Abstract
Emerging roles are being recognized increasingly for apolipoproteins in the pathogenesis and treatment of lung diseases on the basis of their ability to suppress inflammation, oxidative stress, and tissue remodeling, and to promote adaptive immunity and host defense. Apolipoproteins, such as apolipoprotein E (apoE) and apolipoprotein A-I (apoA-I), are important components of lipoprotein particles that facilitate the transport of cholesterol, triglycerides, and phospholipids between plasma and cells. ApoE-containing lipoprotein particles are internalized into cells by low-density lipoprotein receptors (LDLRs), whereas apoA-I can interact with the ATP-binding cassette subfamily A member 1 (ABCA1) transporter to efflux cholesterol and phospholipids out of cells. ApoE and apoA-I also mediate receptor-independent effects, such as binding to and neutralizing LPS. Both apoE and apoA-I are expressed by lung cells, which allows apoE/LDLR- and apoA-I/ABCA1-dependent pathways to modulate normal lung health and the pathogenesis of respiratory diseases, including asthma, acute lung injury, cancer, emphysema, pulmonary fibrosis, and pulmonary hypertension. Data from human studies and research using experimental murine model systems have shown that both apoE and apoA-I pathways play primarily protective roles in lung biology and respiratory disease. Furthermore, apolipoprotein mimetic peptides, corresponding to the LDLR-binding domain of apoE or the class A amphipathic α-helical structure of apoA-I, have antiinflammatory and antioxidant effects that attenuate the severity of lung disease in murine models. Thus, the development of inhaled apolipoprotein mimetic peptides as a novel treatment paradigm could represent a significant advance for patients with respiratory disease who do not respond to current therapies.
Keywords: apolipoprotein E, apolipoprotein A-I, lipid transport, lung disease
Apolipoproteins play important roles in maintaining normal lipid homeostasis by clearing cholesterol, triglycerides, and phospholipids from plasma. Two apolipoproteins that modulate lung disease are apolipoprotein E (apoE) and apolipoprotein A-I (apoA-I). ApoE interacts with the low-density lipoprotein receptor (LDLR) to mediate the transport of cholesterol- and triglyceride-rich lipoprotein particles into cells via receptor-mediated endocytosis (Figure 1) (1, 2). In contrast, apoA-I is the major protein constituent of high-density lipoprotein (HDL) that mediates reverse cholesterol transport out of cells by an initial interaction with the ATP-binding cassette subfamily A member 1 (ABCA1) transporter (3). Although apoE and apoA-I are both synthesized primarily in the liver, they are also expressed by lung cells. This article reviews how apoE/LDLR-dependent (Figure 2) and apoA-I/ABCA1-dependent (Figure 3) pathways play primarily protective roles in lung biology and respiratory disease via their ability to attenuate inflammation, oxidative stress, and tissue remodeling responses, while augmenting adaptive immunity and host defense. Furthermore, the potential use of apoE and apoA-I mimetic peptides for the treatment of lung diseases is discussed (Tables 1 and 2).
Figure 1.
Modulation of lipid trafficking in the lung by apolipoprotein E (apoE) and apolipoprotein A-I (apoA-I). ApoE mediates the cellular uptake of cholesterol, triglycerides, and phospholipids within lipoprotein particles by interacting with low-density lipoprotein receptors (LDLRs) on the cell surface. LDLRs are then internalized into cells within clathrin-coated endocytic vesicles by a process termed receptor-mediated endocytosis. ApoE also mediates the LDLR-dependent internalization of mycobacterial lipid antigens by antigen-presenting cells. Lipid-free and lipid-poor apoA-I can interact with ATP-binding cassette subfamily A member 1 (ABCA1) transporters on the cell surface to efflux cholesterol and phospholipids out of cells and thereby create nascent high-density lipoprotein (HDL) particles. Both apoE and apoA-I can directly bind and neutralize LPS.
Figure 2.
Roles of apoE in normal lung health and respiratory disease. An apoE/low-density lipoprotein (LDL) receptor (LDLR) pathway modulates developmental alveologenesis in male mice, mucous cell metaplasia, and airway hyperresponsiveness in experimental house dust mite–induced asthma, and internalization of mycobacterial lipids for presentation by antigen-presenting cells, such as dendritic cells. Hypercholesterolemia in Apoe-deficient mice fed a high-fat or Western diet induces lung remodeling responses that may be relevant for the pathogenesis of emphysema, sarcoidosis, pulmonary fibrosis, and pulmonary hypertension. Increased inflammation and oxidative stress in Apoe-deficient mice augments cigarette smoke–induced emphysema and acute lung injury with a variety of causative factors. BALF, bronchoalveolar lavage fluid; MMP, matrix metalloproteinase; SIRT1, sirtuin 1.
Figure 3.
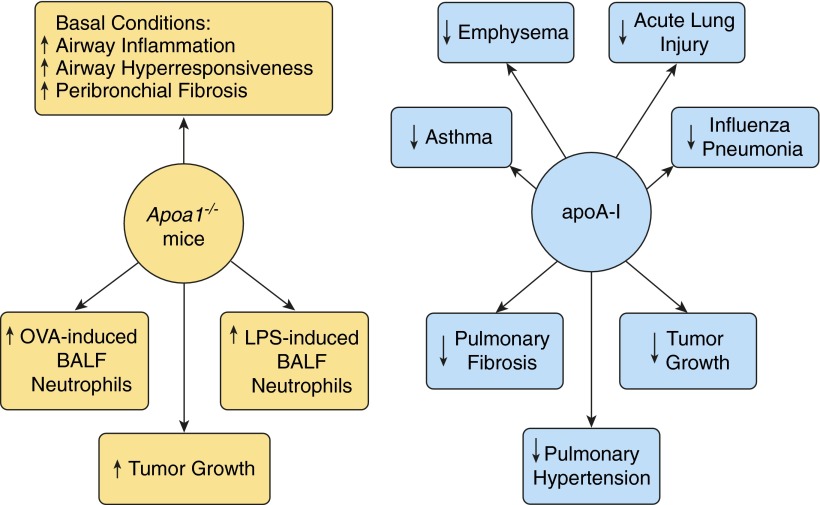
Roles of apoA-I in normal lung health and respiratory disease. Apoa1-deficient mice have increased airway inflammation, airway hyperresponsiveness, and peribronchial fibrosis under basal conditions, as well as enhanced neutrophilic airway inflammation in experimental ovalbumin (OVA)-induced asthma. Apoa1-deficient mice also have a phenotype of increased BALF neutrophils in response to inhaled LPS and increased growth of Lewis lung tumor cells. Murine models have demonstrated that administration of apoA-I or apoA-I mimetic peptides and transgenic expression of apoA-I can attenuate the severity of experimental asthma, emphysema, acute lung injury, viral pneumonia caused by influenza A, pulmonary fibrosis, pulmonary hypertension, and neoplasia.
Table 1.
