Abstract
The mechanisms that contribute to homeostasis of the immune system in sepsis are largely unknown. One study suggests a potential detrimental role for thymic stromal lymphopoietin (TSLP) in sepsis; however, the immune-regulatory effects of TSLP on myeloid cells within the intestinal microenvironment suggest the contrary. Our objective was to clarify TSLP's role in sepsis. Cecal ligation and puncture was performed in mice with total or myeloid-specific deficiency in the TSLP receptor (TSLPR). Survival was monitored closely, peritoneal fluids and plasma were analyzed for markers of inflammation, and myeloid cell numbers and their ability to produce inflammatory mediators was determined. The interaction of TSLP with TSLPR in myeloid cells contributed to mouse survival after septic peritonitis. Mice with TSLPR deficiency in myeloid cells displayed excessive local and systemic inflammation levels (e.g., increased inflammatory cell and cytokine levels) relative to control mice. Moreover, hepatic injury was exacerbated in mice with TSLPR deficiency in their myeloid cells. However, the enhanced inflammatory response did not affect the ability of these mice to clear bacteria. Resident neutrophils and macrophages from septic mice with TSLPR deficiency exhibited an increased ability to produce proinflammatory cytokines. Collectively, our findings suggest that the effects of TSLP on myeloid cells are crucial in reducing the multiple organ failure that is associated with systemic inflammation, which highlights the significance of this cytokine in modulating the host response to infection and in reducing the risks of sepsis development.
Keywords: sepsis, inflammation, myeloid cells, thymic stromal lymphopoietin
Clinical Relevance
Infections can lead to sepsis, a complex, incompletely understood, and often fatal disorder that is considered to reflect dysregulation of the host immune response. Early diagnosis and treatment of sepsis is critical to prevent the cascade of the complex processes that lead to severe sepsis and septic shock. However, the best available diagnostic and treatment strategies have failed, which underscores the need for a better understanding of the pathophysiological mechanisms regulating the inflammatory host response during sepsis. Our study provides evidence that thymic stromal lymphopoietin is part of a mechanism that is initiated by the host to restore homeostasis during the inflammatory response against bacterial infections to reduce the risk of sepsis development.
Effective host immunity against bacterial infection is characterized by pathogen clearance followed by resolution of the inflammatory response. However, infections can lead to sepsis—a complex, incompletely understood, and often fatal disorder that is considered to reflect dysregulation of the host immune response (1). The initial stages of sepsis include a hyperinflammatory response that triggers a cascade of pathophysiological processes for which the affected patient cannot adequately compensate. Indeed, concomitant compensatory or antiinflammatory responses can also aggravate the septic condition by compromising the patient’s ability to combat the infection (2). There are approximately 750,000 new severe sepsis cases each year, with mortality rates ranging from 20 to 50% (3). Early diagnosis and treatment of sepsis is critical to prevent the cascade of the complex processes that lead to severe sepsis and septic shock. However, the best available diagnostic and treatment strategies have failed, which underscores the need for a better understanding of the pathophysiological mechanisms regulating the inflammatory host response during sepsis (4).
Thymic stromal lymphopoietin (TSLP) is a member of the IL-2 cytokine family and is a distant paralog of IL-7 (5). TSLP has been studied extensively in the context of lung and skin allergic disorders, where it promotes Th2 responses (6). However, it is becoming clear that TSLP may also have influences on other disorders and multiple organ systems, including the blockade of Th1/Th17 responses and/or accelerated wound healing in inflammatory bowel disease, promotion of cancer, and exacerbation of autoimmunity (6). TSLP signals via a receptor that includes the IL-7 receptor α-chain (IL-7Rα CD127) and the unique TSLP receptor (TSLPR) chain. TSLPR-deficient mice (7) have been extensively used to determine TSLP–TSLPR signaling contribution to homeostasis and disease (8, 9).
