Abstract
Mice lacking the endogenous β2-adrenoceptor (β2AR) agonist epinephrine (phenylethanolamine N-methyltransferase [PNMT]-knockout mice) are resistant to developing an “asthma-like” phenotype in an ovalbumin sensitization and challenge (Ova S/C) model, and chronic administration of β2AR agonists to PNMT-KO mice restores the phenotype. Based on these and other studies showing differential effects of various β2AR ligands on the asthma phenotype, we have speculated that the permissive effect of endogenous epinephrine and exogenous β2AR agonists on allergic lung inflammation can be explained by qualitative β2AR signaling. The β2AR can signal through at least two pathways: the canonical Gαs–cAMP pathway and a β-arrestin–dependent pathway. Previous studies suggest that β-arrestin-2 is required for allergic lung inflammation. On the other hand, cell-based assays suggest antiinflammatory effects of Gαs–cAMP signaling. This study was designed to test whether the in vitro antiinflammatory effects of phosphodiesterase 4 inhibitors, known to increase intracellular cAMP in multiple airway cell types, attenuate the asthma-like phenotype produced by the β2AR agonists formoterol and salmeterol in vivo in PNMT-KO mice, based on the hypothesis that skewing β2AR signaling toward Gαs–cAMP pathway is beneficial. Airway inflammatory cells, epithelial mucus production, and airway hyperresponsiveness were quantified. In Ova S/C PNMT-KO mice, formoterol and salmeterol restored the asthma-like phenotype comparable to Ova S/C wild-type mice. However, coadministration of either roflumilast or rolipram attenuated this formoterol- or salmeterol-driven phenotype in Ova S/C PNMT-KO. These findings suggest that amplification of β2AR-mediated cAMP by phosphodiesterase 4 inhibitors attenuates the asthma-like phenotype promoted by β-agonists.
Keywords: asthma model, β2-adrenoceptor, biased signaling
Clinical Relevance
Our previous work suggests that β2-adrenoceptor (β2AR) ligands may contribute to the asthma phenotype via a β-arrestin–dependent mechanism. The current study demonstrates that cAMP-augmenting agents, such as phosphodiesterase 4 inhibitors, help reverse this effect. Thus, our data now implicate opposing effects for two pathways activated by β2AR signaling.
Asthma is a chronic inflammatory disease of the airways, characterized by inflammatory cell infiltration into the lungs, excess mucus production, and airway hyperresponsiveness (AHR). The mainstays of asthma therapy include inhaled corticosteroids and β2-adrenoceptor (β2AR) agonists (1). The β2AR agonists used for managing asthma have been modified over the years to enhance their selectivity for the β2AR, to reduce off-target effects, and to increase their duration of action to improve patient adherence. These changes led to the development of preferential and long-acting β2AR agonists (2). Despite these changes, the chronic use of β2AR agonists is associated with a loss of asthma control, and β2AR agonists with long duration of action, such as salmeterol, have shown a small, but significant, increase in asthma-related mortality (3).
We have previously shown, using an ovalbumin (Ova) sensitization and challenge (Ova S/C) murine asthma model, that β2AR-knockout (KO) mice (β2AR-KO mice) and phenylethanolamine N-methyltransferase (PNMT)-KO mice (which lack the enzyme, phenylethanolamine N-methyltransferase, required for the synthesis of epinephrine, the endogenous ligand for the β2AR) have an attenuated “asthma-like” response (4, 5). Those reports showed that ligand activation of the β2AR was required for developing the asthma phenotype. PNMT-KO mice do not exhibit any overt phenotypes. They breed normally, have no developmental defects, and have normal resting blood pressure and body temperature (6–8).
The β2AR can signal through at least two pathways: the canonical Gαs–cAMP pathway and the βarrestin signaling pathway that can activate multiple pathways, including those of the mitogen-activated protein kinase family, such as extracellular signal–regulated kinase (ERK) 1/2 (9–12). Numerous studies have shown that ligands can preferentially activate signaling pathways relative to the activation profile of a reference ligand at that receptor (10, 13). This preferential activation of a signaling pathway of the receptor by a ligand has become known as “biased signaling” (9, 10, 11).
Studies suggest that the βarrestin-2 (also termed arrestin 3) signaling pathway is an important mediator of the asthma phenotype in murine models. For example, βarrestin-2 null mice show an attenuated asthma-like phenotype (14), and, as shown by a recent study, where βarrestin-2 was deleted after the asthma-like phenotype had been established, βarrestin-2 is essential for perpetuation of AHR (15). Furthermore, we have recently identified that certain “β-blockers” (such as carvedilol) that lack agonism toward the Gαs signaling pathway, yet activate ERK1/2 in cell-based assays, restore the asthma-like phenotype in PNMT-KO mice, and are ineffective at attenuating the phenotype in wild-type (WT) mice (16).
