Abstract
Animal model systems are invaluable for examining human diseases. Our laboratory recently established a mouse model of nasal polyps (NPs) and investigated similarities and differences between this mouse model and human NPs. We especially focus on the hypothesis that B cell activation occurs during NP generation in the murine model. After induction of ovalbumin-induced allergic rhinosinusitis, 6% ovalbumin and Staphylococcus aureus enterotoxin B (10 ng) were instilled into the nasal cavity of mice three times per week for 8 weeks. The development of structures that somewhat resemble NPs (which we will refer to as NPs) was confirmed by hematoxylin and eosin staining. The mRNA and protein levels of various inflammatory cell markers and mediators were measured by real-time PCR in nasal tissue and by ELISA in nasal lavage fluid (NLF), respectively. Total Ig isotype levels in NLF were also quantitated using the Mouse Ig Isotyping Multiplex kit (EMD Millipore, Billerica, MA) on a Luminex 200 instrument (Life Technologies, Grand Island, NY). Similar to human NPs, there were significant increases in gene expression of inflammatory cell markers, such as CD19, CD138, CD11c, and mast cell protease-6 in nasal tissue samples of the NP group compared with those of the control group. In further investigations of B cell activation, mRNA expressions of B cell activating factor and a proliferation-inducing ligand were found to be significantly increased in mouse NP tissue. B cell–activating factor protein concentration and IgA and IgG1 levels in NLF were significantly higher in the NP group compared with the control group. In this study, the NP mouse model demonstrated enhanced B cell responses, which are reminiscent of B cell responses in human NPs.
Keywords: animal model, antibodies, B cells, chronic rhinosinusitis, nasal polyps
Clinical Relevance
The present study demonstrates that the nasal polyp (NP) mouse model revealed enhanced B cell responses, reminiscent of human NPs. This mouse model may enhance our understanding of the pathophysiology of NPs and provide a model to test therapeutic targets in vivo.
Chronic rhinosinusitis (CRS) is one of most common chronic diseases worldwide. CRS can be categorized into two types: CRS with nasal polyps (NPs; CRSwNPs) and CRS without NPs (CRSsNPs). The pathogenesis of NPs is incompletely understood, and lack of an in vivo animal model for NPs has been a major hurdle in investigating NP pathogenesis and testing new treatment modalities. Animal model systems are invaluable in vivo models for examining a variety of human diseases and aid in the development of new therapeutic targets. Our laboratory recently established a mouse model of NPs using a modified protocol generated by Kim and colleagues (1), and investigated similarities and differences between this mouse model and human NPs.
There have been several reports that local activation of B cells and production of antibodies are associated with pathogenic mechanisms in airway diseases (2–7). In addition, our group has previously shown that B cell–activating factor (BAFF) of the TNF family, a key B cell survival factor, is highly expressed in NP tissue from patients with CRSwNPs (8). We also reported increased levels of autoantigen-specific antibodies in NP tissue (9, 10). Several reports have also demonstrated elevated levels of various isotypes of Igs, including IgG, IgE, and IgA, in sinus tissue from patients with CRS (11, 12). Taken together, these findings suggest that B cell activation and antibody production may play important roles in the pathogenesis of CRSwNPs. We therefore focused this investigation on B cell activation in the mouse NP model.