Previous observations prompted us to investigate the contribution of TSLP to sepsis outcomes. First, TSLP production in epithelial cells can be induced by stimuli present at the early stages of the septic response, such as microbes, proinflammatory cytokines, including TNF, IL-1β, and IL-17, as well as injury (10). Second, a recent report indicated that mice treated with anti-TSLP antibodies exhibited decreased mortality and improved bacterial clearance when subjected to cecal ligation and puncture (CLP) (11). In contrast to this study, we found that deficiency in TSLP–TSLPR signaling in myeloid cells increased mouse mortality and contributed to an exacerbated inflammatory response in a polymicrobial model of sepsis. Overall, our data demonstrate that TSLP contributes to survival and limits the magnitude of the inflammatory response after septic insult without compromising the host’s ability to fight pathogens.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratories (Sacramento, CA). Tslpr−/− mice on the C57BL/6 background were described previously (7). Mice with transgenic expression of Lys-Cre or Vill-Cre on the C57BL/6 background were purchased from Jackson Laboratories and crossed with mice containing loxP-flanked Tslpr alleles (12). Mice were bred and maintained at the Seattle Children’s Research Institute Animal Facility (Seattle, WA). Unless specified otherwise, all experiments were performed using male mice that were 12 weeks old at the beginning of the experiment. We used male mice to avoid the potential confounding effects of female mouse hormones on the immune response (13). We routinely used 12-week-old mice, because certain cell populations that actively participate in the septic response, such as mast cells (14, 15), reach a plateau in their cell numbers in the peritoneal cavity (infection site) at this age (16).
All animal care and experimentation was conducted in accordance with the current National Institutes of Health guidelines and with the approval of the Seattle Children’s Research Institute Institutional Animal Care and Use Committee.
CLP
CLP was performed as described previously (15). Briefly, mice were deeply anesthetized by an intraperitoneal injection of 100 mg/kg ketamine and 20 mg/kg xylazine. The cecum was exposed by a 1- to 2-cm midline incision on the anterior abdomen, subjected to ligation of the distal half (moderate CLP) of the cecum, and punctured once with a 22-G needle on the ligated segment. The cecum was then placed back into the abdomen, 1 ml of sterile saline (pyrogen-free 0.9% NaCl) was administered into the peritoneal cavity, and the incision was closed using 9-mm steel wound clips. For TSLP treatment, mice were injected once with 2 μg TSLP intraperitoneally at 2 hours after moderate or severe CLP (ligation of the distal two-thirds of the cecum). For treatment with the anti–IL-6R antibody, mice were intraperitoneally injected once with 200 μg anti–IL-6R antibody (clone 15A7; BioXcell, West Lebanon, NH) at 6 hours after moderate CLP. For treatment with the anti-TSLP antibody, mice were intraperitoneally injected once with 100 μg anti-TSLP antibody (clone M702; Amgen, Thousand Oaks, CA) or isotype control the day before CLP. Mouse rectal temperatures were measured at 24 hours after CLP. For survival experiments, mice were observed for mortality at least four times daily for the first 3 days, and then twice each day for up to 7 days. Mice that were clearly moribund were killed by CO2 inhalation.
Statistical Analyses
All statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA). We assessed differences in the survival rates after CLP using the Mantel-Haenszel log-rank test. All other data were analyzed for statistical significance using the Mann-Whitney U test. A P value less than 0.05 was considered statistically significant. Unless otherwise specified, all data are presented as means (±SEM).
The following procedures are described in the online supplement: murine neutrophil isolation; human neutrophil isolation; flow cytometry; flow cytometry of human neutrophils; bacterial CFU quantification; cytokine and chemokine measurements; alanine aminotransferase measurements; quantitative PCR; and collection of human plasma samples from patients with sepsis and healthy control subjects.
Results
TSLP Levels Are Increased and Promote Survival via TSLPR after Experimental Sepsis Induction (CLP) in Mice
To assess whether TSLP levels are increased in a murine sepsis model system, we performed a moderately severe variation of CLP (<10% of the wild-type mice died within 7 d after CLP). Intraperitoneal and plasma TSLP levels were significantly increased in septic mice at 24 hours after surgery and were reduced at 48 hours (Figures 1A and 1B). These data suggest that TSLP levels are elevated during the peak of the inflammatory response against bacteria and decrease once bacteria are cleared and inflammation is resolved.
Figure 1.
Thymic stromal lymphopoietin (TSLP) levels are increased and improve survival after cecal ligation and puncture (CLP). (A and B) TSLP peritoneal lavage fluid (A) and plasma concentrations (B) at the indicated time points after induction of moderate CLP (50% ligation; single puncture with a 22-G needle; n = 3–10 mice/group). (C) Drop in body temperature at 24 hours after moderate CLP in TSLP receptor (Tslpr)+/+ mice (n = 19) and Tslpr−/− mice (n = 17). Data were pooled from five independent experiments, each of which produced similar results. (D) Survival after moderate CLP in Tslpr+/+ mice (n = 14) and Tslpr−/− mice (n = 12). Data were pooled from the three independent experiments, each of which produced similar results. *P < 0.05 versus Tslpr−/−. Data in A–C are presented as means (±SEM).