On the other hand, there are few in vivo data about the role of preferentially activating, the canonical β2AR–Gαs–cAMP pathway, in the development or maintenance of the asthma-like phenotype. This gap in knowledge is due, at least in part, to the fact that no orthosteric Gαs-biased drugs for the β2AR have been identified to date. For the β2AR–Gαs–cAMP pathway, there are no ligands with the opposite signaling profile of carvedilol, a ligand that preferentially activates ERK1/2 via βarrestin-2 (12). However, numerous cell-based studies using normal and asthmatic human bronchial epithelial and airway smooth muscle cells have indirectly examined the role of the β2AR–Gαs–cAMP signaling pathway using phosphodiesterase (PDE) 4 inhibitors to increase β2AR–cAMP accumulation. PDE4 is the isozyme responsible for the breakdown of cAMP in numerous inflammatory cells, and in airway epithelial cells (17). The results of these studies suggest that enhancing cAMP accumulation produces an antiinflammatory effect through various mechanisms, including suppression of inflammatory gene expression (18, 19). Data also suggest that asthmatic airway smooth muscle cells have a decreased cAMP response, and this impaired cAMP accumulation is due to up-regulation of PDE4D-mediated cAMP breakdown (20). Indeed, enhancing antiinflammatory responses by increasing β2AR–Gαs–cAMP accumulation led to the development of GS-5759, a novel bifunctional molecule, where potent β2AR agonists have been covalently linked with a PDE4 inhibitor (GSK256066). This dual ligand (GS-5759) has also shown potent antiinflammatory properties in in vitro assays (21). Although combining a β2AR-agonist with a PDE4 inhibitor is not a classical example of a β2AR–Gαs–cAMP–biased ligand, it represents a convenient means of skewing signaling qualitatively, similar to what would happen with a β2AR–cAMP–biased ligand.
Thus, there are numerous mechanistic studies in human cells suggesting that amplification of the β2AR–Gαs–cAMP pathway would be protective in asthma. There are also reports using murine models showing that administration of roflumilast resulted in attenuation of asthma-like phenotypes (22, 23). However, there is little in vivo evidence to suggest that the attenuation of the asthma phenotype results from biasing signaling toward the β2AR–Gαs–cAMP pathway. The present studies were performed to assess the in vivo role of qualitatively skewing β2AR–Gαs–cAMP signaling by administering PDE4 inhibitors in conjunction with long-acting β2AR agonists compared with administration of the β2AR agonist alone. To assure, as much as possible, that the results were due to PDE4 inhibition increasing β2AR agonist–induced increases in cAMP, two different PDE4 inhibitors were used (roflumilast and rolipram), as well as two clinically relevant β2AR agonists (salmeterol and formoterol). These studies have the added novelty of being the first to examine the interaction of long-acting β2AR agonists and PDE4 inhibition in vivo in mice lacking the endogenous β2AR hormone, epinephrine. This not only eliminated the confounding influence of varying levels of circulating endogenous β2AR agonist, but also allowed a more direct comparison with the conditions used in the in vitro cell-based studies.
Materials and Methods
Experimental Animals
WT SvJ/129 mice (6- to 8-wk old; Jackson Laboratory, Bar Harbor ME) and mating pairs of PNMT-KO mice, obtained as a gift from Stephen Ebert of University of Central Florida (Orlando, FL), were bred in house (6) and used for the experiments. All animal experiments were conducted in conformity to strict rules and regulations of the Institutional Animal Care and Use Committee of the University of Houston (Houston, TX). Mice were randomly assigned to the various treatment or control groups, and the parameters were measured in blinded fashion.
Antigen S/C Protocol
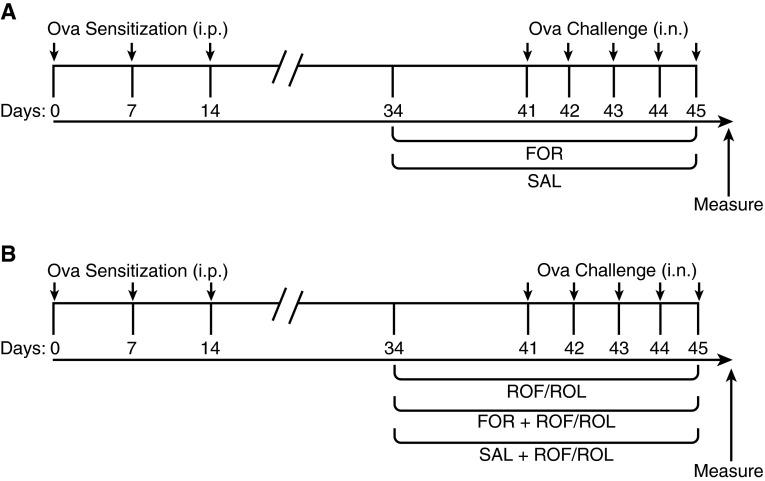
Briefly, mice were sensitized to 2 mg/kg/day Ova with 2 mg alum intraperitoneally on Days 0, 7, and 14, followed by once-daily intranasal challenge with 1 mg/kg/day Ova or saline for 5 consecutive days, as shown in Figures 1A and 1B and as previously described (5). For intranasal challenge, a noninvasive inoculation technique was performed on mice anesthetized with isoflurane.
Figure 1.