Attenuation of Lung Disease in Experimental Murine Models by Administration of ApoE Mimetic Peptides or Full-Length ApoE
| Disease Model | Peptide/Protein | Composition | Effect | Reference |
|---|---|---|---|---|
| House dust mite–induced asthma | apoE (130–149) | apoE amino acids 130 to 149 corresponding to the LDLR-binding domain | Decreased airway hyperresponsiveness, airway inflammation, and mucous cell metaplasia in Apoe-deficient mice | (4) |
| LPS-induced sepsis | apoE (133–149) | apoE amino acids 133 to 149 corresponding to the LDLR-binding domain | Suppressed systemic and brain inflammatory responses to intravenous LPS administration in wild-type mice | (40) |
| Neutrophilic lung inflammation induced by LPS or CXCL1 | COG1410 | apoE amino acids 138 to 149 with aminoisobutyric acid substitutions at amino acids 140 and 145 | Attenuated recruitment of neutrophils and macrophages to bronchoalveolar lavage fluid in wild-type mice | (29) |
| Peritonitis model of sepsis | COG1410 | Described above | Reduced systemic inflammatory responses and mortality in response to cecal ligation and puncture in humanized APOE ε3 and ε4 mice | (41) |
| Pulmonary hypertension | Full-length apoE | Recombinant protein | Inhibited platelet-derived growth factor-BB–induced proliferation of pulmonary artery smooth muscle cells from wild-type and Apoe-deficient mice | (18) |
Definition of abbreviations: apoE, apolipoprotein E; CXCL1, chemokine C-X-C motif, ligand 1; LDLR, low-density lipoprotein receptor.
Table 2.
Attenuation of Lung Disease in Experimental Murine Models by Administration of ApoA-I Mimetic Peptides or Full-Length ApoA-I
| Disease Model | Peptide/Protein | Composition | Effect | Reference |
|---|---|---|---|---|
| Ovalbumin-induced asthma | 5A | Bihelical amphipathic peptide with 5-alanine substitutions in one helix to reduce hydrophobicity | Suppressed neutrophilic airway inflammation in Apoa1-deficient mice primarily by reducing G-CSF expression | (58) |
| D-4F | 18 amino acid peptides derived from a class A, amphipathic α helix of apoA-I and composed of D-amino acids | Reduced AHR, BALF eosinophils, TGF-β1 activation, collagen deposition, and oxidative stress in wild-type mice | (73) | |
| House dust mite–induced asthma | 5A | Described above | Decreased BALF inflammatory cells, mucous cell metaplasia, collagen expression, and AHR in wild-type mice | (72) |
| Human apoA-I | Purified from plasma | Suppressed established airway inflammation, mucus production, AHR, and allergen uptake by lung dendritic cells in wild-type mice; promoted lipoxin A4 production and recovery of epithelial tight junction proteins (Zo-1, occludin) in wild-type mice | (64) | |
| Cigarette smoke–induced emphysema | Human apoA-I | Transgenic mice conditionally expressing human apoA-I in alveolar epithelial cells | Attenuated emphysema, lung inflammation, oxidative stress, apoptosis, and metalloproteinase activation | (77) |
| Neutrophilic lung inflammation induced by LPS, CXCL1, or CXCL2 | L-4F | 18 amino acid peptides derived from a class A, amphipathic α helix of apoA-I and composed of L-amino acids | Suppressed BALF neutrophils in wild-type mice | (29) |
| LPS- and lipoteichioic acid–induced acute lung injury and sepsis | Human apoA-I or adenoviral vector expressing human apoA-I or L-4F | Purified from plasma | Decreased acute lung, renal, and liver injury, as well as mortality in wild-type mice | (78–84) |
| Influenza pneumonia | D-4F | Described above | Reduced inflammation, IL-6 levels, and viral titers in the lung, maintained body temperature, and improved sick behavior | (89) |
| Murine lung cancer | Human apoA-I | Transgenic mice expressing human apoA-I under the control of the human apoA-I promoter | Reduced tumor volume | (91) |
| Pulmonary hypertension | L-4F | Described above | Rescued pulmonary hypertension, reduced plasma levels of oxidized lipids, and restored miR-193 expression | (94) |
| Bleomycin-induced pulmonary fibrosis | Human apoA-I | Purified from plasma | Suppressed BALF inflammatory cells and pulmonary fibrosis in wild-type mice | (59) |
| Silica-induced pulmonary fibrosis | Human apoA-I | Transgenic mice expressing human apoA-I under the control of the surfactant protein C promoter | Attenuated pulmonary fibrosis, silicotic nodules, BALF inflammatory cells, and TGF-β1 | (95) |
Definition of abbreviations: AHR, airway hyperresponsiveness; apoA-I, apolipoprotein A-I; BALF, bronchoalveolar lavage fluid; CXCL, chemokine C-X-C motif; miR, microRNA; G-CSF, granulocyte-colony stimulating factor; TGF, transforming growth factor.
ApoE
Background
ApoE is a 34-kD lipoprotein that mediates the clearance of chylomicron remnants and very low-density lipoprotein from plasma by binding to the LDLR, the LDLR-related protein, and heparan sulfate proteoglycans (1). ApoE has two main structural domains, a LDLR-binding domain at the amino-terminus and a lipid-binding domain at the carboxy-terminus. In the lung, apoE is expressed by alveolar macrophages, type I and type II alveolar epithelial cells (AECs), and pulmonary artery smooth muscle cells (PASMCs), whereas LDLRs are expressed by airway epithelial cells and type I and type II AECs (4–7). Although macrophages, type I AECs, and PASMCs have been shown to secrete apoE, the relative contribution of apoE versus systemic apoE that is produced by these cells in modulating normal lung homeostasis and disease has not been established (5, 7–9).
The human APOE gene is polymorphic, with three common isoforms that encode different amino acids at positions 112 and 158, which greatly modify protein function (1). The most common APOE allele is ε3, which has a cysteine at position 112 and an arginine at position 158, whereas the APOE ε2 allele has cysteines and the APOE ε4 allele has arginines at both positions. As compared with the APOE ε3 allele, the APOE ε2 allele has minimal LDLR binding and results in type III hyperlipoproteinemia and accelerated atherosclerosis, whereas the APOE ε4 allele has altered lipid binding that increases both plasma low-density lipoprotein and atherosclerosis risk. In contrast, there is a single Apoe gene in the mouse (10). To investigate the function of the polymorphic human APOE alleles, mice have been engineered so that the single murine Apoe gene has been replaced with the human APOE ε2, ε3, or ε4 genes (10). Experiments have used both humanized APOE knockin mice, as well as Apoe-deficient mice, which are null for the murine Apoe gene, to investigate the role of apoE in lung disease. In addition to its important role in cardiovascular disease, apoE has also been implicated in normal lung health and in the pathogenesis of multiple respiratory diseases.
ApoE and Normal Lung Health
Murine genetic models have identified a role for the apoE/LDLR pathway in sex-specific lung development. Male, but not female, mice with a deletion of the murine Apoe or Ldlr genes have diminished formation of pulmonary gas–exchange units, consistent with impaired developmental alveologenesis, as well as high airway resistance and more rapid loss of lung recoil with age (11, 12). The sex-specific role of apoE in impaired alveologenesis is consistent with the presence of apoE-independent lung developmental pathways in female mice. Furthermore, LDLRs located on type II AECs facilitate low-density lipoprotein particle internalization, with resultant use of endocytosed lipids and cholesterol for surfactant synthesis and secretion (6). In addition, Apoe-deficient, but not Ldlr-deficient, mice have increased serum levels of both very low-density lipoprotein and triglycerides, with associated increases in alveolar levels of disaturated phosphatidylcholine (13), which is synthesized by type II AECs and functions as the major surfactant lipid that lowers surface tension. This suggests that regulation of circulating triglyceride levels by apoE thereby modulates surfactant phospholipid synthesis by type II AECs.