Materials and Methods
Generation of the Mouse Model of NPs
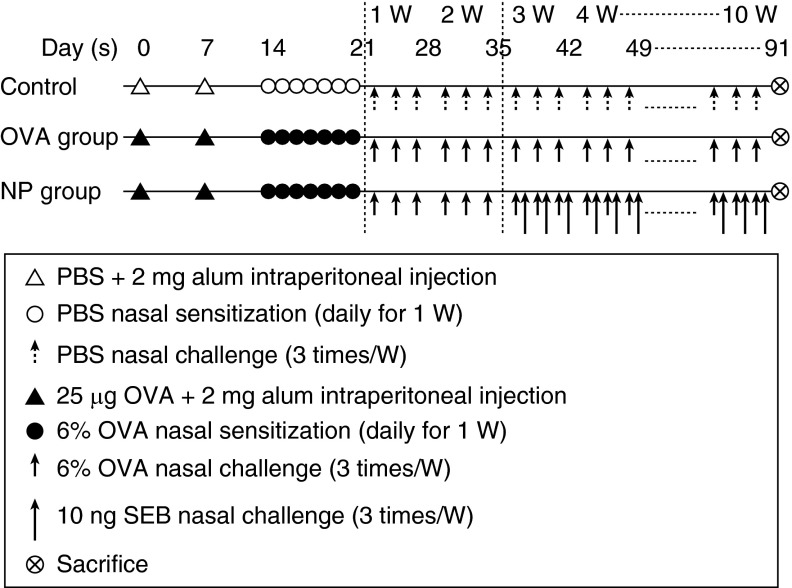
Mice were divided into three groups and each group consisted of 10 mice (control group, ovalbumin [OVA] group, NP group; Figure 1). The procedure for the mouse CRS model is summarized in Figure 1. Briefly, 4-week-old female BALB/c mice were systemically sensitized with an intraperitoneal injection of PBS or 25 μg of OVA (grade V; Sigma, St. Louis, MO) plus 2 mg of aluminum hydroxide gel on Days 0 and 7. After general sensitization, mice were nasally challenged with PBS or 6% OVA daily from Day 14 to Day 20. For the NP group, 6% OVA with Staphylococcus aureus enterotoxin B (SEB; 10 ng) was instilled into the nasal cavity of mice three times per week for 8 weeks after induction of OVA-induced allergic rhinosinusitis. Development of NP-like tissue was confirmed by staining with hematoxylin and eosin (H&E). Negative control mice received neither OVA nor SEB. The OVA group of mice were challenged intranasally with only 6% OVA without SEB. The whole experiment was repeated three times. All animal experiments conducted in this study followed the guidelines and ethics of the Institutional Animal Care and Use Committee at Northwestern University (Chicago, IL).
Figure 1.
Experimental protocol. BALB/c mice were sensitized with PBS or ovalbumin (OVA) plus 2 mg of aluminum hydroxide gel (Alum) on Days 0 and 7 (general sensitization) and then received intranasal PBS or OVA from Day 14 to Day 20. For the nasal polyp (NP) group, 6% OVA with Staphylococcus aureus enterotoxin B (SEB; 10 ng) was instilled into the nasal cavity of mice three times per week for 8 weeks after 2 weeks of the OVA-alone challenge.
Tissue Preparation for H&E Staining, Real-Time RT-PCR, and Nasal Lavage Fluid Collection
For the preparation of nasal cavity samples, we decapitated mice and then, with their heads immobilized, removed the lower jaw together with the tongue. Using the hard palate as a guide, we then used a large scalpel to remove the snout with a transverse cut behind the back molars. After removing the skin and any excess soft tissue, we flushed the external nares with PBS to wash out any blood within the nasal cavity.
For H&E staining, nasal tissues were fixed in 4% paraformaldehyde, decalcified, embedded in paraffin, and sectioned coronally (4 μm thick) approximately 5 mm from the nasal vestibule.
For real-time RT-PCR, the nasal mucosa was taken out meticulously using a small curette after bisecting the nasal tissue sagittally along the nasal septum. Nasal mucosa was immediately immersed in liquid nitrogen and stored at –70°C until use for real-time RT-PCR.
To collect nasal lavage fluid (NLF), 200 μl of PBS was flushed through the nasal cavity from the posterior choanae to the anterior nostrils using a pipette tip after the lower jaw was resected. This was performed twice, and approximately 400 μl of NLF was collected.