Treatment protocol. Phenylethanolamine N-methyltransferase (PNMT)-knockout (KO) and wild-type (WT) mice received ovalbumin (Ova; 2 mg/kg/day in 2 mg of alum) intraperitoneally (i.p.), as indicated, on Days 0, 7, and 14, followed by intranasal (i.n.) challenge with Ova (1 mg/kg/day) or saline on Days 41–45 (arrows). (A) Groups of Ova sensitization and challenge (Ova S/C) PNMT-KO and WT mice received twice-daily formoterol (FOR; 5 μg/kg, intraperitoneally) or salmeterol (SAL; 1.5 μg/kg, intraperitoneally). (B) Groups of Ova S/C PNMT-KO and WT mice were administered roflumilast (ROF; 5 mg/kg) or rolipram (ROL; 5 mg/kg) alone via oral gavage, or groups were coadministered ROF (5 mg/kg) or ROL (5 mg/kg) twice daily with either FOR (5 μg/kg) or SAL (1.5 μg/kg). Duration of all drug administration was 12 days.
Administration of β2AR Agonists and PDE4 Inhibitors
Ova S/C WT and PNMT-KO mice received twice-daily intraperitoneal injections of either 5 μg/kg formoterol (saline: DMSO, 99.95/0.05, vol/vol; Sigma-Aldrich, St. Louis, MO) (24–27) or 1.5 μg/kg salmeterol (saline: methanol, 99.95/0.05, vol/vol; Sigma-Aldrich) (28–30); or an equal volume of the appropriate vehicle via intraperitoneal injection. Ova S/C WT and PNMT-KO mice were coadministrated 5 mg/kg roflumilast in 4% methylcellulose solution (24, 31–33) or 5 mg/kg rolipram in 0.1% methylcellulose (34, 35) (Figure 1B), or an equal volume of the appropriate vehicle, via oral gavage. The doses chosen for all drugs were taken on the low end of what has been used in other murine studies. Our rationale for this was that, for the β2AR agonists, we were attempting to “restore” β2AR agonist signaling in the PNMT-KO mice, not necessarily produce a drug effect (24–29, 32, 36). Similarly, for the PDE4 inhibitors we did not want the dose to be highly antiinflammatory, but simply a concentration that would skew β2AR signaling toward the Gαs–cAMP pathway (22, 31–35).
All drugs were administered for 12 days, as shown in Figures 1A and 1B. Control mice received an equal volume of vehicle solution.
Bronchoalveolar Lavage
Mice were killed with 45 mg/kg pentobarbital intraperitoneal (Sigma-Aldrich), and then 400 μl of cold sterile saline was perfused into the right lung lobe to obtain bronchoalveolar lavage (BAL) fluid. Total and differential cell counts were obtained from the BAL fluid using a hemacytometer (Hausser Scientific, Horsham, PA) and Wright-Giemsa staining (Sigma-Aldrich), as previously described (37).
Mucin Analysis
Formalin-fixed, paraffin-embedded lungs were cut into 5-μm sections transversely and stained with periodic acid fluorescent Schiff’s stain (Sigma-Aldrich). Morphometric quantification of mucin was based upon a previous report (38). Different investigators, who were blinded to the treatment groups, performed image acquisition and morphometric quantification.
AHR
AHR was measured using the forced oscillation method with the FlexiVent (SCIREQ, Montreal, PQ, Canada). Briefly, mice were anesthetized with ketamine (240 mg/kg) and xylazine (48 mg/kg), and then methacholine was nebulized at doses of 3.125, 6.25, 12.5, 25, and 50 mg/ml. Three peak airway resistance values were averaged at each dose to determine the airway resistance (5).
Statistical Analysis
Data are expressed as mean (±SEM) and one-way ANOVA with Tukey’s post hoc test or two-way ANOVA with Holm-Sidak’s post hoc test were performed. Statistical significance was set at P less than 0.05. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA).
Results
Effect of Drug Administration on Total Inflammatory Cells in the Airways
To determine the inflammatory response of mice to Ova S/C with different drug administration, we examined the infiltration of inflammatory cells into the airways (Figures 2A and 2B). Consistent with previously published results, the total cells in the airway markedly increased in vehicle-treated Ova S/C WT mice, but not in vehicle-treated Ova S/C PNMT-KO mice (Figures 2A and 2B). Compared with vehicle treatment, formoterol administration to Ova S/C PNMT-KO mice caused an increase in total cells to levels comparable to that in Ova S/C WT mice (P < 0.05) (Figures 2A and 2B). Salmeterol administration to Ova S/C PNMT-KO mice also increased the total cells in airways relative to control PNMT-KO mice, and this increase was comparable to that after formoterol administration (P < 0.05; Figures 2A and 2B). Ova S/C WT mice administered either formoterol or salmeterol did not show a change in total cells in the airways compared with vehicle-treated Ova S/C WT mice (Figures 2A and 2B).
Figure 2.