ApoE, Hypercholesterolemia, and Pathologic Remodeling of the Lung Parenchyma and Vasculature
ApoE-mediated cholesterol clearance from plasma prevents excessive lipid accumulation in tissues, including the lung. Experimental murine models of hypercholesterolemia and systemic inflammation have demonstrated that cholesterol accumulation in the lung can cause pathologic parenchymal and vascular remodeling, with resultant emphysema, sarcoidosis, pulmonary fibrosis, and pulmonary hypertension (PH). This concept is based on experiments using Apoe-deficient mice fed a high-fat diet (HFD) or a Western diet (WD). In one study, Apoe-deficient mice fed an atherogenic WD for 10 weeks developed emphysema, as well as increases in pulmonary macrophages, granulocyte-colony stimulating factor (G-CSF), matrix metalloproteinase (MMP)-9 and MMP-12, and proinflammatory signaling (14). In another study, feeding a WD to Apoe-deficient mice caused an increase in lung cholesterol content and a progression of pathologic changes that included inflammatory cell infiltration at 4 weeks, lipid-laden lung cells and emphysema at 12 weeks, and granuloma formation at 24 weeks (15). Apoe-deficient mice fed a cholate-containing HFD for 16 weeks also developed granulomatous lung inflammation similar to sarcoidosis, as well as progressive pulmonary fibrosis (16). Hypercholesterolemic Apoe-deficient mice fed an HFD for 12 weeks developed marked pulmonary fibrotic changes and lung inflammation (17). In addition, feeding an HFD to Apoe-deficient mice induced both PH and insulin resistance, which suggests a role for the metabolic syndrome in disease pathogenesis (18). Similarly, PH was more severe in Apoe-deficient and IL-1–receptor type 1 double knockout mice fed a Paigen atherogenic diet (19). Collectively, these murine studies suggest an important role for systemic apoE in maintaining normal lung homeostasis by preventing the pathological lung and vascular remodeling changes related to systemic inflammation and oxidative stress caused by hypercholesterolemia (11).
ApoE and Asthma
Murine models of experimental asthma have demonstrated a potential protective role of an apoE/LDLR pathway in disease pathogenesis. Lung apoE is increased by house dust mite (HDM) challenges, whereas HDM-challenged Apoe- and Ldlr-deficient mice have selective increases in airway hyperresponsiveness (AHR) and mucous cell metaplasia (MCM), but not airway inflammation (4). Administration of an apoE mimetic peptide, corresponding to the LDLR-binding domain of holo-apoE, suppressed HDM-induced AHR and MCM as well as eosinophilic airway inflammation in Apoe-deficient mice, but not in Ldlr-deficient mice (4). Collectively, this supports the concept that the administration of apoE mimetic peptides to interact with LDLRs on airway epithelial cells might represent a new treatment approach for asthma. The effects of apoE mimetic peptides, however, are likely to be independent of lipid transport, because the peptides do not contain a lipid-binding domain. Consistent with this, apoE mimetic peptides have been shown to interact with SET, which is a physiological binding partner of protein phosphatase 2A. The apoE-SET interaction thereby liberates protein phosphatase 2A, which can then inhibit proinflammatory signaling pathways (20). Lastly, the Gata5 transcription factor has been shown to function as an upstream regulator of apoE (21). Ovalbumin (OVA)-challenged Gata5-deficient mice have reduced expression of apoE in the lung and, like Apoe- and Ldlr-deficient mice, increased HDM-induced AHR and MCM, but not airway inflammation.
Experiments using humanized APOE knockin mice have demonstrated that human APOE ε3 had a protective effect on the severity of HDM-induced airway disease (22). In addition, human APOE ε4 had an intermediary effect, whereas human APOE ε2 did not diminish disease severity. This suggests that the three common human polymorphic APOE alleles differentially modify the manifestations of allergic asthma. In addition, aged Apoe-deficient mice fed an HFD had decreased β-adrenergic receptor expression in the lung, which may represent an additional mechanism by which hypercholesterolemia modifies bronchodilator responses (23).
ApoE and Emphysema
ApoE plays a local protective role in the pathogenesis of experimental emphysema caused by cigarette smoke–induced oxidative stress and inflammation. Apoe-deficient mice fed a regular diet and exposed to cigarette smoke for 3 days had increased oxidative stress, lipid peroxidation, neutrophilic lung inflammation, MMP expression, airspace enlargement, and lung compliance (24). These changes were associated with a decrease in the level and activity of NAD+-dependent deacetylase sirtuin 1, which may predispose the lung to endothelial dysfunction because of increased acetylation and inactivation of endothelial nitric oxide synthase.
ApoE, Acute Lung Injury, and Sepsis
ApoE has antiinflammatory effects that can attenuate the pathogenesis of acute lung injury (ALI). For example, Apoe-deficient mice have increased neutrophilic inflammation and oxidative stress in murine models of ALI induced by acid aspiration, hyperoxia, or pulmonary nanoparticle exposure (25–27). In addition, hyperoxia and nitrogen mustard exposure increased apoE in the lung, whereas intratracheal administration of apoE to rat lungs prevented hyperoxia-mediated ALI (9, 27).
The antiinflammatory effects of apoE can also protect against ALI secondary to LPS, a cell wall component of gram-negative bacteria. Apoe-deficient mice have enhanced neutrophil recruitment to the lungs in response to LPS or CXCL1, whereas administration of an apoE mimetic peptide to wild-type mice inhibited LPS-mediated increases in bronchoalveolar lavage fluid (BALF) neutrophils and alveolar macrophages (28). LPS-challenged, Apoe-deficient mice fed an HFD also developed increased pulmonary capillaritis and alveolar hemorrhage (29). The mechanism by which apoE suppresses LPS-induced neutrophilic inflammation and septic shock involves the direct binding and neutralization of LPS (30–32). During endotoxemia, LPS bound by apoE in plasma is redirected from macrophages to liver parenchymal cells, where it is secreted into the bile and inactivated (30, 31, 33). This prevents LPS-induced cytokine production and mortality caused by systemic inflammation. Furthermore, plasma apoE is cleared primarily by LDLRs in LPS-treated mice (32). This suggests a role for LDLRs in mediating the clearance of apoE-LPS complexes. Consistent with a protective role for apoE in endotoxemic shock, mortality was reduced in wild-type mice that received intravenous LPS plus apoE as compared with mice that received LPS alone (30). Similarly, Apoe-deficient mice had increased mortality, augmented tumor necrosis factor (TNF)-α production, and more bacterial outgrowth from their organs after intravenous LPS administration or bacterial infection as compared with wild-type mice (34–36). These studies show that apoE-deficient mice have both excessive inflammatory responses to LPS and impaired host defense against bacterial infections.
Not all studies, however, have found a protective effect for apoE in sepsis. In a rat model of polymicrobial sepsis secondary to cecal ligation and puncture, repeated administration of recombinant apoE3 increased mortality (37). The mechanism involved the enhanced presentation of endogenous lipid antigens by dendritic cells to activate natural killer T (NKT) cells, which was associated with increases in Th1 cytokines and liver injury (37, 38). Thus, the role of apoE in bacterial sepsis is likely to be context dependent and may reflect a balance of its protective effects on inflammation and host defense rather than its ability to augment immune responses via lipid antigen presentation.