Measurement of mRNAs in Nasal Tissue, and BAFF Protein and Total Ig Isotype Levels in NLF
The mRNA levels of various cell markers and inflammatory mediators were measured by real-time RT-PCR in nasal tissue (8). Total RNA was prepared from the nasal mucosa with TriZol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using Superscript reverse transcriptase (Invitrogen) and oligo(dT) primers (Fermentas, Burlington, ON, Canada). For analysis of CD19 (Mm00515420_m1), CD138 (Mm00448918_m1), CD11c (Mm00498701_m1), mast cell protease-6 (MCP-6) (Mm01301240_g1), BAFF (Mm00446347_m1), a proliferation-inducing ligand (APRIL; Mm03809849_s1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1), and Pre-Developed Assay Reagent kits of primers and probes were purchased from Applied Biosystems (Foster City, CA). Amplification of CD19, CD138, CD11c, MCP-6, BAFF, APRIL, and GAPDH cDNA was performed in MicroAmp optical 96-well reaction plates (Applied Biosystems). The reaction was performed using an ABI PRISM 7,000 Sequence Detection System (Applied Biosystems). The average transcript levels of genes were then normalized to GAPDH.
The protein level of BAFF was quantitated by ELISA in NLF. Total Ig isotype levels in NLF were also quantitated using the Mouse Ig Isotyping Multiplex kit (EMD Millipore, Billerica, MA) on a Luminex 200 instrument (Life Technologies, Grand Island, NY), and values were normalized to total protein.
Results
Histologic Confirmation of NP Generation
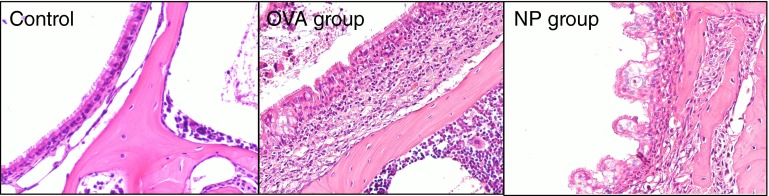
The H&E staining (Figure 2) of nasal tissue revealed that mice challenged with OVA plus SEB (NP group) developed multiple edematous, polyp-like lesions with heavy eosinophilic infiltration, whereas mice challenged with only OVA (OVA group) showed eosinophilic infiltrations, but no polypoid lesions. We streamlined the original protocol of the mouse NP model (1) by changing the OVA-challenge period from 4 weeks to 2 weeks (Figure 1). Even after omitting 2 weeks of the OVA-challenge period, we still obtained a similar pattern of polypoid lesions by H&E staining (data not shown). This finding can contribute to shortening the duration of the NP generation.
Figure 2.
Histology of a mouse model of NPs. Representative hematoxylin and eosin staining images show that mice challenged with OVA plus SEB developed multiple edematous polypoid lesions with heavy eosinophilic infiltrations (NP group), whereas control mice and mice challenged with only OVA (OVA group) did not. Magnification, ×200.