Effect of ROF or ROL administration, with or without FOR or SAL coadministration, on total cell count. Total cells in bronchoalveolar lavage (BAL) fluid of saline challenged (control [CTL]) and vehicle (VEH)-treated Ova S/C PNMT-KO (solid bars) and WT mice (open bars). Groups of Ova S/C PNMT-KO and WT mice were administered different drug combinations. In these groups of mice, either (A) ROF (5 mg/kg) or (B) ROL (5 mg/kg) were administered once daily via oral gavage, or coadministered twice-daily dosages of either FOR (5 μg/kg, intraperitoneally) or SAL (1.5 μg/kg, intraperitoneally) for a duration of 12 days. The lungs were lavaged with 400 μl of sterile saline through a sterile cannula, and BAL fluid was collected 24 hours after the last challenge. Cells were counted using a hemacytometer. Data represent the mean (±SEM) from n = 5–8 mice in each group. *P < 0.05 significance compared with respective PNMT-KO or WT CTL; #P < 0.05 significance compared with respective PNMT-KO or WT treatment groups in the absence of ROF or ROL.
Total cell number in BAL fluid of Ova S/C PNMT-KO mice or Ova S/C WT mice administered roflumilast or rolipram was not different from vehicle-treated Ova S/C PNMT-KO mice or vehicle-treated Ova S/C WT mice, respectively (Figures 2A and 2B). The increase in total cell infiltration produced by administration of formoterol or salmeterol to Ova S/C PNMT-KO mice was attenuated when either of the two long-acting β2AR agonists (formoterol or salmeterol) was coadministered with either roflumilast or rolipram (P < 0.05; Figures 2A and 2B).
Effect of Drug Administration on Eosinophilic Infiltration in the Airways
The Ova murine model of asthma is mainly an eosinophil-driven inflammatory model. Therefore, we quantified the number of eosinophils in BAL fluid using the Wright-Giemsa staining. As shown in previous studies, vehicle-treated Ova S/C PNMT-KO mice, unlike Ova S/C WT mice, did not demonstrate significant eosinophilia in the BAL fluid (P < 0.05; Figures 3A and 3B). However, administration of formoterol restored the number of eosinophils in BAL fluid from Ova S/C PNMT-KO mice (P < 0.05; Figures 3A and 3B), comparable to those seen in vehicle-treated Ova S/C WT mice. Similar to formoterol, salmeterol also significantly restored the number of eosinophils in Ova S/C PNMT-KO mice (P < 0.05; Figures 3A and 3B). Ova S/C WT mice administered formoterol or salmeterol exhibited no change in eosinophils compared with vehicle-treated Ova S/C WT mice (Figures 3A and 3B).
Figure 3.
Effect of ROF or ROL administration, with or without FOR or SAL coadministration, on airway eosinophil count. Eosinophils in BAL fluid of saline-challenged (CTL) and VEH-treated Ova S/C PNMT-KO (solid bars) and WT mice (open bars). Groups of Ova S/C PNMT-KO (solid bars) and WT mice (open bars) were administered with different drug combinations as indicated. In these groups of mice, either (A) ROF (5 mg/kg) or (B) ROL (5 mg/kg) were administered once daily via oral gavage or coadministered with twice-daily FOR (5 μg/kg, intraperitoneally) or SAL (1.5 μg/kg, intraperitoneally) for 12 days. Differential counting of cells was done with Wright-Geimsa staining. Data represent the mean (±SEM) from n = 5–7 mice in each group. *P < 0.05 significance compared with respective PNMT-KO or WT CTL; #P < 0.05 significance compared with respective PNMT-KO or WT treatment groups in the absence of ROF or ROL.
There was no difference in eosinophil cell counts between Ova S/C PNMT-KO mice administered with roflumilast or rolipram compared with their respective control mice (Figures 3A and 3B). In Ova S/C WT mice, rolipram, but not roflumilast, lowered eosinophil counts in the airways (Figures 3A and 3B). The increased eosinophil cell count in the airways observed in Ova S/C PNMT-KO and Ova S/C WT mice treated with formoterol or salmeterol was attenuated by the coadministration of roflumilast or rolipram (P < 0.05; Figures 3A and 3B).
Effect of Drug Administration on Mucus Production in the Airways
As shown in Figures 4A and 4B, vehicle-treated Ova S/C WT mice (P < 0.05), but not vehicle-treated Ova S/C PNMT-KO mice, exhibited an increase in mucus production (assessed by morphometric quantification of periodic acid fluorescent Schiff’s staining; Figure 4C). In Ova S/C PNMT-KO mice, either formoterol or salmeterol administration restored mucus production in the airways (P < 0.05; Figures 4A and 4B). There was no difference in mucus production between Ova S/C PNMT-KO mice administered roflumilast (Figure 4A) or rolipram (Figure 4B) compared with their respective control mice. The mucus production in Ova S/C WT mice administered either roflumilast (Figure 4A) or rolipram (Figure 4B) was not different from the mucus production in vehicle-treated Ova S/C WT mice (Figure 4D).
Figure 4.
