeruginosa infection. Wild-type (WT), Mmp7−/−, and Sdc1−/− mice were intubated with 2 × 109 colony-forming units of live P. aeruginosa, and bronchoalveolar lavage (BAL) was collected at the indicated times. (A) Total neutrophils (PMN) were quantified on stained cytospins (n = 4, mean ± SE; P < 0.01, WT relative to #Mmp7−/− and *Sdc1−/−). (B) Representative pictures at 9 and 24 hours after infection. (C) CXCL1 (KC) levels in BAL were determined by ELISA. *P < 0.01. (D) WT BAL was immunoprecipitated with control rat IgG (solid bars) or syndecan-1 ectodomain antibody 281-2 (open bars). KC levels remaining in BAL were determined by ELISA. *P < 0.01 compared with IgG control.
Activated Type II Pneumocytes Stimulate Neutrophil Activation
Mmp7−/− mice are markedly protected from lethality due to lung or colon injury, which we attributed to impaired neutrophil movement (9, 10). However, we did not think that the lack of transepithelial neutrophil influx alone caused the significant difference in mortality. Rather, we hypothesized that the state of neutrophil activation differed between genotypes. Because neutrophils had egressed from the vasculature and into the lung and colon tissues (9, 10), it is likely that early stages of activation—such as increased glucose uptake in circulating neutrophils (19)—were identical between genotypes. Indeed, we saw no significant difference in the uptake of the glucose analog [18F]fluorodeoxyglucose—a parameter that correlates with neutrophil influx (18, 19)—in circulating neutrophils from bleomycin-injured wild-type and Mmp7−/− mice (data not shown). Because neutrophils and endothelial cells do not express MMP7, we speculated that control over neutrophil activity by this proteinase was mediated by epithelial cells.
To test this idea, we developed a coculture model to assess if MMP7 shedding of syndecan-1/CXCL1 complexes from wounded epithelium stimulates neutrophil activation. For this, alveolar type II epithelial cells from wild-type, Mmp7−/−, and Sdc1−/− mice were plated at a high density to quickly achieve confluency, and cultured for 4–5 days to overcome the stress of isolation. We then wounded the monolayers by repeated scraping. MMP7 is not expressed by intact type II cells, but is induced in response to injury (22).
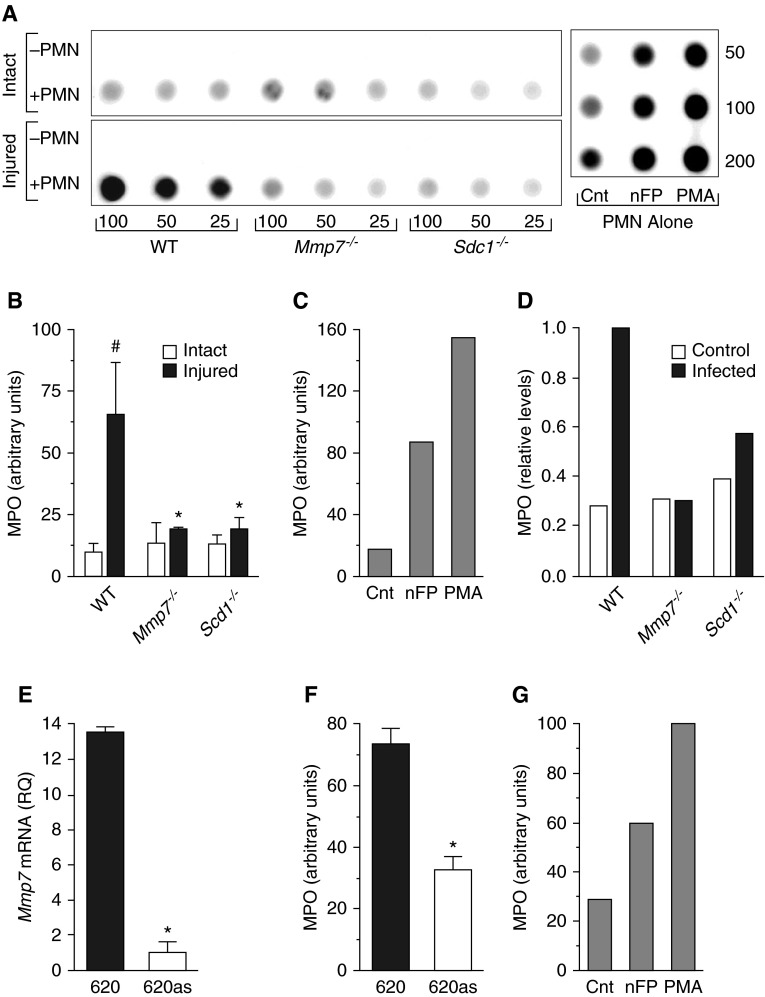
After allowing 2 hours for the cells to respond to injury, normal human neutrophils were added, and medium was collected 3 hours later. Release of MPO, a marker of neutrophil respiratory burst (23), was used to gauge the degree of activation. Neutrophils were not activated when added to intact type II cultures regardless of genotype (Figures 2A and 2B). However, neutrophils were robustly activated when cocultured with injured wild-type cells (Figures 2A and 2B) to a level comparable to that induced by nFMLP, a bacterial peptide and a potent neutrophil agonist (Figure 2C). Activation was not stimulated in neutrophils added to wounded Mmp7−/− or Sdc1−/− monolayers (Figures 2A and 2B; note: the variation in MPO activation levels among experiments [e.g., Figure 2C versus Figure 2G] likely reflects differences in basal and stimulated neutrophil activation from different donors).