Increased Expression of Inflammatory Cell Markers in CRS and the NP Model
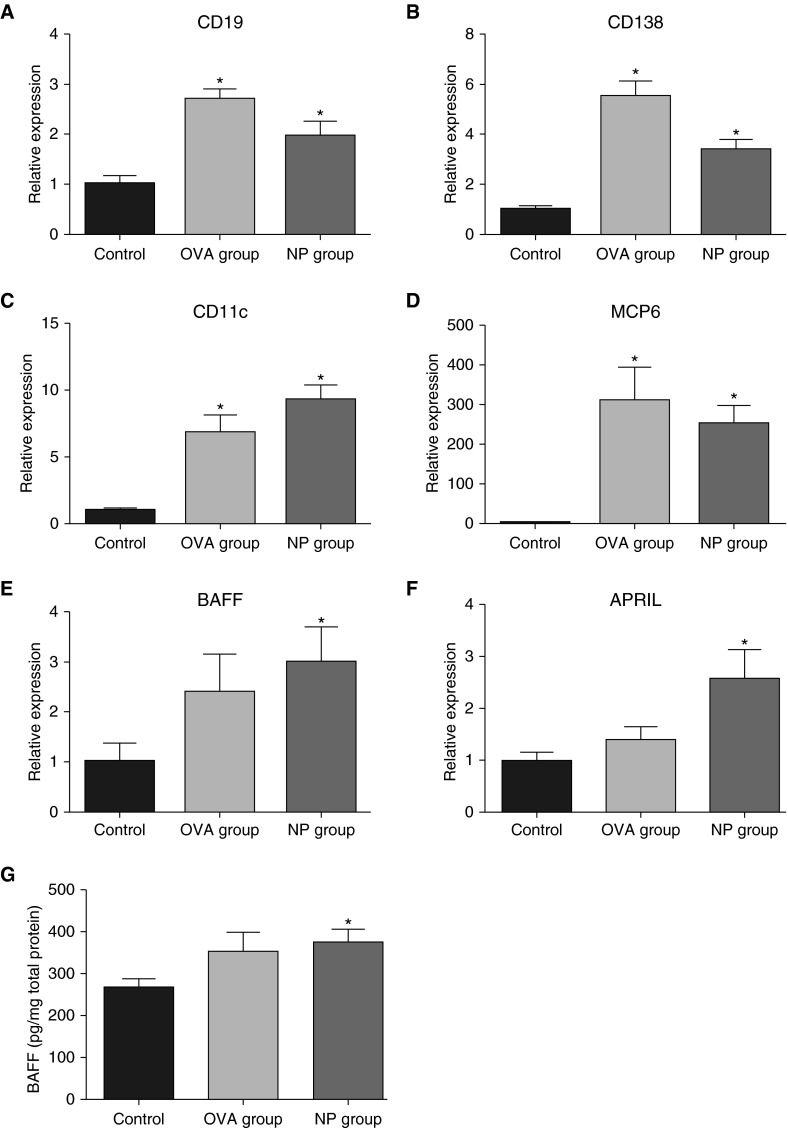
As in human NPs, there were significant increases in expression of inflammatory cell marker genes, such as CD19 (B cell marker, twofold; Figure 3A), CD138 (plasma cell marker, 3.5-fold; Figure 3B), CD11c (dendritic cell marker, ninefold; Figure 3C), and MCP-6 (mast cell marker, 250-fold; Figure 3D), in the nasal tissue samples from the NP group compared with those of the control group (P < 0.05). However, CD3e, a T cell marker, was not significantly different between the groups (data not shown). In the case of CD19, CD138, CD11c, and MCP-6, mRNA expression levels were also significantly increased in the OVA group compared with those seen in the control group (Figures 3A–3D). These results suggest that these markers are not specific to NPs, but rather indicators of allergic inflammation.
Figure 3.
Expression of inflammatory and B cell markers in the NP group. Total RNA was extracted from nasal tissues, and expression of CD19 (B cell marker [A]), CD138 (plasma cell marker [B]), CD11c (dendritic cell marker [C]), and mast cell protease-6 (MCP6) (mast cell marker [D]) was analyzed using real-time PCR. In more analyses of B cell activation, mRNA expression of B cell–activating factor (BAFF) (E) and a proliferation-inducing ligand (APRIL) (F) was found to be significantly increased in mouse NP tissue. BAFF protein concentration in nasal lavage fluid (NLF) was also significantly higher in the NP group than in the control (G). Data presented are means ± SEM. *P < 0.05 versus control.
Increased Production of BAFF mRNA and Protein in the NP Model
Upon further analysis of B cell activation, mRNA expression levels of BAFF (threefold; Figure 3E) and APRIL (2.5-fold; Figure 3F) were found to be significantly increased in mouse NP tissue compared with those seen in control (P < 0.05). However, the OVA group did not show significant differences in mRNA expression of either BAFF or APRIL compared with control. Because we previously found that BAFF, but not APRIL, is significantly elevated in CRS and associated with elevation of Igs in the airways (8, 13), we measured BAFF protein concentration in NLF. BAFF levels were significantly higher in the NP group than those in the control group (1.5-fold, P < 0.05; Figure 3G).