A recent report indicated that pretreatment, 1 day before CLP, with an anti-TSLP neutralizing antibody (clone MAB555) improved bacterial clearance and mouse survival after CLP (11). We performed the same experiment described by Kuethe and colleagues (11) using a different anti-TSLP antibody clone (clone M702) that has been extensively used for in vivo studies (17–19). We found that mice treated with this anti-TSLP antibody exhibited increased morbidity rates, as reflected in their pronounced drop in body temperature, and no improvement in bacterial clearance when compared with mice treated with an isotype control antibody after CLP (see Figure E1 in the online supplement). According to these results, we decided to investigate the contribution of TSLP to sepsis using a genetic approach to prevent any potential off-targets effects and the possibility of not using the optimal dose or administration time for the anti-TSLP antibody treatment blockade. For this purpose, we performed CLP on TSLPR-deficient (Tslpr−/−) mice. Naive Tslpr−/− mice do not exhibit obvious phenotypic defects (7) that may confound the outcome of the polymicrobial model of sepsis. The Tslpr−/− mice had an increased morbidity (Figure 1C) and mortality rate (Figure 1D) after CLP when compared with their littermate controls.
To demonstrate that receptor ligation with TSLP is protective, we decided to administer TSLP to septic mice. TSLP administration to mice that underwent CLP of moderate severity did not exhibit any significant reduction in mouse morbidity. This result was not surprising, considering that moderate CLP does not induce a strong inflammatory response that can increase the risk of sepsis progression. We decided to administer TSLP to mice that were subjected to CLP that induced severe sepsis in which more than 50% of the mice died within 4 days after CLP. These TSLP-treated mice exhibited reduced morbidity rates (Figure E2). These data indicate that exogenous TSLP can reduce morbidity after severe CLP, suggesting that receptor ligation with TSLP is protective.
Overall, these findings indicate that TSLP generation may be triggered by the inflammatory response against bacteria and contributes to survival by interacting with TSLPR after CLP.
TSLP Reduces Inflammation without Impairing Bacteria Clearance after Experimental Sepsis Induction
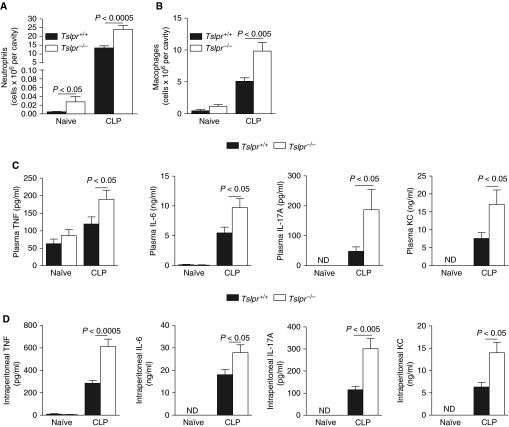
The increased mortality rate in the Tslpr−/− mice could not be explained by defective bacterial clearance, because local (Figure 2A) and systemic (Figure 2B) CFU levels were significantly decreased in the Tslpr−/− mice after CLP. Because the Tslpr−/− mice could effectively contain the infections, we decided to investigate whether a dysregulation in the inflammatory host response could cause the increased mortality that was observed in the Tslpr−/− mice. As shown in Figures 3A and 3B, the Tslpr−/− mice exhibited a 78.2 and 192.5% increase in intraperitoneal neutrophil and macrophage numbers, respectively, at 24 hours after CLP. Moreover, plasma and intraperitoneal levels of proinflammatory cytokines, such as TNF, IL-6, and IL-17A, and chemokines, such as keratinocyte chemoattractant (KC), were also increased in the Tslpr−/− mice after CLP (Figures 3C and 3D). In agreement with these data, we found that mice treated with exogenous TSLP exhibited a reduction in intraperitoneal neutrophil numbers and plasma and intraperitoneal levels of proinflammatory cytokines, indicating that receptor ligation with TSLP reduces inflammation after severe CLP (Figure E3). Interestingly, TSLP-treated mice also showed a trend for increased plasma CFU levels (Figure E3E), suggesting that TSLPR ligation with TSLP may impair bacteria clearance in concordance with data shown in Figure 2B.