Figure 2.
MMP7 and syndecan-1 are required for epithelial-mediated neutrophil activation. (A and B) Alveolar type II epithelial cells were isolated from WT, Mmp7−/−, and Sdc1−/− mice, grown to confluence, scratch wounded, incubated for 2 hours to respond to injury, then cocultured for 3 hours with 2 × 106 normal human neutrophils. Myeloperoxidase (MPO) levels in the media samples were determined by immunoblot assay. Shown is a representative immunoblot for MPO. Values (25–100 μl, 50–200 μl) are the volumes of cell-free conditioned media spotted per dot. Neutrophils (2 × 106; PMN alone) were stimulated for 3 hours with vehicle (Cnt), 1 μM N-formyl-L-methionyl-L-leucyl-L-phenylalanine (nFMLP [nFP]), or 1 μM phorbol-12-myristate-13-acetate (PMA). (B) MPO immunoblot signal was expressed in arbitrary units. Data are mean ± SE of cells isolated from 5 mice/genotype. #P < 0.01, relative to intact within the same genotype; *P < 0.05, relative to WT within same condition. (C) Data are the means of MPO release from neutrophils from duplicate experiments. (D) Confluent monolayers of alveolar type II epithelial cells were infected with 50 multiplicity of infection P. aeruginosa for 1 hour, washed with antibiotics, and cocultured for 3 hours with 2 × 106 normal human neutrophils. Data are from one experiment. (E) Mmp7 mRNA levels in parental SW620 (620) and Mmp7 knockdown (620AS) human colon carcinoma cells were measured by quantitative RT-PCR and expressed as relative quantification (RQ). *P < 0.01. (F) Parental and Mmp7 knockdown human colon carcinoma cells were grown to confluence and cocultured with 2 × 106 human neutrophils for 3 hours. MPO release into conditioned media was assessed by immuno-dot blot and expressed in arbitrary units. *P < 0.01. (G) Neutrophils (2 × 106) were stimulated for 3 hours with vehicle (Cnt), 1 μM nFP, or 1 μM PMA.
To determine if infection with P. aeruginosa induces the ability of type II cells to stimulate neutrophil activation, confluent cultures of wild-type, Mmp7−/−, and Sdc1−/− type II cells were infected with P. aeruginosa for 1 hour, washed with antibiotics, and cocultured with neutrophils. The basal levels of MPO release were again roughly equivalent among the genotypes (Figure 2D). However, after exposure to bacteria, wild-type type II cells stimulated neutrophil activation, whereas Mmp7−/− and Sdc1−/− cells did not (Figure 2D).