Effect of ROF or ROL administration, with or without FOR or SAL coadministration, on mucus production. (A and B) Morphometric quantification of the mucin volume density, and (C and D) representative microscopic images of mucin (red) content in the airway epithelia (green) assessed from saline-challenged (CTL) and VEH-treated Ova S/C PNMT-KO and WT mice. Groups of Ova S/C PNMT-KO (solid bars) and WT mice (open bars) were administered different drug combinations, as indicated. In these groups of mice, either (A) ROF (5 mg/kg) or (B) ROL (5 mg/kg) were administered alone via oral gavage or coadministered with twice-daily dosages of either FOR (5 μg/kg, intraperitoneally) or SAL (1.5 μg/kg, intraperitoneally) for 12 days. Data represent the mean (±SEM) from n = 5–8 in each group (PNMT-KO) and from n = 5–13 in each group (WT). *P < 0.05 significance as compared with respective CTL; #P < 0.05 significance as compared with respective treatment groups in the absence of ROF or ROL. (C) Representative images of mucin content by either FOR or SAL administration. (D) Representative images of mucin content effect after phosphodiesterase 4 inhibition by either ROF or ROL administration. Scale bars: 50 μM.
The restored mucus production observed with formoterol or salmeterol administration in Ova S/C PNMT-KO mice was attenuated when coadministered with either roflumilast (Figure 4A) (P < 0.05) or rolipram (P < 0.05) (Figure 4B). Ova S/C WT mice administered formoterol and roflumilast, as well as salmeterol with either roflumilast or rolipram, also displayed similar reduction in mucus production to that observed in similarly treated PNMT-KO mice (P < 0.05) (Figures 4A–4D).
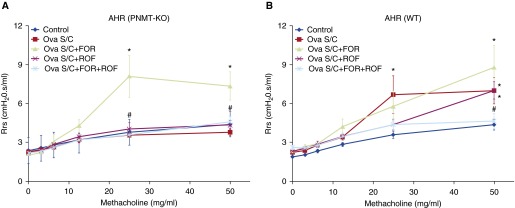
Effect of Roflumilast Administration, with or without Formoterol Coadministration, on AHR
As shown in Figures 5A and 5B, 25 and 50 mg/ml of methacholine increased airway resistance in vehicle-treated Ova S/C WT, but not in vehicle-treated Ova S/C PNMT-KO mice (P < 0.05). In Ova S/C PNMT-KO mice, formoterol administration restored airway resistance (P < 0.05; Figure 5A). There was no difference in airway resistance between Ova S/C PNMT-KO mice administered roflumilast compared with control mice (Figure 5A). Airway resistance in Ova S/C WT mice administered roflumilast was not different from vehicle-treated Ova S/C WT mice (Figure 5B). The increase in airway resistance observed with formoterol administration in Ova S/C PNMT-KO mice was attenuated when coadministered with roflumilast (P < 0.05; Figure 5A).
Figure 5.
Effect of ROF administration, with or without FOR coadministration, on airway resistance. (A and B) Airway resistance responses to increasing doses of nebulized methacholine (0–50 mg/ml) were measured using forced oscillation technique in Ova S/C PNMT-KO (A) and WT (B) mice administered different drug combinations. Airway resistance was determined by averaging the three highest resistance responses recorded for each mouse at each methacholine dose. In these groups of mice, ROF (5 mg/kg) was administered once daily via oral gavage or coadministered twice daily with FOR (5 μg/kg, intraperitoneally) for 12 days. Data represent the mean (±SEM) from n = 4–9 mice in each group. *P < 0.05 significance compared with respective PNMT-KO or WT CTL; #P < 0.05 significance compared with respective PNMT-KO or WT treatment groups in the absence of ROF. AHR, airway hyperresponsiveness.
Discussion
We have previously shown that β2AR signaling is required for development of the asthma-like phenotype in murine models, because either (1) chronic treatment with certain β2AR inverse agonists (such as nadolol and ICI 118,551), or (2) genetic deletion of the β2AR, attenuated the asthma phenotype (4, 37). We also demonstrated, through both pharmacologic and genetic approaches, that ligand activation of the β2AR was required for development of the asthma phenotype (5). Furthermore, we have used ligands that modify the asthma-phenotype and shown that their effect is not observed in β2AR-KO mice. Finally, we have also shown that nadolol blocks the effect of alprenolol in maintaining the asthma-like phenotype, suggesting that the ligands are competing for the β2AR (4). Thus, numerous lines of evidence indicate that ligand binding to the β2AR is responsible for promoting the development of the asthma phenotype.
However, the β2AR can signal through multiple effector pathways downstream of the receptor after agonist binding (10). There are two signaling pathways that have been well characterized for the β2AR. One is the canonical Gαs–cAMP pathway, which, by increasing cAMP, leads to PKA activation. The other is activation of mitogen-activated protein kinases (usually ERK1/2), which are most often activated in a βarrestin-dependent manner (9, 10, 12, 39).