Figure 2.
TSLPR deficiency enhances bacteria clearance after CLP. (A and B) Colony-forming units (CFUs) in the peritoneal lavage fluid (A) and blood (B) at 24 hours after moderate CLP in Tslpr+/+ mice (n = 11–25) and Tslpr−/− mice (n = 7–25). Data were pooled from six independent experiments.
Figure 3.
TSLP–TSLPR interactions down-regulate inflammation after CLP. Neutrophil numbers in the peritoneal cavity (A), macrophage numbers in the peritoneal cavity (B), amounts of TNF, IL-6, IL-17, and keratinocyte chemoattractant (KC) in the plasma (C), and amounts of TNF, IL-6, IL-17, and KC in the peritoneal lavage fluids (D) at 24 hours after moderate CLP in Tslpr+/+ mice (n = 12–25) and Tslpr−/− mice (n = 12–25). ND, not detected. Data were pooled from six independent experiments. Data are presented as means (±SEM).
Overall, these observations indicate that TSLP–TSLPR interactions protect against sepsis by limiting the magnitude of the inflammatory response to infection.
TSLP Limits the Production of Proinflammatory Mediators by Myeloid Cells from Septic Mice
TSLP is known to have wide-ranging impacts on cell lineages that have been shown to contribute to sepsis outcomes, including dendritic cells (DCs) (20) and B cells (21). In agreement with previous reports (22–24), we found that CD4, CD8, B cell, and DC numbers were significantly reduced in mice subjected to moderate CLP. However, we did not observe any difference in the numbers of these cells between littermate controls and the Tslpr−/− mice (Figure E4). There is extensive literature on the effects of TSLP on lymphocytes and DCs (25–27). In contrast, less is known about the effects of TSLP on neutrophils and macrophages. Notably, we found a strong correlation between neutrophil numbers and TSLP levels at 24 hours after CLP (Spearman r = 0.5573, P < 0.05). Moreover, we observed that neutrophils expressed TSLPR mRNA and up-regulated its expression after CLP (Figure E5). Neutrophils play a critical role in sepsis, as their accumulation in organs during the sepsis hyperinflammatory phase has been shown to contribute to organ injury and lethality (28). Macrophages also express TSLPR (29) and produce TSLP in inflammatory conditions (30). Moreover, macrophage-derived proinflammatory cytokines can also lead to tissue destruction, vascular damage, septic shock, multiple organ failure, and lethality during the sepsis hyperinflammatory stage (2). Consequently, we investigated whether neutrophils and macrophages are main target cells for TSLP during sepsis. For this purpose, we generated mice with loxP-flanked Tslpr alleles (12) that also expressed Cre recombinase at the myeloid-specific gene, Lys (31) (Lys-Cre+; Tslprfl/fl mice), or did not express Cre at Lys (Cre−; Tslprfl/fl mice). TSLPR expression was reduced in neutrophils and macrophages obtained from the Lys-Cre+; Tslprfl/fl mice when compared with their Cre− counterparts (Figure 4A). We observed that the macrophage and neutrophil frequencies were similar in naive Cre−; Tslprfl/fl mice and Lys-Cre+; Tslprfl/fl mice, indicating that TSLPR deficiency in myeloid cells does not affect their development.
Figure 4.
Myeloid cells with TSLPR deficiency exhibit increased production of proinflammatory cytokines after CLP. (A) TSLPR expression, as assessed by flow cytometry in bone marrow–derived neutrophils (Gr-1+; CD11b+) and peritoneal macrophages (F4/80+; CD11b+) from Cre−; Tslprfl/fl and Lys-Cre+; Tslprfl/fl mice. Data are representative of three independent experiments. (B) mRNA expression for Il-6, Tnf, Il-1b, Il12p40, and Il-23p19 in peritoneal macrophages that were obtained from Cre−; Tslprfl/fl and Lys-Cre+; Tslprfl/fl mice at 24 hours after CLP (n = 5). (C) Representative flow cytometry plots and frequencies for peritoneal neutrophils and macrophages obtained from Cre−; Tslprfl/fl and Lys-Cre+; Tslprfl/fl mice at 24 hours after moderate CLP and reactivated by phorbol myristate acetate (PMA)/ionomycin for 4 hours in the presence of GolgiPlug. Cells were immunostained for IL-6 and surface granulocyte-differentiation antigen-1 (Gr-1) and CD11b for peritoneal neutrophils and F4/80 and CD11b for macrophages. Data are representative of three independent experiments. FSC, forward scatter; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Data are presented as means (±SEM).