To examine if the observed effect could be mediated by epithelial cells from other mucosal tissues, we assessed the ability of SW620 cells, a human colon carcinoma cell line that constitutively expresses MMP7, to promote neutrophil activation. SW620AS is an established line with near complete ablation of MMP7 production due to overexpression of a specific antisense transcript (15), which we verified by quantitative RT-PCR (Figure 2E). When normal human neutrophils were added to intact SW620 monolayers, MPO release was stimulated more than twofold above the levels detected in cocultures with SW620AS cells (Figure 2F). This level of activation was slightly greater than that reached by neutrophils exposed directly to nFMLP (Figure 2G). The levels of MPO released from neutrophils added to SW620AS cells did not differ from the levels released from control neutrophils (Figures 2F and 2G). Together, data from these different models demonstrate that epithelial cells activated by injury or infection—processes that induce MMP7 expression and, in turn, syndecan-1 shedding—stimulate neutrophil activation.
CXCL1 Expression and Release
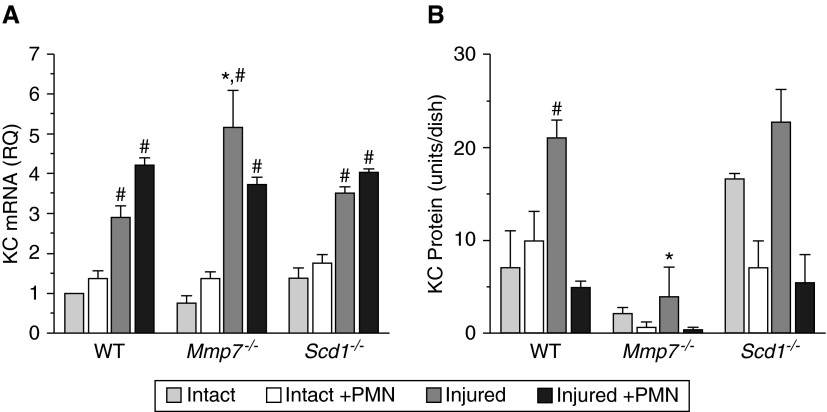
We assessed the expression of CXCL1 and its release into the medium of injured type II cells (Figure 3). As determined by quantitative RT-PCR, injury stimulated CXCL1 expression to similar levels in type II cells of all genotypes (Figure 3A), and the addition of neutrophils had no significant effect on expression (neutrophils were washed away before harvesting type II cells for RNA). However, the patterns of CXCL1 protein released into the medium among genotypes differed. In wild-type cultures, CXCL1 release increased in response to injury (Figure 3B). Despite expression of its mRNA (Figure 3A), the levels of CXCL1 protein in the medium were consistently low in Mmp7−/− cultures (Figure 3B), which we attribute to the lack of shedding of syndecan-1/CXCL1 complexes. In contrast, the levels of CXCL1 protein in uninjured Sdc1−/− cultures were greater than in wild-type cultures (Figure 3B), indicating that, in the absence of syndecan-1, CXCL1 is freely released into the medium. Indeed, in injured Sdc1−/− cells, the levels of CXCL1 released into the medium were comparable to those detected in injured wild-type cultures. The presence of neutrophils led to a drop in soluble CXCL1 levels under most conditions (Figure 3B), likely due to binding of CXCL1 to its receptor on these granulocytes. If so, this interaction was apparently not sufficient to stimulate measurable neutrophil activation in the absence of syndecan-1 (Figure 2B), which highlights the importance of the shed syndecan-1/CXCL1 complex in mediating neutrophil activation.
Figure 3.
CXCL1 released from injured epithelium is dependent on MMP7. Type II cells were isolated from WT, Mmp7−/−, and Sdc1−/− mice and grown, injured, and co-cultured with neutrophils, as described in Figure 2. (A) Cxcl1 (KC) mRNA was quantified by quantitative RT-PCR. RQ was normalized to the WT/intact values. #P < 0.01, relative to intact conditions within the same genotype; *P < 0.01, relative to WT within the same condition. (B) CXCL1 (KC) protein levels in media samples were quantified by ELISA. Data are mean ± SE of cells isolated from three mice/genotype. #P < 0.01, relative to intact conditions within the same genotype; *P < 0.01 relative to WT within the same condition. The legend is for both panels.
Neutrophil Activation In Vivo
We reported that the efflux of neutrophils from the vasculature into the lung or colon in response to acute injury did not differ significantly between wild-type and Mmp7−/− mice, but, rather, that the ability of these granulocytes to advance through the epithelial layer into the alveolar space or gut lumen was markedly impaired in the absence of MMP7 (9, 10). We saw a similar defect in neutrophil movement in injured (9) or infected (Figure 1) Sdc1−/− mice. Furthermore, the inability of neutrophils to cross these epithelial layers was associated with marked protection from lethality associated with the acute insults (9, 10). Hence, we assessed the degree of neutrophil activation in vivo using bleomycin as a model of acute lung injury.