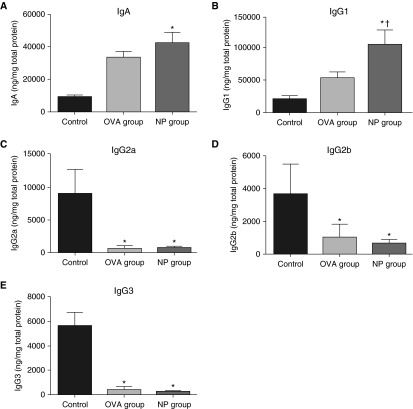
Increased Levels of IgA and IgG1 in the Mouse Model of NPs
Although B cells can play various roles in ongoing inflammation, an important activity is the production and secretion of antibodies (14). We therefore evaluated Ig isotype levels in NLF using the Mouse Ig Isotyping Multiplex kit. In accordance with our previous results of increased IgA, IgG1, and IgG4 in human NPs (13), NLF from the NP mouse group contained significantly higher levels of IgA (Figure 4A) and IgG1 (Figure 4B) than those seen in the control group (P < 0.05). IgG1 levels were also significantly increased in the NP group compared with the OVA group (P < 0.05). On the other hand, IgG2a, IgG2b, and IgG3 levels were significantly lower in the NP group compared with those in control group (P < 0.05; Figures 4C–4E). Regarding the inflammatory changes in mice, IgG1 is typically associated with Th2 immune responses, whereas IgG2a, IgG2b, and IgG3 are associated with Th1 immune responses (15).
Figure 4.
Antibody levels in NLF. Total Ig isotype assay by the Mouse Ig Isotyping Multiplex kit demonstrated that NLF of the NP group contained significantly increased levels of IgA (A) and IgG1 (B) compared with those seen in control. IgG1 level was also significantly higher in the NP group than that in the OVA group. The levels of IgG2a (C), IgG2b (D), and IgG3 (E) were significantly lower in the NP group than those in control. Data presented are means ± SEM. *P < 0.05 versus control; †P < 0.05 versus OVA group.
Discussion
Because one of the most representative signs of B cell activation is enhanced production and secretion of antibodies, our group and others have previously evaluated antibody secretion in human NPs (13, 16). Increased levels of several specific autoantibodies were found in NP tissue compared with levels seen in tissues of control subjects and in patients with CRSsNPs. In addition, nuclear-targeted autoantibodies, such as anti–double-stranded DNA IgG and IgA antibodies, were found at significantly increased levels in NPs, particularly in NPs from patients requiring revision surgery for recurrence (9). In addition, we previously measured the levels of various antibody isotypes in NP and uncinate tissue, as well as in serum. NPs had significantly higher levels of all antibody isotypes (except IgG3) compared with normal uncinate tissue, accompanied by enriched infiltrates of B cells and plasma cells in NPs. In contrast, there were no differences in circulating antibody levels (13). Taken together, activated B cells and plasma cells in NPs vigorously produce antibodies locally, and may be involved in chronic inflammation in patients with CRSwNPs. Our mouse NP model appears to reproduce major elements of findings in the B cell compartment observed in human NPs, showing significantly increased levels of IgA and IgG1 in NLF. Although we could not measure IgE level in NLF due to the absence of the IgE item in the multiplex kit and the insufficient amount of NLF, the original report of the protocol of the mouse NP model (1) already described increased levels of IgE in NLF. Furthermore, a number of studies have confirmed a significantly higher local concentration of IgE in human NP than control tissues (17–21).
This mouse NP model has some limitations. First, as it is based on allergic inflammation with eosinophilic infiltration, which is biased to Th2 responses, it might better reflect mechanisms of formation of Western polyps, but not Asian polyps, which are often not eosinophilic (22). Second, the morphology of mouse NPs is somewhat different from that of human NPs, which often become quite large and expand to fill the nasal cavity. This could be due to a reduced distribution of respiratory epithelium in the nasal airways of the mouse compared with the human nasal airways, where the NP-like structures that we have observed originated. Third, the necessity of prolonged challenges to make the model is a practical obstacle for investigators, although our protocol has been simplified by the modification of the previous protocol. Finally, this mouse model is driven by antigen, and requires the enterotoxin adjuvant to develop NPs, and is thus antigen dependent. Human nasal polyposis has not been definitively shown to be driven by antigens, although, as mentioned previously here, studies from many laboratories support a role of local Igs of various isotypes in CRS pathogenesis.