We found that macrophages from CLP-treated Lys-Cre+; Tslprfl/fl expressed increased mRNA levels for IL-6 and IL-12p40 compared with macrophages from control mice, whereas TNF, IL-1β, and IL-23p19 mRNA levels were not significantly different between the two groups (Figure 4B). Next, we validated our data at the protein level by performing intracellular staining and flow cytometry for IL-6 production in peritoneal myeloid cells from septic mice. We found that peritoneal macrophages and neutrophils from CLP-treated Lys-Cre+; Tslprfl/fl mice produced increased IL-6 protein levels when reactivated with phorbol myristate acetate/ionomycin (Figure 4C). Moreover, we determined that human peripheral blood neutrophils (CD15+ cells) express TSLPR mRNA (Figure 5A), and they produced reduced mRNA and protein amounts of IL-6 when preconditioned with TSLP (Figures 5B–5D).
Figure 5.
TSLP reduces IL-6 production in human neutrophils. (A) TSLPR mRNA expression in human blood neutrophils (CD15+ cells) activated for 1 hour by either LPS (100 ng/ml) or GM-CSF (granulocyte/macrophage colony–stimulating factor; 100 ng/ml). (B–D) IL-6 mRNA expression (B), flow cytometry plots and frequencies (C), and IL-6 amounts in the supernatants (D) of activated human blood neutrophils that were pretreated with TSLP (100 ng/ml) and activated with LPS (100 ng/ml) for either 1 hour (B) or 4 hours (C and D). For the IL-6 intracellular staining (C), the cells were incubated in the presence of GolgiPlug for 4 hours and then immunostained for IL-6 and surface CD15. Data were pooled from four independent experiments. Data are presented as means (±SEM).
Overall, these observations indicate that lack of TSLP–TSLPR signaling causes a dysregulation in myeloid cell function that is reflected in their ability to produce increased amounts of proinflammatory mediators.
TSLP–TSLPR Signaling in Myeloid Cells Contributes to Survival and Reduced Morbidity by Limiting Inflammation after Experimental Sepsis Induction
We assessed next how deficiency in TSLP–TSLPR signaling in myeloid cells contributes to CLP outcomes. Lys-Cre+; Tslprfl/fl mice exhibited reduced survival rates (Figure 6A) after moderate CLP when compared with the Cre−; Tslprfl/fl mice. In addition, moderate CLP was associated with increased liver dysfunction in the Lys-Cre+; Tslprfl/fl mice (Figure 6B). Similar to the Tslpr−/− mice, the Lys-Cre+; Tslprfl/fl mice were more efficient in their ability to clear bacteria at the infection site (Figure 6C). Moreover, the Lys-Cre+; Tslprfl/fl mice exhibited increased peritoneal neutrophil and macrophage numbers, (Figures 6D and 6E), and increased systemic proinflammatory cytokine levels (Figure 6F) after CLP.
Figure 6.
TSLP–TSLPR interactions in myeloid cells improve survival and reduce inflammation after CLP. (A) Survival after moderate CLP (50% ligation; single puncture with a 22-G needle) in Cre−; Tslprfl/fl (n = 17) and Lys-Cre+; Tslprfl/fl mice (n = 12). Data were pooled from three independent experiments. (B) Plasma alanine aminotransferase (ALT) levels were assessed in naive Cre−; Tslprfl/fl (n = 3–7) and Lys-Cre+; Tslprfl/fl (n = 3–9) mice and after moderate CLP. (C–F) CFUs in the peritoneal lavage fluid and blood (C), neutrophil numbers in the peritoneal cavity (D), macrophage numbers in the peritoneal cavity (E), and amounts of plasma TNF, IL-6, IL-17, and KC (F) at 24 hours after moderate CLP in Cre−; Tslprfl/fl (n = 7–17) and Lys-Cre+; Tslprfl/fl mice (n = 13–24). Data were pooled from seven independent experiments. Data in B–F are presented as means (±SEM).