We assessed early neutrophil priming by quantifying the uptake of the glucose analog [18F]FDG, which correlates closely with neutrophil influx (18, 19). Although [18F]FDG uptake was elevated in circulating neutrophils at 1 day after bleomycin injury, there was no difference between wild-type and Mmp7−/− mice (data not shown). We interpret these data to indicate that early stages of neutrophil activation are not influenced by MMP7. In contrast, we did detect differences in neutrophil activation within injured lungs.
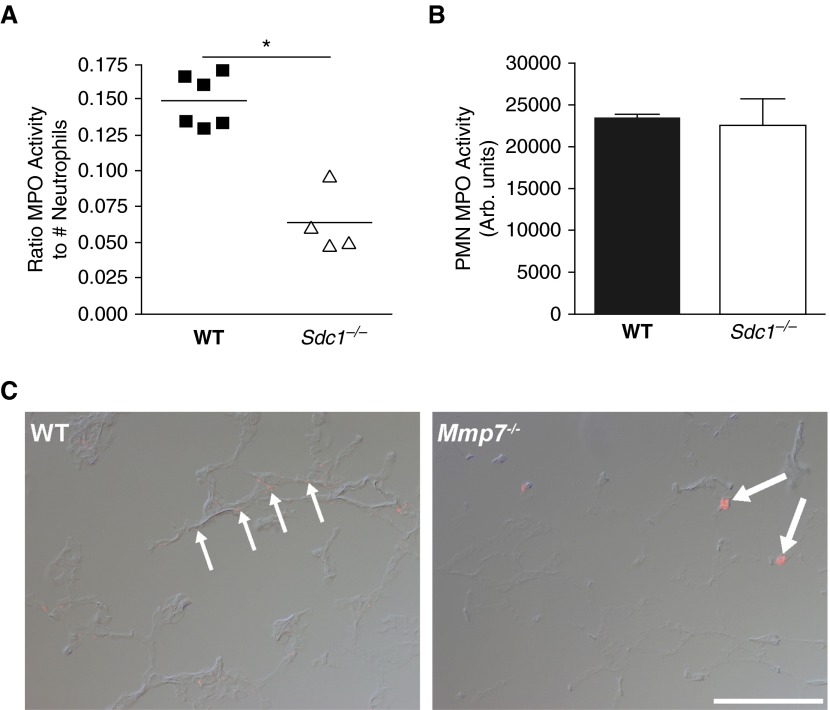
MPO activity in BAL normalized to total neutrophils was approximately twofold greater in wild-type compared with Sdc1−/− mice (Figure 4A). These data indicate that the amount of MPO released per neutrophil was reduced in Sdc1−/− mice, even though the amount of stored MPO was equivalent between wild-type and Sdc1−/− neutrophils (Figure 4B). We saw a similar reduction in soluble MPO levels in Mmp7−/− mice (10). MPO is a component of the chromatin extracellular traps that are dispersed by activated neutrophils (24). In wild-type lungs, immunofluorescence signal for MPO was detected in a punctuated pattern consistent with the enzyme being released from disrupted neutrophils (Figure 4C). In contrast, in Mmp7−/− mice, signal for MPO was seen only with intact cells. Together, these data indicate that neutrophil activation within the alveolar wall tissue was impaired in Mmp7−/− and Sdc1−/− mice.
Figure 4.
Neutrophil activation is dependent on syndecan-1. WT and Sdc1−/− mice (n = 4–6/genotype) were intubated with 4 U/kg bleomycin, and BALs were harvested 4 days later. (A) Total MPO activity in cell-free BAL fluids was measured and expressed relative to the total neutrophil numbers in the BAL (*P = 0.01). (B) Total MPO activity was measured in unstimulated bone marrow–derived neutrophils from WT and Sdc1−/−mice. (C) Lung sections from WT and Mmp7−/− mice were immunostained for MPO, which demonstrated fine, dispersed signal throughout lungs of WT mice (thin arrows), while being confined to individual cells in Mmp7−/− lungs (thick arrows). Scale bar: 100 μm.