The polymorphic human APOE alleles can modify LPS-induced inflammatory responses. In experimental sepsis models induced by intravenous LPS administration or cecal ligation and puncture, proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, IL-12, G-CSF), hypothermia, hepatic injury, and mortality were increased in knockin mice expressing the human APOE ε4 allele as compared with mice expressing the human APOE ε3 allele (39–40). In addition, apoE mimetic peptides suppressed these LPS-induced inflammatory responses. Human subjects heterozygous for APOE ε3/ε4 have enhanced innate immune responses to intravenous LPS administration as compared with individuals homozygous for APOE ε3 (41). Blood from APOE ε3/ε4 subjects stimulated ex vivo with the Toll-like receptor 4 (TLR4) agonist, LPS, or the TLR2 agonist, PAMCSK4, produced increased amounts of cytokines, chemokines, and G-CSF as compared with blood from APOE ε3/ε3 subjects. Furthermore, CD14hi monocytes from APOE ε3/ε4 subjects had an increase in lipid rafts as compared with monocytes from APOE ε3/ε3 subjects, which may have contributed to the enhanced TLR responses. APOE ε3/ε4 subjects that were challenged in vivo with LPS also displayed sustained increases in body temperature and increased plasma levels of TNF-α. The APOE ε4 allele has also been associated with increased coagulation system failure in European American patients with severe sepsis. Collectively, these results suggest that the human APOE ε4 allele may regulate innate immune responses to LPS and may promote more severe organ injury during sepsis. Similarly, in a study of 343 patients undergoing major elective noncardiac surgery, carriers of the APOE ε3 allele (one or two copies) had a lower incidence of severe sepsis and fewer intensive care unit days as compared with individuals without at least one copy of APOE ε3 (42).
ApoE and Host Defense to Mycobacteria and Chlamydia
ApoE enhances host defense to mycobacterial infection by facilitating the presentation of exogenous lipid antigens, which is important for adaptive immune responses during tuberculosis. Binding of mycobacterial glycolipid antigens by apoE precedes receptor-mediated endocytosis via the LDLR into antigen-presenting cells, such as dendritic cells, and subsequent presentation by the major histocompatibility complex–like molecule, CD1, to activate NKT cells (43). Similarly, activated human B cells expressing the LDLR use apoE to capture lipid antigens and thereby present antigens and activate NKT cells via CD1d (44).
The manifestations of Chlamydia pneumoniae infection may also be modulated by apoE. C. pneumoniae is a gram-negative, obligate, intracellular, respiratory pathogen that has been associated with asthma and chronic obstructive pulmonary disease, as well as with atherosclerosis, inflammatory arthritis, and giant cell arteritis (45–47). The human APOE ε4 allele increases both the attachment of C. pneumoniae elementary bodies to host cells and the risk of bacterial dissemination (45, 46). Furthermore, serum apoE levels are increased in pediatric patients with bacterial pneumonia, meningitis, and sepsis and may represent a biomarker of bacterial infection (48, 49).
ApoE and Lung Cancer
Proteomic profiling of human plasma has identified elevated levels of apoE in current smokers as compared with nonsmokers (50). Similarly, a significant positive correlation was observed between plasma apoE levels and premalignant squamous metaplasia. In one study, 50% of 56 lung adenocarcinomas with malignant pleural effusions had increased apoE expression (51). Furthermore, survival was significantly reduced in patients with lung adenocarcinomas that displayed increased apoE expression. Transfection of a lung adenocarcinoma cell line with small interfering RNAs to knockdown apoE expression increased cis-platinum–mediated reductions in cellular proliferation and impaired cell migration. Similarly, pleural fluid apoE levels were higher in malignant as compared with benign pleural effusions, especially those associated with adenocarcinomas (52). Collectively, these studies suggest a role for apoE in promoting lung cancer, perhaps by enhancing cholesterol transport into tumor cells (50). Consistent with this concept, LDLR expression is up-regulated in the A549 human lung adenocarcinoma cell line and increases when cholesterol is depleted (53). Thus, in contrast to its protective effect regarding inflammatory respiratory diseases, apoE may promote tumor cell growth in the lung.
ApoE and PH
ApoE has a protective effect in PH by suppressing platelet-derived growth factor-BB (PDGF-BB)–induced PASMC proliferation (18). This is mediated by the binding of apoE to the LDLR-related protein, with subsequent internalization of the PDGF-BB receptor, PDGFR-β (7). Both bone morphogenetic protein-2 and peroxisome proliferator-activated receptor-γ agonists stimulate apoE production by PASMCs, which is consistent with a bone morphogenetic protein/peroxisome proliferator-activated receptor-γ/apoE-dependent pathway that suppresses PDGF-BB–mediated PASMC proliferation and prevents PH (7).
ApoA-I
Background
ApoA-I is a 28-kD protein with 10 conserved, class A amphipathic α-helices that mediate high-affinity interactions with lipids (54). Lipid-free apoA-I associates with ABCA1 to efflux unesterified cholesterol and phospholipids from cells, which generates nascent HDL particles (3, 54, 55). Lecithin-cholesterol acyltransferase then associates with HDL and esterifies cholesterol, which is transferred from the surface to the particle core, and generates a mature HDL. HDL can subsequently be transferred to the liver, where cholesteryl esters are oxidized into bile acids and excreted from the body. In addition to its important role in the prevention of atherosclerosis, apoA-I modulates normal lung lipid homeostasis and the pathogenesis of respiratory disease.
ApoA-I and Normal Lung Lipid Homeostasis
ApoA-I is expressed in the lung and can be detected by gestational Day 15.5 in mice, with higher levels of expression in females (56). ApoA-I expression in the adult mouse lung is markedly reduced as compared with the developing lung, which suggests that it may modulate lipid metabolism during embryonic development. Alveolar macrophages, AECs, airway smooth muscle cells, and pulmonary vascular endothelial cells all express ABCA1, whereas alveolar macrophages and AECs also express apoA-I (57–62). Furthermore, apoA-I protein has been detected in both human and murine BALF, which suggests that apoA-I may be secreted by lung cells into epithelial lining fluid (57, 58, 63). However, the direct secretion of apoA-I by lung cells has not yet been demonstrated.
Under basal conditions, Apoa1-deficient mice have increased AHR, subepithelial collagen deposition, and lung oxidative stress, as well as increased numbers of lung inflammatory cells (64). Plasma levels of the antioxidant protein, paraoxonase 1, were also decreased, whereas plasma HDL oxidized at a faster rate, suggesting that the pulmonary phenotype of Apoa1-deficient mice is caused by increased oxidative stress. ABCA1 on type I AECs can release lipids to apoA-I and generate particles that resemble nascent HDL, which supports a role for ABCA1 in maintaining normal lung lipid homeostasis by transporting lipids out of lung cells (60). Consistent with this, Abca1-deficient mice have a phenotype of alveolar proteinosis with lipid-laden alveolar macrophages and type II AECs that lack normal surfactant-containing lamellar bodies (65, 66).
ApoA-I and Asthma
Studies using genetic murine models have identified a role for the apoA-I/ABCA1 pathway in mediating neutrophilic airway inflammation, which may be relevant for the pathogenesis of type 2-low asthma (67). Apoa1-deficient mice that were sensitized and challenged with OVA demonstrated a selective increase in BALF neutrophils that was primarily G-CSF dependent (57). The increased neutrophilic airway inflammation in OVA-challenged Apoa1-deficient mice was also associated with increases in type 1 (IFN-γ) and type 17 (IL-17A) cytokines, but not type 2 cytokines (IL-4, IL-5, and IL-13). Similarly, OVA-challenged mice that overexpressed the human ABCA1 transporter under the Tie2 promoter in pulmonary vascular endothelial cells and macrophages had attenuated increases in BALF neutrophils and G-CSF as compared with wild-type mice (59). Collectively, these studies showed that an apoA-I/ABCA1-dependent pathway inhibited experimental OVA-induced neutrophilic airway inflammation by reducing G-CSF production.