To assess the contribution of the exaggerated inflammatory response to shock, hypothermia, and death in the absence of TSLP–TSLPR signaling in myeloid cells, we decided to target the IL-6 signaling pathway. We decided on targeting the IL-6 pathway to reduce inflammation, because it has been shown that IL-6 contributes to the induction of the IL-17/KC axis (32), it has been shown to lead to neutrophil accumulation during the septic inflammatory response (33, 34), and because it has been reported that IL-6 can cause hypothermia within 24 hours of severe sepsis (35). Lys-Cre+; Tslprfl/fl mice treated with anti–IL-6R antibodies exhibited reduced morbidity (Figure E6A), peritoneal neutrophil numbers (Figure E6B), and plasma IL-17 and KC levels (Figure E6C), whereas Cre−; Tslprfl/fl mice only showed a slight reduction in morbidity and inflammatory cell accumulation (Figures E6A and E6B).These observations suggest that interventions aimed at reducing inflammation in the absence of TSLP–TSLPR signaling can improve CLP outcomes.
Collectively, these data indicate that TSLP can directly reduce the ability of myeloid cells to produce large amounts of proinflammatory mediators that can lead to a dysregulation of the immune response to bacterial infection, and hence to sepsis.
Discussion
TSLP was initially studied as a B cell growth factor (36), but successive studies have demonstrated that multiple hematopoietic and nonhematopoietic cells express TSLPR and respond to TSLP, thus highlighting the potential of this cytokine to influence disease development and to maintain homeostasis. Accordingly, strong evidence supports a role for TSLP in the promotion of Th2-type inflammation in the skin, airways, and gastrointestinal tract, hence favoring the progression of allergic diseases, such as atopic dermatitis, asthma, and food allergies (37). Less is known about the beneficial and/or prohomeostatic effects of TSLP.
The present study provides evidence that TSLP contributes to survival and promotes homeostasis in sepsis. First, we observed that TSLP levels were increased during experimental sepsis in mice. Second, we demonstrated that TSLP–TSLPR signaling contributed to mouse survival, reduced morbidity, and reduced inflammation after moderate CLP. Third, we showed that TSLP acts directly on myeloid cells to limit their capacity to produce large amounts of proinflammatory cytokines, such as IL-6.
Besides the potential off-target effects of the anti-TSLP antibody treatment used in the study by Kuethe and colleagues (11), we can speculate on other reasons for the contradictory effects reported in that study and our work. For example, we found that TSLPR can be up-regulated in myeloid cells at the peak of the inflammatory response in sepsis (Figure E3). Consequently, we hypothesize that the effects of TSLP neutralization will greatly depend on when the antibody is administered, as not all of the cells associated with inflammation and bacterial clearance express high TSLPR levels throughout the septic response. In agreement with the report by Kuethe and colleagues, we did find that TSLP–TSLPR signaling impairs the efficiency of mice to clear bacteria (Figure 2). The reduction in the CFU levels in the TSLPR-deficient mice could be explained by the hyperinflammatory response (e.g., increased macrophages, neutrophils, and proinflammatory mediators) that these mice develop after CLP. Although, this may be seen as an advantage for the TSLPR-deficient septic mouse, the hyperinflammation that TSLPR-deficient mice experienced can lead to increased risk of organ dysfunction and sepsis progression, despite the increased bacteria clearance. Notably, a similar phenotype was observed with IL-10–deficient mice, which were more efficient in their ability to clear bacteria via increased neutrophil mobilization to the peritoneal cavity than IL-10–competent mice. Similarly to TSLPR-deficient mice, IL-10–deficient mice succumbed to sepsis due to severe multiple organ damage (38).
Overall, our observations indicate that an exaggerated inflammatory response in the absence of TSLP–TSLPR signaling in myeloid cells can lead to shock, hypothermia, and death. We demonstrated this hypothesis by showing that interventions aimed at reducing inflammation in the absence of TSLP–TSLPR signaling, like blocking the IL-6 pathway, can improve CLP outcomes. Our findings provide, for the first time, evidence for a TSLP-mediated down-regulatory effect on hyperinflammation during sepsis. The implications of our observations to understand the pathophysiological mechanisms of sepsis may only apply to patients that develop hyperinflammation, a clinical phenotype that may or may not be observed across patients and over time in each patient (4).