There are numerous in vitro and in vivo studies implicating the βarrestin–ERK1/2 pathway as proinflammatory (reviewed in Ref. 40). In vivo studies have reported that βarrestin-2 null mice have shown an attenuated asthma phenotype, and we have recently shown that administration of β2AR ligands capable of activating ERK1/2 in vitro (10, 12) restore the asthma-like phenotype in PNMT-KO mice, and do not attenuate the asthma-like phenotype in WT mice (16). There are even small clinical studies in subjects with asthma showing administration of nadolol, a ligand that inhibits β2AR-mediated activation of the ERK1/2 pathway (12), causes a dose-dependent reduction in AHR (reflected as an increase in the provocative concentration of methacholine causing a 20% drop in FEV1) in subjects with mild asthma (41, 42). Conversely, administration of propranolol, a ligand that activates the ERK1/2 pathway via the β2AR (12), had no effect in a different subset of subjects with asthma concurrently using inhaled corticosteroids (43).
Whereas the existence of well established βarrestin-biased β2AR ligands has enabled a clear picture of the proinflammatory effect of skewing β2AR signaling toward βarrestin signaling, similar studies assessing the effects of skewing β2AR signaling toward the β2AR–Gαs–cAMP signaling pathway are lacking. An ideal strategy for such studies would be to use a β2AR ligand that preferentially activated the Gαs–cAMP pathway, while inhibiting the βarrestin pathway (i.e., a β2AR–Gαs–cAMP–biased ligand). However, no such compound has yet been identified. In addition, although it would seem tempting to have studied the Gαs signaling pathway in the βarrestin-2 null mice, thereby isolating the pathway, it must be recalled that such mice already have a greatly attenuated asthma phenotype, and thus allow little room to measure any further improvement or attenuation of the asthma phenotype by an alternate pathway (14).
In the absence of a Gαs-biased β2AR ligand, we chose the strategy used in cell-based assays to increase cAMP levels downstream of the β2AR using PDE4 inhibitors. PDEs, including PDE4, break down cAMP and cGMP and play a key role in inflammatory and immune responses (44). PDE4s are the major isozymes highly expressed in immune cells, airway epithelial cells, and smooth muscle cells (17). The antiinflammatory effects of combining a β2AR with a PDE4 inhibitor were promising enough that a new chemical entity (GS-5759) for possible use in obstructive airway diseases was created by Gilead Sciences by covalently binding the β2AR agonists, indacaterol and carmoterol, to a potent PDE4 inhibitor, GSK256066 (21, 45). In our studies, to more rigorously test whether the results that we observed were due to the “amplified” β2AR–cAMP resulting from coadministration of a PDE4 inhibitor, we tested four different combinations of a PDE4 inhibitor and a β2AR agonist (rolipram or roflumilast in combination with either formoterol or salmeterol), and compared the results to administration of the β2AR agonist alone (Figures 1A and 1B).
We observed that, similar to our previous results assessing formoterol (5), another long-acting β2AR agonist, salmeterol, also restored inflammatory cell infiltration (Figures 2 and 3) and mucus production (Figure 4) in the airways of Ova S/C PNMT-KO mice. However, all concurrent administrations of either PDE4 inhibitor, roflumilast or rolipram, with either β2AR agonist, formoterol or salmeterol, to Ova S/C PNMT-KO mice attenuated the increases in inflammatory cell infiltration (Figures 2 and 3) and mucus production (Figure 4) that occurred with treatment with the β2AR agonists alone.
Ova S/C WT mice treated with roflumilast or rolipram alone did not show any significant reduction in inflammatory cell infiltration (Figures 2 and 3) or mucus production (Figure 4) in the airways. In addition, cotreatment with either formoterol and roflumilast or salmeterol and rolipram did not significantly reduce total inflammatory cells in the airways of Ova S/C WT mice (Figures 2A and 2B). The reason for this discrepancy of a lack of significant effect of roflumilast and rolipram when administered in the presence of endogenous epinephrine rather than formoterol or salmeterol is unclear. However, the β2AR agonists, salmeterol and formoterol, have been shown to be βarrestin–ERK1/2 activation biased relative to epinephrine (13). It is possible that the “balancing” of signaling produced by amplification of the Gαs–cAMP pathway of β2AR signaling may have a more protective effect in the presence of a βarrestin–ERK1/2–biased ligand than in the presence of epinephrine, which, by definition, is the reference-neutral (unbiased) ligand. Furthermore, one of the major advantages of our studies is the ability to study the β2AR agonist/PDE4 inhibition in the absence of the confounding influence of the circulating β2AR agonist, epinephrine. Given the natural variability and circadian fluctuations of the endogenous β2AR hormone, it is perhaps not surprising that differences between PNMT-KO and WT mice treated with PDE4 inhibitors would occur. Especially noteworthy is that we only observed quantitative, not qualitative, differences (Figures 2–4).
In all of our previous studies, the effect of drug therapies on inflammatory cell infiltration and mucus production has paralleled the effect on AHR and lung function parameters. In the present study, we also observed that concurrent administration of roflumilast and formoterol to Ova S/C PNMT-KO mice significantly reduced airway resistance compared with administration of formoterol alone (Figure 5A).
In summary, we have demonstrated that amplification of specific β2AR–Gαs–cAMP signaling by two distinct PDE4 inhibitors attenuates cardinal features of the asthma-like phenotype when PNMT-KO mice were chronically treated with formoterol or salmeterol. Although further studies are needed to fully comprehend the role of the various β2AR signaling pathways, taken together with the results implicating the βarrestin-2 pathway as proinflammatory (14, 16, 46), our results suggest that the ideal β2AR drug for asthma management may be a Gαs-biased ligand.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants 1R01AI110007 (J.K.L.W., R.B.P., and R.A.B.).