ApoA-I also has antiinflammatory and immunomodulatory properties that may be relevant for adaptive immune responses in allergic asthma. For example, apoA-I can suppress adaptive immunity by inhibiting the differentiation, maturation, and function of dendritic cells (68–70). Furthermore, the ability of apoA-I to decrease the content of cholesterol and major histocompatibility complex class II proteins within plasma lipid raft domains can attenuate antigen presentation by immune cells (68).
ApoA-I mimetic peptides have an amphipathic α-helical structure that simulates the biologic activity of apoA-I by mediating cholesterol efflux out of cells (54). Furthermore, apoA-I mimetic peptides have antiinflammatory effects. Several recent studies have shown that apoA-I mimetic peptides can reduce disease activity in experimental murine asthma models. For example, apoA-I mimetic peptides prevented increases in neutrophilic airway inflammation in OVA-challenged Apoa1-deficient mice (57). Similarly, administration of apoA-I mimetic peptides or human apoA-I to murine models of experimental allergic asthma suppressed airway inflammation with decreases in BALF inflammatory cells (e.g., eosinophils, neutrophils, and lymphocytes), type 2 and type 17 cytokines, airway epithelial cytokines (IL-25, IL-33, thymic stromal lymphopoietin), C-C chemokines, and alternative macrophage activation, and increased lipoxin A4 (63, 71, 72). In addition, apoA-I mimetic peptides or human apoA-I attenuated increases in AHR, MCM, transforming growth factor (TGF)-β, and lung collagen deposition, and improved airway epithelial cell barrier function. Collectively, these studies suggest that apoA-I mimetic peptides might be developed into a novel treatment approach for asthma.
Serum apoA-I levels have also been positively correlated with less severe airflow obstruction in both those with asthma and individuals without lung disease. For example, a positive correlation was found between serum apoA-I levels, as well as HDL, and higher FEV1 in a cohort of 159 subjects with atopic asthma, whereas a similar positive correlation was found in an analysis of the 14,135 subjects without respiratory disease who participated in the Third National Health and Nutrition Examination Survey (73, 74). These studies suggest that circulating levels of apoA-I may have a protective effect on airflow obstruction in asthma.
ApoA-I and Emphysema
Levels of apoA-I are reduced in both the sputum and lungs of patients with chronic obstructive pulmonary disease, as well as in the lungs of cigarette smoke–exposed mice (75, 76). Transgenic mice that conditionally overexpressed human apoA-I in AECs were protected from developing neutrophilic lung inflammation and cigarette smoke–induced emphysema (76). Collectively, this suggests that apoA-I may play a protective role in cigarette smoke–induced emphysema.
ApoA-I, ALI, and Sepsis
The antiinflammatory properties of apoA-I also protect against ALI and end-organ damage in septic shock. For example, neutrophil recruitment to the lung is increased in Apoa1-deficient mice after LPS inhalation (28). Administration of purified human apoA-I or an adenoviral vector that expressed human apoA-I to mice challenged with LPS or lipoteichoic acid, a cell wall component of gram-positive bacteria, protected against LPS-induced ALI, renal and liver injury, and mortality (77–81). In addition, apoA-I reduced BALF cytokines (e.g., TNF-α, IL-1β, IL-6) and TLR4 expression by vascular endothelial cells. ApoA-I mimetic peptides have similar protective effects against LPS-induced ALI. Intravenous administration of apoA-I mimetic peptides to wild-type mice suppressed LPS-induced neutrophilic airway inflammation and protected against ALI, liver injury, and mortality in rat models (28, 82, 83). Furthermore, an apoA-I mimetic peptide directly suppressed CXCR2-directed neutrophil chemotaxis to the lung, which suggests that apoA-I mimetic peptides might be used therapeutically in ALI (28).
Data from human studies similarly support a role for apoA-I in ameliorating ALI. Patients with an increased risk of ALI and 30-day mortality after cardiopulmonary bypass surgery had a higher frequency of the APOA1 -75 G/A polymorphism in the APOA1 promoter, which is associated with reduced apoA-I expression (84). An apoA-I mimetic peptide also suppressed IL-6 production by LPS-stimulated human blood, preserved paraoxonase 1 activity, and inhibited the ability of serum from patients with adult respiratory distress syndrome to activate human neutrophils ex vivo (82).
The antiinflammatory functions of apoA-I that protect against LPS-induced ALI involve several mechanisms. Domains located in the carboxy-terminal half of apoA-I can directly bind and neutralize LPS and thereby modulate innate immune responses to bacterial infection and attenuate sepsis and ALI (78, 80, 85, 86). ApoA-I mimetic peptides can similarly bind LPS and protect against ALI, whereas apoA-I can also bind lipoteichoic acid (79, 82). In addition, the LPS-binding protein, which binds and neutralizes LPS, associates with apoA-I in human plasma (87).
ApoA-I and Host Defense against Influenza
On the basis of the finding that influenza infection in mice causes HDL to lose its antiinflammatory properties, studies have assessed the role of apoA-I mimetic peptides in modulating immune responses to influenza A infection (88). Administration of an apoA-I mimetic peptide to WD-fed Ldlr-deficient mice that were infected with influenza A virus reduced lung viral titers and inflammation. ApoA-I mimetic peptide treatment also inhibited increases in plasma low-density lipoprotein cholesterol and IL-6, reduced macrophage trafficking into large arteries, and increased plasma HDL cholesterol and paraoxonase activity. Furthermore, treatment of influenza-infected A549 cells with an apoA-I mimetic peptide inhibited viral replication, caspase activation, and the production of cytokines and proinflammatory oxidized phospholipids (89).
ApoA-I and Lung Cancer
Experimental murine models have shown that apoA-I has antitumorigenic effects mediated by multiple immunomodulatory mechanisms. These include the conversion of tumor-promoting M2 macrophages into an antitumor M1 phenotype, increased tumor infiltration by cytotoxic CD8+ T cells, and decreased angiogenesis, MMP-9 activity, and survivin expression (90). Transgenic mice that overexpressed human apoA-I displayed decreased tumor growth when Lewis lung tumor cells were injected subcutaneously. Similarly, administration of apoA-I to Apoa1-deficient mice that received B16F10L melanoma tumor cells showed significant inhibition of tumor growth and metastasis, as well as enhanced tumor regression and survival. These findings suggest that apoA-I–based treatments might be developed for cancer.
Human translational studies have also provided evidence regarding a potential role of apoA-I in lung cancer. Patients with advanced stage non-small cell lung cancer and persistently high serum apoA-I levels had the highest overall median survival at 36 months, whereas patients with persistently low serum apoA-I levels had the lowest median overall survival. This suggests that higher serum apoA-I levels were associated with a lower rate of disease progression in non-small cell lung cancer (91). In contrast, serum levels of pro–apoA-I have been found to be specifically and selectively increased only in patients with lung cancer that had metastasized to the brain (92).
ApoA-I and PH
In rat models of monocrotaline- and hypoxia-induced PH, administration of an apoA-I mimetic peptide improved cardiac and pulmonary structure and function and suppressed plasma levels of oxidized lipids (93). There was also increased expression of miR-193, which may have then decreased the expression of lipoxygenases and insulin-like growth factor-1 receptor. This suggests that apoA-I mimetic peptides might be used as a treatment for PH.