Our study provides evidence for a TSLP-mediated modulatory effect on inflammation during an acute response to insult. In agreement with our findings, it has been shown that TSLP can also modulate proinflammatory cytokine production in chronic intestinal inflammatory disorders, such as inflammatory bowel disease, without suppressing Th2 responses (17). The molecular mechanisms underlying the antiinflammatory effects of TSLP remain unclear. TSLP has been shown to induce signal transducer and activator of transcription (STAT) 1, STAT3, STAT4, STAT5, and STAT6 phosphorylation in human DCs, whereas it only activates STAT5 in mouse DCs (37, 39). However, signaling pathways that are activated downstream of TSLPR may differ among murine myeloid cells and their phenotypes, as well as under dissimilar conditions. For example, it has been shown that TSLP can induce a specific gene expression profile change; however, it does not activate STAT5 in selective DC subsets that were generated by FMS-like tyrosine kinase ligand (Flt3L) and express B220 and CD11c (FL-pDCs) or CD11c, CD11b, and CD24 (FL-CD24-DCs) (39). We observed, in preliminary studies, that peritoneal macrophages from septic Lys-Cre+; Tslprfl/fl mice exhibited reduced STAT3 phosphorylation (data not shown). Mice lacking STAT3 in myeloid cells succumb to septic peritonitis and exhibit substantial systemic inflammation, accompanied by increases in proinflammatory cytokines, including IL-6 and IL-12p40 (40), which is similar to what we observed in septic Lys-Cre+; Tslprfl/fl mice. This evidence suggests that TSLP-induced STAT3 activation in myeloid cells may limit the production of proinflammatory cytokines in sepsis.
The down-regulatory effects of TSLP on inflammation may not be limited to its direct effects on myeloid cell function. Recent studies indicate that injury is associated with increased inflammation and worsens mortality in septic mice that do not express the transcription factor, NF-kB, in their intestinal epithelium (41). Injury is also one of the main triggers for TSLP production by epithelial cells (10), which requires the activation of the NF-kB signaling pathway (42). Taking into account these observations, we hypothesized that TSLP may also have modulatory effects on intestinal epithelial cells (IECs) that may lead to down-regulation of the inflammatory response. Our studies with mice that do not express TSLPR in IECs (Vill-Cre+; Tslprfl/fl mice) indicate that TSLP–TSLPR interactions indeed contribute to reduced morbidity and inflammation after moderate CLP. These observations seem to support the hypothesis that a loss of balance between the gut’s epithelium, the immune system, and the endogenous microflora can lead to multiple organ dysfunction during sepsis (43). Studies are underway to dissect the mechanisms behind these modulatory effects of TSLP on IECs in sepsis.
The relevance of our murine findings regarding the contribution of TSLP to the regulation of inflammation in human sepsis still requires investigation. We performed a pilot study in which we assessed TSLP levels in 40 patients with severe sepsis from which plasma samples were taken within 24 hours of admission to the intensive care unit, and in 39 age- and sex-matched control subjects (Table E1). TSLP levels were significantly higher in the patients with severe sepsis (1,533 pg/ml [0–8,320 pg/ml]) than in the control subjects (523.3 pg/ml [0–2,004 pg/ml]) (P = 0.013), suggesting that TSLP levels are up-regulated during disease. This study, together with our finding that TSLP can reduce IL-6 production by human neutrophils activated by LPS (Figure 6), seems to indicate that TSLP up-regulation in sepsis has the potential to influence the outcome of the disease in humans.
Taking into consideration the beneficial effects of TSLP that are reported in this study, we speculate that TSLP up-regulation during sepsis is an immunoregulatory mechanism that is initiated by the host to restore homeostasis. However, we should point out that excessive TSLP amounts may have important implications for the development of immunosuppression if TSLP levels remain high at later stages of the disease, which could compromise the host’s ability to fight potentially lethal secondary infections. Consequently, increased TSLP levels may be potentially beneficial to a healthy young adult who will likely respond with a profound cytokine-storm–mediated response to infection. Conversely, increased amounts of TSLP will likely increase the risk of succumbing to sepsis due to immunosuppression in an elderly patient with a less effective immune system (undergoing immunosenescence) and increased comorbidities (4).
In summary, our data demonstrate a previously unrecognized down-regulatory role for TSLP on acute inflammation that is driven by myeloid cell production of proinflammatory mediators in sepsis.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health (NIH) grant HL113351-01 and the American Heart Association grant 12GRNT9680021 (laboratory of A.M.P.), and by NIH grants AI068731, HL098067, and AR059058 (laboratory of S.F.Z.).