In summary, this study demonstrates novel findings of enhanced expression of B cell and plasma cell markers, and elevated levels of IgA and IgG1 as a consequence of B cell activation in a new murine model of NPs. Up-regulation of BAFF and APRIL may play an important role in the B cell activation that occurs during NP generation. Based upon this study and previous work in human NPs, testing of an anti-BAFF human monoclonal antibody (belimumab) may be warranted in the treatment of NPs. This animal model of CRS and NPs can be used to explore pathogenic mechanisms of the disease, as well as to test potential therapeutic targets for the pharmacologic interventions.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grant 1K23AI110731 and American Heart Association grant 11SDG7590063 (S.H.C.), and National Institutes of Health grants R37HL068546, R01HL078860, and U19AI106683, and funds from the Ernest Bazley Foundation (R.P.S.), and from the Research Resettlement Fund for the new faculty of Seoul National University (D.-Y.K.).
Author Contributions: Conception and design—D.-Y.K., R.P.S., and S.H.C.; acquisition of data—D.-Y.K., S.H.L., and S.H.C.; analysis and interpretation of data—D.-Y.K., R.G.C., A.K., R.P.S., and S.H.C.; drafting the manuscript or revising it critically for important intellectual content—D.-Y.K., S.H.L., R.G.C., A.K., R.P.S., and S.H.C.; final approval of the version to be published—D.-Y.K., S.H.L., R.G.C., A.K., R.P.S., and S.H.C.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0002RC on May 10, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, Lee CH, Rhee CS. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011;25:e255–e261. doi: 10.2500/ajra.2011.25.3727. [DOI] [PubMed] [Google Scholar]
- 2.Takhar P, Smurthwaite L, Coker HA, Fear DJ, Banfield GK, Carr VA, Durham SR, Gould HJ. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–5032. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima S, Gillespie DN, Gleich GJ. Differences between IgA and IgE as secretory proteins. Clin Exp Immunol. 1975;21:306–317. [PMC free article] [PubMed] [Google Scholar]
- 4.Merrett TG, Houri M, Mayer AL, Merrett J. Measurement of specific IgE antibodies in nasal secretion—evidence for local production. Clin Allergy. 1976;6:69–73. doi: 10.1111/j.1365-2222.1976.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 5.Small P, Barrett D, Frenkiel S, Rochon L, Cohen C, Black M. Local specific IgE production in nasal polyps associated with negative skin tests and serum RAST. Ann Allergy. 1985;55:736–739. [PubMed] [Google Scholar]
- 6.Shatkin JS, Delsupehe KG, Thisted RA, Corey JP. Mucosal allergy in the absence of systemic allergy in nasal polyposis and rhinitis: a meta-analysis. Otolaryngol Head Neck Surg. 1994;111:553–556. doi: 10.1177/019459989411100503. [DOI] [PubMed] [Google Scholar]
- 7.Takhar P, Corrigan CJ, Smurthwaite L, O’Connor BJ, Durham SR, Lee TH, Gould HJ. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, Conley D, Grammer LC, Kern R, Schleimer RP. Evidence of a role for B cell–activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–1392. doi: 10.1016/j.jaci.2008.03.002. 1392.e1–1392.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, Zhou J, Norton J, Carter R, Hinchcliff M, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–1206.e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, Hulse KE, Norton J, Conley DB, Chandra RK, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–2111. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, Lin P, Bousquet J, Van Steen K. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–968. doi: 10.1016/j.jaci.2010.07.007. 968.e1–968.e6. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Segura A, Brieva JA, Rodríguez C. Regulation of immunoglobulin secretion by plasma cells infiltrating nasal polyps. Laryngoscope. 2000;110:1183–1188. doi: 10.1097/00005537-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, Kern RC, Conley DB, Chandra RK, Tan BK, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–1083. doi: 10.1016/j.jaci.2013.01.043. 1083.e1–1083.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kölsch E, Podlaski FJ, Gately MK, Rüde E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 16.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–1847. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein JM, Gorfien J, Noble B, Yankaskas JR. Nasal polyposis: immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps) J Allergy Clin Immunol. 1997;99:165–175. doi: 10.1016/s0091-6749(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 18.Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–290. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 19.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 20.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–79. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 21.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, Acke F, De Ruyck N, Banfield G, Kariyawasam HH, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 22.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.