ApoA-I and Pulmonary Fibrosis
ApoA-I was first proposed to play a role in the pathogenesis of idiopathic pulmonary fibrosis on the basis of a proteomic analysis of BALF that found reduced levels in patients as compared with healthy subjects (58). Furthermore, in the subjects with idiopathic pulmonary fibrosis, BALF apoA-I levels were inversely correlated with the percentage of BALF foamy macrophages, as well as with fibrosis scores. In addition, intranasal administration of human apoA-I to a murine model of bleomycin-induced fibrosis attenuated increases in BALF inflammatory cells and lung collagen deposition. Similarly, transgenic mice that conditionally expressed human apoA-I in AECs were protected from lung fibrosis in an experimental model of pulmonary silicosis, with decreased collagen deposition, lung inflammation, fibrotic nodule formation, and TGF-β, whereas lipoxin A4 was increased (94). Collectively, these results support the concept of a protective role of apoA-I in pulmonary fibrotic disease.
Conclusions
An emerging body of literature has identified important roles for apoE and apoA-I in modulating normal lung homeostasis, as well as in the pathogenesis of many respiratory diseases. Apolipoprotein mimetic peptides that correspond to the LDLR-binding domain of apoE (Table 1) or the class A, amphipathic α-helical structure of apoA-I (Table 2) have antiinflammatory, antioxidant, and antineoplastic effects, and they also attenuate pathogenic lung remodeling responses (54). In particular, administration of apoE mimetic peptides to mice has attenuated disease severity in experimental models of asthma, ALI, and sepsis, whereas administration of apoA-I mimetic peptides has reduced the manifestations of experimental asthma, ALI, viral pneumonia, and PH. In addition, the manifestations of experimental lung disease have been suppressed in transgenic mice that expressed human apoA-I, as well as by administration of full-length recombinant or purified apoE or apoA-I to mice. Collectively, these results may support the advancement of apoE and apoA-I mimetic peptides from preclinical animal studies to human clinical trials that can assess the safety and effectiveness of inhalational administration to attenuate disease severity.
Three clinical studies have already demonstrated that administration of the L-4F and D-4F apoA-I mimetic peptides to human subjects with cardiovascular disease by systemic and oral routes, respectively, is feasible and well tolerated (95–97). In addition, our laboratory is working toward developing a 5A apoA-I mimetic peptide as an inhalational preparation for the treatment of asthma. The potential advantages of inhalational apolipoprotein mimetic peptides for the treatment of pulmonary disease include ease of administration and site-directed delivery to lung cells. A potential disadvantage of inhalational administration could be limited efficacy if the relevant site of action turns out to be plasma or an extrapulmonary organ. This has been shown to be relevant, for example, in the case of atherosclerotic disease in which the relevant site of action of apoA-I mimetic peptides appears to be the small intestine (98). Nevertheless, the development of apolipoprotein mimetic peptides as a new treatment paradigm could represent a significant advance to benefit patients with respiratory diseases unresponsive to current therapies.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Dr. Joel Moss for his critical reading of the manuscript.
Footnotes
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health.
Author Contributions: All authors contributed to the writing and editing of this manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0060TR on April 13, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50:S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov. 2014;13:445–464. doi: 10.1038/nrd4279. [DOI] [PubMed] [Google Scholar]
- 4.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, et al. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CT, Xu YF, Wu JY, Chan L. Immunoreactive apolipoprotein E is a widely distributed cellular protein. Immunohistochemical localization of apolipoprotein E in baboon tissues. J Clin Invest. 1986;78:947–958. doi: 10.1172/JCI112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voyno-Yasenetskaya TA, Dobbs LG, Erickson SK, Hamilton RL. Low density lipoprotein- and high density lipoprotein-mediated signal transduction and exocytosis in alveolar type II cells. Proc Natl Acad Sci USA. 1993;90:4256–4260. doi: 10.1073/pnas.90.9.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARγ/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takemura R, Werb Z. Modulation of apoprotein E secretion in response to receptor-mediated endocytosis in resident and inflammatory macrophages. J Exp Med. 1984;159:167–178. doi: 10.1084/jem.159.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Chen Z, Chintagari NR, Bhaskaran M, Jin N, Narasaraju T, Liu L. Alveolar type I cells protect rat lung epithelium from oxidative injury. J Physiol. 2006;572:625–638. doi: 10.1113/jphysiol.2005.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N. Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res. 2009;50:S178–S182. doi: 10.1194/jlr.R800070-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L991–L997. doi: 10.1152/ajplung.00013.2008. [DOI] [PubMed] [Google Scholar]
- 12.Massaro D, Massaro GD. Developmental alveologenesis: new roles for ApoE and LDL receptor. Pediatr Res. 2011;70:458–461. doi: 10.1203/PDR.0b013e31822f24df. [DOI] [PubMed] [Google Scholar]
- 13.Ryan AJ, Medh JD, McCoy DM, Salome RG, Mallampalli RK. Maternal loading with very low-density lipoproteins stimulates fetal surfactant synthesis. Am J Physiol Lung Cell Mol Physiol. 2002;283:L310–L318. doi: 10.1152/ajplung.00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, Lemaître V, D’Armiento J. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–L1208. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang Q, Huang Z, Lin H, Ni J, Lu H, Chen X, Wang Z, Lin L. Apolipoprotein E deficiency and high-fat diet cooperate to trigger lipidosis and inflammation in the lung via the toll-like receptor 4 pathway. Mol Med Rep. 2015;12:2589–2597. doi: 10.3892/mmr.2015.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samokhin AO, Bühling F, Theissig F, Brömme D. ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am J Pathol. 2010;176:1148–1156. doi: 10.2353/ajpath.2010.090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naura AS, Hans CP, Zerfaoui M, Errami Y, Ju J, Kim H, Matrougui K, Kim JG, Boulares AH. High-fat diet induces lung remodeling in ApoE-deficient mice: an association with an increase in circulatory and lung inflammatory factors. Lab Invest. 2009;89:1243–1251. doi: 10.1038/labinvest.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-γ activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 19.Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, Pickworth J, Kiely DG, Crossman DC, Francis SE. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011;179:1693–1705. doi: 10.1016/j.ajpath.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen DJ, Ohkubo N, Oddo J, Van Kanegan MJ, Neil J, Li F, Colton CA, Vitek MP. Apolipoprotein E and peptide mimetics modulate inflammation by binding the SET protein and activating protein phosphatase 2A. J Immunol. 2011;186:2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Moore TV, Li Z, Sperling AI, Zhang C, Andrade J, Rodriguez A, Bahroos N, Huang Y, Morrisey EE, et al. Gata5 deficiency causes airway constrictor hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2014;50:787–795. doi: 10.1165/rcmb.2013-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X, Dai C, Fredriksson K, Lam J, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Jeffries N, et al. Human apolipoprotein E genotypes differentially modify house dust mite-induced airway disease in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L206–L215. doi: 10.1152/ajplung.