Author Contributions: Participated in research design—G.S.F., V.J.T., N.A.-S., B.J.K., J.K.L.W., R.B.P., and R.A.B.; conducted experiments—G.S.F., D.V., T.P., and P.A.G.; performed data analysis—G.S.F., H.K., D.V., S.P., T.P., R.B.P., and R.A.B.; wrote or contributed to the writing of the manuscript—G.S.F., H.K., V.J.T., N.A.-S., D.V., R.J., B.J.K., J.K.L.W., R.B.P., and R.A.B..
Originally Published in Press as DOI: 10.1165/rcmb.2015-0373OC on February 24, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barnes PJ. Drugs for asthma. Br J Pharmacol. 2006;147:S297–S303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack D. The 1990 Lilly Prize Lecture: a way of looking at agonism and antagonism: lessons from salbutamol, salmeterol and other β-adrenoceptor agonists. Br J Clin Pharmacol. 1991;31:501–514. doi: 10.1111/j.1365-2125.1991.tb05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thanawala VJ, Forkuo GS, Al-Sawalha N, Azzegagh Z, Nguyen LP, Eriksen JL, Tuvim MJ, Lowder TW, Dickey BF, Knoll BJ, et al. β2-Adrenoceptor agonists are required for development of the asthma phenotype in a murine model. Am J Respir Cell Mol Biol. 2013;48:220–229. doi: 10.1165/rcmb.2012-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the Cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: a new model for studying the developmental distribution of adrenergic cells. Dev Dyn. 2004;231:849–858. doi: 10.1002/dvdy.20188. [DOI] [PubMed] [Google Scholar]
- 7.Sharara-Chami RI, Joachim M, Mulcahey M, Ebert S, Majzoub JA. Effect of epinephrine deficiency on cold tolerance and on brown adipose tissue. Mol Cell Endocrinol. 2010;328:34–39. doi: 10.1016/j.mce.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler MG, Milic M, Sun P, Tang CM, Elayan H, Bao X, Cheung WW, O’Connor DT. Endogenous epinephrine protects against obesity induced insulin resistance. Auton Neurosci. 2011;162:32–34. doi: 10.1016/j.autneu.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. Beta-arrestin–biased agonism at the β2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 10.van der Westhuizen ET, Breton B, Christopoulos A, Bouvier M. Quantification of ligand bias for clinically relevant β2-adrenergic receptor ligands: implications for drug taxonomy. Mol Pharmacol. 2014;85:492–509. doi: 10.1124/mol.113.088880. [DOI] [PubMed] [Google Scholar]
- 11.Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding β(2)-adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011;163:18–28. doi: 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Hegde A, Choi YH, Theriot BS, Premont RT, Chen W, Walker JK. Genetic deletion of β-arrestin-2 and the mitigation of established airway hyperresponsiveness in a murine asthma model. Am J Respir Cell Mol Biol. 2015;53:346–354. doi: 10.1165/rcmb.2014-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanawala VJ, Valdez DJ, Joshi R, Forkuo GS, Parra S, Knoll BJ, Bouvier M, Leff P, Bond RA. β-Blockers have differential effects on the murine asthma phenotype. Br J Pharmacol. 2015;172:4833–4846. doi: 10.1111/bph.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 18.Moodley T, Wilson SM, Joshi T, Rider CF, Sharma P, Yan D, Newton R, Giembycz MA. Phosphodiesterase 4 inhibitors augment the ability of formoterol to enhance glucocorticoid-dependent gene transcription in human airway epithelial cells: a novel mechanism for the clinical efficacy of roflumilast in severe chronic obstructive pulmonary disease. Mol Pharmacol. 2013;83:894–906. doi: 10.1124/mol.112.083493. [DOI] [PubMed] [Google Scholar]
- 19.Patel BS, Prabhala P, Oliver BG, Ammit AJ. Inhibitors of phosphodiesterase 4, but not phosphodiesterase 3, increase β2-agonist–induced expression of antiinflammatory mitogen-activated protein kinase phosphatase 1 in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2015;52:634–640. doi: 10.1165/rcmb.2014-0344OC. [DOI] [PubMed] [Google Scholar]
- 20.Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, Liggett SB, Black JL, Oliver BG. β2-Agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS One. 2011;6:e20000. doi: 10.1371/journal.pone.0020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmon M, Tannheimer SL, Gentzler TT, Cui ZH, Sorensen EA, Hartsough KC, Kim M, Purvis LJ, Barrett EG, McDonald JD, et al. The in vivo efficacy and side effect pharmacology of GS-5759, a novel bifunctional phosphodiesterase 4 inhibitor and long-acting β 2-adrenoceptor agonist in preclinical animal species. Pharmacol Res Perspect. 2014;2:e00046. doi: 10.1002/prp2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbert C, Hettiaratchi A, Webb DC, Thomas PS, Foster PS, Kumar RK. Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma. Clin Exp Allergy. 2008;38:847–856. doi: 10.1111/j.1365-2222.2008.02950.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim SW, Kim JH, Park CK, Kim TJ, Lee SY, Kim YK, Kwon SS, Rhee CK, Yoon HK.Effect of roflumilast on airway remodeling in a murine model of chronic asthma Clin Exp Allergy[online ahead of print] 5 Nov 2015DOI: 10.1111/cea.12670 [DOI] [PubMed] [Google Scholar]