Author Contributions: A.M.P., A.L., P.T., M.C., and N.J.S. performed the experiments and analyzed the data; H.H. generated the Cre−; Tslprfl/fl mice; A.M.P. designed the study; S.F.Z. analyzed the data and provided Tslpr−/− and Cre−; Tslprfl/fl mice; A.M.P., A.L., N.J.S., and S.F.Z. wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0380OC on March 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 6.Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H, Ziegler SF. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91:877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, Ihle JN. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 9.Reardon C, Lechmann M, Brüstle A, Gareau MG, Shuman N, Philpott D, Ziegler SF, Mak TW. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–235. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 11.Kuethe JW, Prakash PS, Midura EF, Johnson BL, III, Kasten KR, Caldwell CC. Thymic stromal lymphopoietin mediates the host response and increases mortality during sepsis. J Surg Res. 2014;191:19–24. doi: 10.1016/j.jss.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Hongwei H, Thelen TD, Comeau MR, Ziegler SF. TSLP-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest. 2014;124:5442–5452. doi: 10.1172/JCI77798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1–induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 15.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. Mast cell–derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jippo T, Morii E, Ito A, Kitamura Y. Effect of anatomical distribution of mast cells on their defense function against bacterial infections: demonstration using partially mast cell–deficient tg/tg mice. J Exp Med. 2003;197:1417–1425. doi: 10.1084/jem.20022157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Dakhama A, Jia Y, Wang M, Zeng W, Takeda K, Shiraishi Y, Okamoto M, Ziegler SF, Gelfand EW. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J Allergy Clin Immunol. 2012;130:1175–1186. doi: 10.1016/j.jaci.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S, Morris S, Lukacs NW. TSLP promotes induction of Th2 differentiation but is not necessary during established allergen-induced pulmonary disease. PLoS One. 2013;8:e56433. doi: 10.1371/journal.pone.0056433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scumpia PO, McAuliffe PF, O’Malley KA, Ungaro R, Uchida T, Matsumoto T, Remick DG, Clare-Salzler MJ, Moldawer LL, Efron PA. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 21.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, Wynn JL, Lee PY, Kumagai Y, Efron PA, et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;208:1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scumpia PO, Delano MJ, Kelly-Scumpia KM, Weinstein JS, Wynn JL, Winfield RD, Xia C, Chung CS, Ayala A, Atkinson MA, et al. Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood. 2007;110:3673–3681. doi: 10.1182/blood-2007-04-087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 24.Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, Hotchkiss R, Clare-Salzler M, Moldawer LL. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol. 2004;173:3035–3043. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- 25.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal–derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4+ T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, de Waal Malefyt R, Liu YJ. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 28.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han H, Headley MB, Xu W, Comeau MR, Zhou B, Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol. 2013;190:904–912. doi: 10.4049/jimmunol.1201808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong HJ, Nam SY, Oh HA, Han NR, Kim YS, Moon PD, Shin SY, Kim MH, Kim HM. Interleukin-32-induced thymic stromal lymphopoietin plays a critical role in macrophage differentiation through the activation of caspase-1 in vitro. Arthritis Res Ther. 2012;14:R259. doi: 10.1186/ar4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 32.Bosmann M, Ward PA. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin Transl Med. 2012;1:4. doi: 10.1186/2001-1326-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γΔ T-cell IL-17 production in a murine model of sepsis. Infect Immun. 2010;78:4714–4722. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauvi DM, Williams MR, Bermudez JA, Armijo G, De Maio A. Elevated expression of IL-23/IL-17 pathway–related mediators correlates with exacerbation of pulmonary inflammation during polymicrobial sepsis. Shock. 2014;42:246–255. doi: 10.1097/SHK.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 37.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sewnath ME, Olszyna DP, Birjmohun R, ten Kate FJ, Gouma DJ, van Der Poll T. IL-10–deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J Immunol. 2001;166:6323–6331. doi: 10.4049/jimmunol.166.10.6323. [DOI] [PubMed] [Google Scholar]
- 39.Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, Wagner KU, Kaplan DH, Reizis B, Hennighausen L, et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat Immunol. 2013;14:364–371. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol. 2003;171:6198–6205. doi: 10.4049/jimmunol.171.11.6198. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez JA, Samocha AJ, Liang Z, Burd EM, Farris AB, Coopersmith CM. Inhibition of IKKβ in enterocytes exacerbates sepsis-induced intestinal injury and worsens mortality. Crit Care Med. 2013;41:e275–e285. doi: 10.1097/CCM.0b013e31828a44ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214–223. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.