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang ZL, Li YF, Song JY, Guo YQ. Effects of β3-adrenoceptor activation on the interaction between adrenoceptors and angiotensin II receptors in apolipoprotein E knockout mouse lung. Eur J Pharmacol. 2014;742:75–80. doi: 10.1016/j.ejphar.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Arunachalam G, Sundar IK, Hwang JW, Yao H, Rahman I. Emphysema is associated with increased inflammation in lungs of atherosclerosis-prone mice by cigarette smoke: implications in comorbidities of COPD. J Inflamm (Lond) 2010;7:34. doi: 10.1186/1476-9255-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita CM, Fessler MB, Vasanthamohan L, Lac J, Madenspacher J, McCaig L, Yao L, Wang L, Puntorieri V, Mehta S, et al. Apolipoprotein E-deficient mice are susceptible to the development of acute lung injury. Respiration. 2014;87:416–427. doi: 10.1159/000358438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen NR, Møller P, Jensen KA, Vogel U, Ladefoged O, Loft S, Wallin H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part Fibre Toxicol. 2009;6:2. doi: 10.1186/1743-8977-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced lung injury and fibrosis. Am J Respir Cell Mol Biol. 2016;54:436–446. doi: 10.1165/rcmb.2015-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madenspacher JH, Azzam KM, Gong W, Gowdy KM, Vitek MP, Laskowitz DT, Remaley AT, Wang JM, Fessler MB. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J Biol Chem. 2012;287:43730–43740. doi: 10.1074/jbc.M112.377192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni JQ, Ouyang Q, Lin L, Huang Z, Lu H, Chen X, Lin H, Wang Z, Xu D, Zhang Y. Role of Toll-like receptor 4 on lupus lung injury and atherosclerosis in LPS-challenge ApoE-/- mice. Clin Dev Immunol. 2013;2013:476856. doi: 10.1155/2013/476856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Oosten M, Rensen PC, Van Amersfoort ES, Van Eck M, Van Dam AM, Breve JJ, Vogel T, Panet A, Van Berkel TJ, Kuiper J. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J Biol Chem. 2001;276:8820–8824. doi: 10.1074/jbc.M009915200. [DOI] [PubMed] [Google Scholar]
- 31.Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J Clin Invest. 1997;99:2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Thompson PA, Kitchens RL. Infection induces a positive acute phase apolipoprotein E response from a negative acute phase gene: role of hepatic LDL receptors. J Lipid Res. 2008;49:1782–1793. doi: 10.1194/jlr.M800172-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Read TE, Harris HW, Grunfeld C, Feingold KR, Calhoun MC, Kane JP, Rapp JH. Chylomicrons enhance endotoxin excretion in bile. Infect Immun. 1993;61:3496–3502. doi: 10.1128/iai.61.8.3496-3502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 35.de Bont N, Netea MG, Demacker PN, Kullberg BJ, van der Meer JW, Stalenhoef AF. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest. 2000;30:818–822. doi: 10.1046/j.1365-2362.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- 36.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- 37.Kattan OM, Kasravi FB, Elford EL, Schell MT, Harris HW. Apolipoprotein E-mediated immune regulation in sepsis. J Immunol. 2008;181:1399–1408. doi: 10.4049/jimmunol.181.2.1399. [DOI] [PubMed] [Google Scholar]
- 38.Chuang K, Elford EL, Tseng J, Leung B, Harris HW. An expanding role for apolipoprotein E in sepsis and inflammation. Am J Surg. 2010;200:391–397. doi: 10.1016/j.amjsurg.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Christensen DJ, Vitek MP, Sullivan PM, Laskowitz DT. APOE genotype affects outcome in a murine model of sepsis: implications for a new treatment strategy. Anaesth Intensive Care. 2009;37:38–45. doi: 10.1177/0310057X0903700111. [DOI] [PubMed] [Google Scholar]
- 41.Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moretti EW, Morris RW, Podgoreanu M, Schwinn DA, Newman MF, Bennett E, Moulin VG, Mba UU, Laskowitz DT Perioperative Genetics and Safety Outcomes Study (PEGASUS) Investigative Team. APOE polymorphism is associated with risk of severe sepsis in surgical patients. Crit Care Med. 2005;33:2521–2526. doi: 10.1097/01.ccm.0000186368.96146.fb. [DOI] [PubMed] [Google Scholar]
- 43.van den Elzen P, Garg S, León L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 44.Allan LL, Hoefl K, Zheng DJ, Chung BK, Kozak FK, Tan R, van den Elzen P. Apolipoprotein-mediated lipid antigen presentation in B cells provides a pathway for innate help by NKT cells. Blood. 2009;114:2411–2416. doi: 10.1182/blood-2009-04-211417. [DOI] [PubMed] [Google Scholar]
- 45.Gérard HC, Fomicheva E, Whittum-Hudson JA, Hudson AP. Apolipoprotein E4 enhances attachment of Chlamydophila (Chlamydia) pneumoniae elementary bodies to host cells. Microb Pathog. 2008;44:279–285. doi: 10.1016/j.micpath.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Gérard HC, Wang GF, Balin BJ, Schumacher HR, Hudson AP. Frequency of apolipoprotein E (APOE) allele types in patients with Chlamydia-associated arthritis and other arthritides. Microb Pathog. 1999;26:35–43. doi: 10.1006/mpat.1998.0242. [DOI] [PubMed] [Google Scholar]
- 47.Nazzal D, Therville N, Yacoub-Youssef H, Garcia V, Thomsen M, Levade T, Segui B, Benoist H. Apolipoprotein E-deficient mice develop an anti-Chlamydophila pneumoniae T helper 2 response and resist vascular infection. J Infect Dis. 2010;202:782–790. doi: 10.1086/655700. [DOI] [PubMed] [Google Scholar]
- 48.Fu P, Wang AM, He LY, Song JM, Xue JC, Wang CQ. Elevated serum ApoE levels are associated with bacterial infections in pediatric patients. J Microbiol Immunol Infect. 2014;47:122–129. doi: 10.1016/j.jmii.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Wang Y, Wang A, Fu P, Yang Y. The diagnostic value of apolipoprotein E in pediatric patients with invasive bacterial infections. Clin Biochem. 2012;45:215–218. doi: 10.1016/j.clinbiochem.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Rice SJ, Liu X, Miller B, Joshi M, Zhu J, Caruso C, Gilbert C, Toth J, Reed M, Rassaei N, et al. Proteomic profiling of human plasma identifies apolipoprotein E as being associated with smoking and a marker for squamous metaplasia of the lung. Proteomics. 2015;15:3267–3277. doi: 10.1002/pmic.201500029. [DOI] [PubMed] [Google Scholar]
- 51.Su WP, Chen YT, Lai WW, Lin CC, Yan JJ, Su WC. Apolipoprotein E expression promotes lung adenocarcinoma proliferation and migration and as a potential survival marker in lung cancer. Lung Cancer. 2011;71:28–33. doi: 10.1016/j.lungcan.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Chen Z, Chen J, Pan J, Zhang W, Pan Q, Ding H, Lin X, Wen X, Li Y, et al. The diagnostic value of apolipoprotein E in malignant pleural effusion associated with non-small cell lung cancer. Clin Chim Acta. 2013;421:230–235. doi: 10.1016/j.cca.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Gueddari N, Favre G, Hachem H, Marek E, Le Gaillard F, Soula G. Evidence for up-regulated low density lipoprotein receptor in human lung adenocarcinoma cell line A549. Biochimie. 1993;75:811–819. doi: 10.1016/0300-9084(93)90132-c. [DOI] [PubMed] [Google Scholar]
- 54.Leman LJ, Maryanoff BE, Ghadiri MR. Molecules that mimic apolipoprotein A-I: potential agents for treating atherosclerosis. J Med Chem. 2014;57:2169–2196. doi: 10.1021/jm4005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 56.Provost PR, Boucher E, Tremblay Y. Apolipoprotein A-I, A-II, C-II, and H expression in the developing lung and sex difference in surfactant lipids. J Endocrinol. 2009;200:321–330. doi: 10.1677/JOE-08-0238. [DOI] [PubMed] [Google Scholar]