- 24.Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul Disord. 2007;17:47–55. doi: 10.1016/j.nmd.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Koopman R, Gehrig SM, Léger B, Trieu J, Walrand S, Murphy KT, Lynch GS. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. J Physiol. 2010;588:4811–4823. doi: 10.1113/jphysiol.2010.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Léger B, Koopman R, Walrand S, Gehrig SM, Murphy KT, Lynch GS. Chronic formoterol administration reduces cardiac mitochondrial protein synthesis and oxidative capacity in mice. Int J Cardiol. 2011;146:270–272. doi: 10.1016/j.ijcard.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 27.Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of β-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 2008;149:2853–2865. doi: 10.1210/en.2007-1202. [DOI] [PubMed] [Google Scholar]
- 28.Maris NA, van der Sluijs KF, Florquin S, de Vos AF, Pater JM, Jansen HM, van der Poll T. Salmeterol, a β2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1122–L1128. doi: 10.1152/ajplung.00125.2003. [DOI] [PubMed] [Google Scholar]
- 29.Qian L, Wu HM, Chen SH, Zhang D, Ali SF, Peterson L, Wilson B, Lu RB, Hong JS, Flood PM. β2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway. J Immunol. 2011;186:4443–4454. doi: 10.4049/jimmunol.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singam R, Jena PK, Behera S, Hellermann GR, Lockey RF, Ledford D, Mohapatra SS. Combined fluticasone propionate and salmeterol reduces RSV infection more effectively than either of them alone in allergen-sensitized mice. Virol J. 2006;3:32. doi: 10.1186/1743-422X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M, Webb DC, Foster PS. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003;307:349–355. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- 32.Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172:848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Sato K, Aoshiba K, Azuma A, Mizushima T. Superiority of PC-SOD to other anti-COPD drugs for elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1250–L1261. doi: 10.1152/ajplung.00019.2012. [DOI] [PubMed] [Google Scholar]
- 34.Miotla JM, Teixeira MM, Hellewell PG. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am J Respir Cell Mol Biol. 1998;18:411–420. doi: 10.1165/ajrcmb.18.3.2913. [DOI] [PubMed] [Google Scholar]
- 35.Yamaki K, Li X, Uchida H, Alam AH, Hossain MA, Yanagisawa R, Takano H, Taneda S, Hayashi H, Mori Y, et al. Effects of the phosphodiesterase IV inhibitor rolipram on Th1 and Th2 immune responses in mice. J Pharm Pharmacol. 2004;56:877–882. doi: 10.1211/0022357023655. [DOI] [PubMed] [Google Scholar]
- 36.Riesenfeld EP, Sullivan MJ, Thompson-Figueroa JA, Haverkamp HC, Lundblad LK, Bates JH, Irvin CG. Inhaled salmeterol and/or fluticasone alters structure/function in a murine model of allergic airways disease. Respir Res. 2010;11:22. doi: 10.1186/1465-9921-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, Ho SB, Ehre C, Kesimer M, Knoll BJ, et al. Chronic exposure to β-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol. 2012;842:279–295. doi: 10.1007/978-1-61779-513-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–7059. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 40.Walker JK, DeFea KA. Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma. Curr Opin Pharmacol. 2014;16:142–147. doi: 10.1016/j.coph.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanania NA, Mannava B, Franklin AE, Lipworth BJ, Williamson PA, Garner WJ, Dickey BF, Bond RA. Response to salbutamol in patients with mild asthma treated with nadolol. Eur Respir J. 2010;36:963–965. doi: 10.1183/09031936.00003210. [DOI] [PubMed] [Google Scholar]
- 42.Hanania NA, Singh S, El-Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss S, Shardonofsky F, et al. The safety and effects of the β-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther. 2008;21:134–141. doi: 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Short PM, Williamson PA, Anderson WJ, Lipworth BJ. Randomized placebo-controlled trial to evaluate chronic dosing effects of propranolol in asthma. Am J Respir Crit Care Med. 2013;187:1308–1314. doi: 10.1164/rccm.201212-2206OC. [DOI] [PubMed] [Google Scholar]
- 44.Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;106:269–297. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Tannheimer SL, Sorensen EA, Cui ZH, Kim M, Patel L, Baker WR, Phillips GB, Wright CD, Salmon M. The in vitro pharmacology of GS-5759, a novel bifunctional phosphodiesterase 4 inhibitor and long acting β2-adrenoceptor agonist. J Pharmacol Exp Ther. 2014;349:85–93. doi: 10.1124/jpet.113.210997. [DOI] [PubMed] [Google Scholar]
- 46.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.