Syndecan-1 and Neutrophil Activation
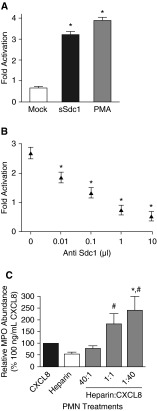
To examine the role of shed syndecan-1 in neutrophil activation, we constructed a variant of syndecan-1 (Sec-Sdc1) truncated at the juxtamembrane site, leading to a constitutively secreted form. We stably expressed Sec-Sdc1 in COS7 monkey kidney epithelial cells, which lack endogenous syndecan-1 but constitutively express CXCL8/IL-8 (25), the functional ortholog of mouse CXCL1. Conditioned media from cells expressing Sec-Sdc1 potently activated neutrophils (Figure 5A). Immunoprecipitation of Sec-Scd1 prevented the activation of neutrophils (Figure 5B). These findings suggest that, independent of epithelial cell damage, shedding of syndecan-1 ectodomains, which likely form complexes with CXCL8, is sufficient to stimulate neutrophil activation.
Figure 5.
Differential neutrophil response to membrane-bound versus soluble syndecan-1 complexes. (A) Neutrophils (0.5 × 106 cells) were incubated with 1 μM PMA or conditioned medium from COS7 cells stably transfected with constitutively secreted syndecan-1 (sSdc1) or control vector (mock), and released MPO levels were measured by an antibody assay after 4 hours. *P < 0.01 relative to mock. (B) Syndecan-1 ectodomain released by COS7 cells expressing constitutively secreted syndecan-1 were immunoprecipitated with increasing amounts of anti–syndecan-1 (Sdc1) antibody. Neutrophils were incubated with immunodepleted media samples, and levels of released MPO were measured after 4 hours. *P < 0.01 relative to control. (C) Neutrophils were cultured with low–molecular weight heparin, CXCL8, or various combinations of the two, and neutrophil activation assessed by MPO activity (#P < 0.05 relative to heparin; *P < 0.05 relative to CXCL8).
We hypothesized that the HS side-chains on soluble syndecan-1 served to present CXCL8 (or CXCL1 in mice) to neutrophils to signal activation. To test this hypothesis, we incubated neutrophils with CXCL8 alone, low–molecular weight heparin alone, or the two in combination. CXCL8 and heparin stimulated neutrophil activation more potently than either CXCL8 or heparin alone (Figure 5C). Neutrophil activation was greatest when CXCL8 was in molar excess compared with the heparin.
Discussion
The data we present here indicate that MMP7 shedding of syndecan-1 functions as part of an epithelial mechanism that regulates both neutrophil advancement across a wounded mucosal surface and their subsequent activation. Combined with findings we reported previously (9, 10, 26, 27), we propose that cell-bound syndecan-1 with CXCL1 bound to its GAG chains functions as a checkpoint to prevent premature neutrophil activation. If still intact on the cell surface, syndecan-1/CXCL1 complexes would support neutrophil binding, yet are not sufficient to facilitate neutrophil activation (Figure 6). MMP7 shedding and release of these complexes provides neutrophils with a go signal permitting activation, ideally at a “safer” distance from the epithelial layer. We propose that this mechanism evolved to bar unwanted damage and cell death due to neutrophil activation occurring too close to a mucosal surface. However, in settings with exuberant inflammation and neutrophil influx, such collateral damage to host tissues ends up being hard to prevent, and contributes to local damage and cell death. We do not propose that this is the only mechanism involved in the transepithelial migration of neutrophils. For example, hepoxilin A3, an epithelial-derived eicosanoid, promotes neutrophil movement through an epithelial barrier in response to bacterial infection (28), and triggering receptor expressed on myeloid cells 1 on neutrophils also functions to facilitate their ability to cross an epithelial barrier (29).
The MMP7/syndecan-1 control of neutrophil movement and activation appears to function in other tissues as well. We have seen impaired neutrophil migration at the mucosal–interstitial interface and markedly reduced levels of CXCL1 and neutrophils in the intestinal lumen in Mmp7−/− mice with acute colon injury (10). Furthermore, Mmp7−/− mice were markedly protected from death compared with wild-type mice. These data suggest that, in wild-type colons, activation of the infiltrated neutrophils would worsen the tissue damage caused by the experimental osmotic injury (dextran sodium sulfate), and this augmented mucosal damage likely contributes to the acute lethality seen in this injury model. Similarly, in a sterile model of acute lung injury, we saw reduced mortality, decreased lumenal (i.e., BAL) CXCL1 levels, and retarded neutrophil influx in Mmp7−/− mice compared with wild-type mice (9). However, when we stimulated the transepithelial movement of neutrophils by instillation of bacterial peptides, lethality was “restored” in Mmp7−/− mice, exceeding wild-type levels. Thus, the observations that mucosal injury in the lungs and colons of Mmp7−/− mice is associated with diminished mortality led us to predict that MMP7 shedding of syndecan-1 controls neutrophil activation.