- 57.Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Dagur PK, McCoy JP, Remaley AT, Levine SJ. Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am J Respir Cell Mol Biol. 2012;47:186–195. doi: 10.1165/rcmb.2011-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, Jung S, Jang AS, Park SW, Uh ST, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182:633–642. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 59.Dai C, Yao X, Vaisman B, Brenner T, Meyer KS, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, et al. ATP-binding cassette transporter 1attenuates ovalbumin-induced neutrophilic airway inflammation. Am J Respir Cell Mol Biol. 2014;51:626–636. doi: 10.1165/rcmb.2013-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bates SR, Tao JQ, Yu KJ, Borok Z, Crandall ED, Collins HL, Rothblat GH. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2008;38:283–292. doi: 10.1165/rcmb.2007-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L869–L878. doi: 10.1152/ajplung.00077.2003. [DOI] [PubMed] [Google Scholar]
- 62.Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L949–L957. doi: 10.1152/ajplung.90394.2008. [DOI] [PubMed] [Google Scholar]
- 63.Park SW, Lee EH, Lee EJ, Kim HJ, Bae DJ, Han S, Kim D, Jang AS, Uh ST, Kim YH, et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin Exp Allergy. 2013;43:914–927. doi: 10.1111/cea.12143. [DOI] [PubMed] [Google Scholar]
- 64.Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, Feroah T, Weihrauch D, Schulte ML, Jones DW, et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res. 2010;51:2560–2570. doi: 10.1194/jlr.M004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 67.Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med. 2015;192:660–668. doi: 10.1164/rccm.201504-0763PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang SH, Yuan SG, Peng DQ, Zhao SP. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 2012;225:105–114. doi: 10.1016/j.atherosclerosis.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 69.Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005;338:1126–1136. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 70.Tiniakou I, Drakos E, Sinatkas V, Van Eck M, Zannis VI, Boumpas D, Verginis P, Kardassis D. High-density lipoprotein attenuates Th1 and th17 autoimmune responses by modulating dendritic cell maturation and function. J Immunol. 2015;194:4676–4687. doi: 10.4049/jimmunol.1402870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJ, et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J Immunol. 2011;186:576–583. doi: 10.4049/jimmunol.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011;52:499–508. doi: 10.1194/jlr.M012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barochia AV, Kaler M, Cuento RA, Gordon EM, Weir NA, Sampson M, Fontana JR, MacDonald S, Moss J, Manganiello V, et al. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med. 2015;191:990–1000. doi: 10.1164/rccm.201411-1990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155:842–848. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 75.Nicholas BL, Skipp P, Barton S, Singh D, Bagmane D, Mould R, Angco G, Ward J, Guha-Niyogi B, Wilson S, et al. Identification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1049–1060. doi: 10.1164/rccm.200906-0857OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim C, Lee JM, Park SW, Kim KS, Lee MW, Paik S, Jang AS, Kim J, Uh S, Kim Y, et al. Attenuation of cigarette smoke-induced emphysema in mice by apolipoprotein A-1 overexpression. Am J Respir Cell Mol Biol. 2016;54:91–102. doi: 10.1165/rcmb.2014-0305OC. [DOI] [PubMed] [Google Scholar]
- 77.Yan YJ, Li Y, Lou B, Wu MP. Beneficial effects of ApoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sci. 2006;79:210–215. doi: 10.1016/j.lfs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Ma J, Liao XL, Lou B, Wu MP. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim Biophys Sin (Shanghai) 2004;36:419–424. doi: 10.1093/abbs/36.6.419. [DOI] [PubMed] [Google Scholar]
- 79.Jiao YL, Wu MP. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine. 2008;43:83–87. doi: 10.1016/j.cyto.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Van Linthout S, Spillmann F, Graiani G, Miteva K, Peng J, Van Craeyveld E, Meloni M, Tölle M, Escher F, Subasigüller A, et al. Down-regulation of endothelial TLR4 signalling after apo A-I gene transfer contributes to improved survival in an experimental model of lipopolysaccharide-induced inflammation. J Mol Med (Berl) 2011;89:151–160. doi: 10.1007/s00109-010-0690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Dong JB, Wu MP. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur J Pharmacol. 2008;590:417–422. doi: 10.1016/j.ejphar.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 82.Sharifov OF, Xu X, Gaggar A, Grizzle WE, Mishra VK, Honavar J, Litovsky SH, Palgunachari MN, White CR, Anantharamaiah GM, et al. Anti-inflammatory mechanisms of apolipoprotein A-I mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS One. 2013;8:e64486. doi: 10.1371/journal.pone.0064486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwon WY, Suh GJ, Kim KS, Kwak YH, Kim K. 4F, apolipoprotein AI mimetic peptide, attenuates acute lung injury and improves survival in endotoxemic rats. J Trauma Acute Care Surg. 2012;72:1576–1583. doi: 10.1097/TA.0b013e3182493ab4. [DOI] [PubMed] [Google Scholar]
- 84.Tu J, Zhang B, Chen Y, Liang B, Liang D, Liu G, He F. Association of apolipoprotein A1 -75 G/A polymorphism with susceptibility to the development of acute lung injury after cardiopulmonary bypass surgery. Lipids Health Dis. 2013;12:172. doi: 10.1186/1476-511X-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emancipator K, Csako G, Elin RJ. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henning MF, Herlax V, Bakás L. Contribution of the C-terminal end of apolipoprotein AI to neutralization of lipopolysaccharide endotoxic effect. Innate Immun. 2011;17:327–337. doi: 10.1177/1753425910370709. [DOI] [PubMed] [Google Scholar]
- 87.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M, Fogelman AM. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106:1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 89.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 90.Zamanian-Daryoush M, Lindner D, Tallant TC, Wang Z, Buffa J, Klipfell E, Parker Y, Hatala D, Parsons-Wingerter P, Rayman P, et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288:21237–21252. doi: 10.1074/jbc.M113.468967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng T, Dai X, Zhou DL, Lv Y, Miao LY. Correlation of apolipoprotein A-I kinetics with survival and response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Med Oncol. 2015;32:407. doi: 10.1007/s12032-014-0407-8. [DOI] [PubMed] [Google Scholar]
- 92.Marchi N, Mazzone P, Fazio V, Mekhail T, Masaryk T, Janigro D. ProApolipoprotein A1: a serum marker of brain metastases in lung cancer patients. Cancer. 2008;112:1313–1324. doi: 10.1002/cncr.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130:776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee Eh, Lee EJ, Kim Hj, Jang As, Koh Es, Uh ST, Kim Yh, Park SW, Park CS. Overexpression of apolipoprotein A1 in the lung abrogates fibrosis in experimental silicosis. PLoS One. 2013;8:e55827. doi: 10.1371/journal.pone.0055827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunbar RL, Bloedon LT, Duffy D, Norris RB, Movva R, Navab M, Fogelman AM, Rader DJ. Daily oral administration of the apolipoprotein A-I mimetic peptide D-4F in patients with heart disease or equivalent risk improves high-density lipoprotein anti-inflammatory function [abstract] J Am Coll Cardiol. 2007;49:366A. [Google Scholar]
- 98.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.