In our model, CXCL1 functions (i.e., ligating CXCR2 on neutrophils) by being a cargo on the HS chains of syndecan-1. Similarly, studies with CXCL8/IL-8, the human functional ortholog of CXCL1, have indicated that its proper function and compartmentalization are dependent on its ability to bind GAGs (26, 30). Although neutrophils can bind free CXCL1, as demonstrated in direct binding studies (26), our findings (Figures 2, 3, and 5) imply that an interaction with CXCL1 in the absence of syndecan-1 is not sufficient to stimulate neutrophil activation. Although the presence of neutrophils led to a drop in soluble CXCL1 levels in both control and wounded Mmp7−/− and, more so, Sdc1−/− type II cultures (Figure 3B), activation of the neutrophils, which was based on release of MPO, did not occur. Thus, we believe this can be interpreted as ligand–receptor interaction functioning as a sink, but not necessarily resulting in a signaling event. As we reported in collaborative studies (26), CXCL1 stimulates neutrophil chemotaxis, but chemotaxis and activation are distinct processes. Our data with injured and infected type II cells support our hypothesis that MMP7 shedding of syndecan-1/CXCL1 complexes functions to control the state of neutrophil activation.
The precise mechanism through which MMP7-mediated shedding of the syndecan-1/CXCL1 complex, or lack thereof, regulates neutrophil activation remains to be determined. Several possibilities exist. Multiple studies have demonstrated that specific MMPs regulate inflammation by controlling chemokine activity (31). This control by MMPs can be through direct processing of these molecules (32–36), resulting in enhanced, inactive, or antagonistic chemokine activities, or indirectly, by processing substrates that bind, retain, or concentrate the chemokines to particular locations (9, 37–39). Thus, it is possible that, in addition to MMP7 shedding the syndecan-1/CXCL1 complex from the cell surface, it may also directly cleave CXCL1, leading to enhanced neutrophil activation.
Another possibility is that MMP7, through processing of cell surface receptors, could regulate epithelial cell gene expression, and thereby control expression of a factor in response to injury or infection that stimulates neutrophils. Our previous studies, however, suggest that this may not be likely, as comparison of global gene expression between wild-type and Mmp7−/− organotypic airway epithelial cultures in response to injury (40) and acute (1 h) and extended (24 h) P. aeruginosa infection (20) did not reveal marked differences in patterns of gene expression between genotypes.
Yet another possibility—and one that we favor—is that neutrophils bind to a surface protein on epithelial cells that actively blocks activation. In this instance, shedding of syndecan-1/CXCL1 complexes would enable neutrophils to disengage from the repressive interaction providing, in effect, a green light to proceed with complete activation (i.e., respiratory burst). Thus, in the absence of MMP7, neutrophils would retain ligation to the negative signal. This model implies that MMP7 shedding of syndecan-1 could affect the activity of the putative repressor, and, indeed, shedding of syndecans does affect the activation state of other receptors, such as integrins (11, 41–45). Although this possible mechanism appears attractive, the specific means by which epithelial cells can constrain neutrophil activation remain to be determined. Our data, however, demonstrate that the MMP7-controlled mechanism appears to function as a checkpoint to prevent premature neutrophil activation.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Dr. Daniel Schuster (deceased), Washington University (St. Louis, MO), for help with the glucose uptake studies, Ying Wang for maintaining the mouse colonies, Dr. Lynn Matrisian for the SW620 cell lines, and Brian Johnson for assistance with immunofluorescence.
Footnotes
This work was supported by National Institutes of Health grants HL082658 (W.C.P.), HL098067 (W.C.P.), AI084218 (S.T.N.), HL084396 (P.C.), HL103868 (P.C.), and fellowships from the Canadian Institutes of Health Research and the Parker B. Francis Foundation (S.E.G.).
Author Contributions: conception and design—S.E.G., P.W.P., P.C., and W.C.P.; conducted experiments—S.E.G., S.T.N., Q.L., and P.W.P.; analysis and interpretation—S.E.G., S.T.N., C.W.F., P.W.P., P.C., and W.C.P.; drafting the manuscript for important intellectual content—S.E.G., P.C., and W.C.P.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0193OC on March 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Slebos DJ, Postma DS, Koëter GH, Van Der Bij W, Boezen M, Kauffman HF. Bronchoalveolar lavage fluid characteristics in acute and chronic lung transplant rejection. J Heart Lung Transplant. 2004;23:532–540. doi: 10.1016/j.healun.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 3.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ellis TN, Beaman BL. Interferon-γ activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-Boado YS, Wilson CL, Hooper LV, Gordon JI, Hultgren SJ, Parks WC. Bacterial exposure induces and activates matrilysin in mucosal epithelial cells. J Cell Biol. 2000;148:1305–1315. doi: 10.1083/jcb.148.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 10.Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS One. 2009;4:e6565. doi: 10.1371/journal.pone.0006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 13.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol. 2010;176:64–73. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994;54:4805–4812. [PubMed] [Google Scholar]
- 16.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 17.López-Boado YS, Wilson CL, Parks WC. Regulation of matrilysin expression in airway epithelial cells by Pseudomonas aeruginosa flagellin. J Biol Chem. 2001;276:41417–41423. doi: 10.1074/jbc.M107121200. [DOI] [PubMed] [Google Scholar]
- 18.Chen DL, Mintun MA, Schuster DP. Comparison of methods to quantitate 18F-FDG uptake with PET during experimental acute lung injury. J Nucl Med. 2004;45:1583–1590. [PubMed] [Google Scholar]
- 19.Chen DL, Schuster DP. Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L834–L840. doi: 10.1152/ajplung.00339.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 24.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2012;91:369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 25.Hsu TC, Tzang BS, Huang CN, Lee YJ, Liu GY, Chen MC, Tsay GJ. Increased expression and secretion of interleukin-6 in human parvovirus B19 non-structural protein (NS1) transfected COS-7 epithelial cells. Clin Exp Immunol. 2006;144:152–157. doi: 10.1111/j.1365-2249.2006.03023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol. 2010;184:2677–2685. doi: 10.4049/jimmunol.0903274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klesney-Tait J, Keck K, Li X, Gilfillan S, Otero K, Baruah S, Meyerholz DK, Varga SM, Knudson CJ, Moninger TO, et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest. 2013;123:138–149. doi: 10.1172/JCI64181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, Wells TN, Wight TN, Martin TR. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28:464–472. doi: 10.1165/rcmb.2002-0084OC. [DOI] [PubMed] [Google Scholar]
- 31.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 32.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 33.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 34.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 35.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270:3739–3749. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 36.Kruidenier L, MacDonald TT, Collins JE, Pender SL, Sanderson IR. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. 2006;130:127–136. doi: 10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, Masure S, Willemze R, Opdenakker G. Prevention of interleukin-8–induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci USA. 1999;96:10863–10868. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, Werb Z, Kheradmand F. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J. 2004;18:995–997. doi: 10.1096/fj.03-1412fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gharib SA, Altemeier WA, Van Winkle LS, Plopper CG, Schlesinger SY, Buell CA, Brauer R, Lee V, Parks WC, Chen P. Matrix metalloproteinase-7 coordinates airway epithelial injury response and differentiation of ciliated cells. Am J Respir Cell Mol Biol. 2013;48:390–396. doi: 10.1165/rcmb.2012-0083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. Int Rev Cytol. 2001;207:113–150. doi: 10.1016/s0074-7696(01)07004-8. [DOI] [PubMed] [Google Scholar]
- 42.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates vβ3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B–induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology. 2007;17:492–503. doi: 10.1093/glycob/cwm009. [DOI] [PubMed] [Google Scholar]
- 44.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates αvβ3 and vβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapraeger AC, Ell BJ, Roy M, Li X, Morrison OR, Thomas GM, Beauvais DM. Vascular endothelial–cadherin stimulates syndecan-1–coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J. 2013;280:2194–2206. doi: 10.1111/febs.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.